Abstract
There are limited data on the relationship of acute dental infections with hospitalisation and new-onset atrial fibrillation (AF). This study aimed to assess the relationship between acute periapical abscess and incident AF. This was a retrospective cohort study from a French national database of patients hospitalized in 2013 (3.4 million patients) with at least five years of follow up. In total, 3,056,291 adults (55.1% female) required hospital admission in French hospitals in 2013 while not having a history of AF. Of 4693 patients classified as having dental periapical abscess, 435 (9.27%) developed AF, compared to 326,241 (10.69%) without dental periapical abscess that developed AF over a mean follow-up of 4.8 ± 1.7 years. Multivariable analysis indicated that dental periapical abscess acted as an independent predictor for new onset AF (p < 0.01). The CHA2DS2VASc score in patients with acute dental periapical abscess had moderate predictive value for development of AF, with Area Under the Curve (AUC) 0.73 (95% CI, 0.71–0.76). An increased risk of new onset AF was identified for individuals hospitalized with dental periapical abscess. Careful follow up of patients with severe, acute dental periapical infections is needed for incident AF, as well as investigations of possible mechanisms linking these conditions.
1. Introduction
Atrial Fibrillation (AF) is the commonest cardiac rhythm disorder (affecting about 1–3% of the population) [1,2]. Sufferers are 3–5 times more times likely to develop stroke, heart failure and myocardial infarction [3]. Although people with AF can suffer a variety of symptoms such as chest pain, dizziness and fatigue, many patients are asymptomatic and only present late with advanced and serious heart problems or a stroke [4]. Population screening programmes are therefore important to pick up cases early. Although AF represents a significant cause of population mortality and morbidity, as well as health care expenditure, the latter is set to escalate due to an ageing population profile given that AF is more prevalent in older people [4]. There are many risk factors for AF, such as: ageing, sex (male), diabetes mellitus, and hypertension [5]. However, many of the risk factors associated with AF are also linked to poor oral health. Indeed, the latter may be a potential causative factor for AF since inflammatory markers generated by oral diseases are found to have a direct effect on the cellular function and electrophysiological remodelling of the heart [6,7].
The main oral disease which has so far been studied in relation to CVD is periodontitis, which is defined as inflammation of the gums and destruction of the surrounding bone. Most adults have some form of gum disease and 11.2% have suffer with severe periodontitis [8]. Severe periodontal disease and tooth loss together affects around 3.9 billion people globally and is estimated as the 6th most frequent global disease [9,10]. Studies show biomarkers such as IL-6 and CRP, which are implicated in both cardiovascular disease (CVD) and periodontitis, are linked to AF [11,12]. Other studies also report a reduction in inflammation following periodontal treatment [13,14], giving rise to a consensus that there is moderate evidence of a decrease in inflammatory biomarkers following treatment of periodontitis [15].
More recently, oral diseases related to CVD apart from periodontitis have also been investigated, such as apical (or periapical) periodontitis, which is an infection of the tooth and root canal system leading to destruction of the surrounding tissues [16]. More than half the population have been found to have apical periodontitis on at least one tooth, which is often presented as a chronic disease [17]. A systematic review reported an association between CVD and chronic apical infection, which suggests that while oral infections elicit a local tissue response, there are wider, systemic effects [18]. However, the primary studies included in the review only studied asymptomatic and chronic apical infections diagnosed through radiographic imaging, and AF was not included as separate entity within the study of CVD effects. Studies investigating oral diseases with CVD do not generally include AF as an outcome for analysis, although one recent retrospective study found that patients with a history of AF had a statistically significant incidence of diagnosed apical infection [19]. Of note, there is a positive association for reduced endothelial function with chronic apical infections [19,20] (and participants were also free of periodontitis), and given the associations between AF and endothelial dysfunction, this may have implications for increasing AF incidence [21].
There are a few retrospective cohort studies linking oral diseases with AF [22,23,24]. A recent 17-year longitudinal study has also found severe periodontitis with higher associations with AF (multivariable adjusted HR, 1.31, 95% CI, 1.06–1.62) [25]. One case report described a patient admitted with acute dental abscess and urticaria, that had also developed persistent AF, although the AF diagnosis could have been a coincidence [26].
Thus far, studies have only focused on asymptomatic, chronic low-grade inflammatory processes associated with oral disease, such as chronic periodontitis, rather than symptomatic or severe dental infections [23], although a recent systematic review [27] concluded there is a need for further research in this area. Incidence of acute dental infections and hospital attendance are common; 5–46% of all people suffer with an acute periapical abscess in their lifetime and 0.7% of all emergency attendances are related to dental problems [28,29,30]. Acute periapical abscess is more prevalent for people in disadvantaged groups and can have a significant impact causing sleepless nights, missed work and reduced quality of life [31].
In the present study, we aimed to investigate the relationship of acute dental periapical abscess with new-onset AF in a nationwide cohort study. We tested the hypothesis that acute periapical abscess is associated with incident AF, in relation to risk strata (using the CHA2DS2VASc score) using data from the French national hospital database from the Program de Médicalisation des Systèmes d’Information (PMSI) [32].
2. Materials and Methods
2.1. Study Design
This was a retrospective longitudinal cohort study which covers the full population and admissions across 1546 hospitals in France. All patients in France are discharged using the PMSI database and categorised with codes relating to diagnosis and treatment, as in the US Medicare system [33]. Patients are linked with unique identifiers for multiple admissions, ensuring the data remains anonymised. The inpatient International Classification of Diseases (ICD) codes were captured and commonly used for gathering large amounts of data [34].
The population for analysis was taken from French hospitals using the PMSI database, which included adults who were admitted from 01 January until 31 December 2013 and had at least five years of follow up, unless deceased. The Tenth Revision codes, International Classification of Diseases (ICD-10 codes) were used to identify primary and secondary diagnosis specified as infection inside and surrounding the root of the tooth and including hospital admission (dental periapical abscess K04.6, K04.7) [35]. After exclusion of patients with a history of previous AF, patients were followed up for new-onset AF with hospitalisation using ICD-10 codes. Identifying AF using an electronic database is considered reliable, and PMSI data have been evaluated in previous studies [32,36]. Baseline characteristics from patients hospitalised during 2013 with or without dental periapical abscesses were recorded, in addition to age, gender, and medical history. Events and predictors for AF were monitored with at least 5 years follow up. We also calculated the CHA2DS2VASc score (congestive heart failure, high blood pressure, diabetes, stroke, heart disease, age, gender) which is used for stroke risk stratification, but used this to stratify risk for new-onset AF where acute periapical abscess was present [34,35,36,37].
2.2. Ethical Approval
The research database involved a collection of non-identifiable information used for data analysis with external researchers. The study was conducted retrospectively and, as patients were not involved in its conduct, there was no impact on their care. Studies such as this study have been labelled as clinical audits, therefore sponsorship was not required from the University of Liverpool or the University of Tours [32,37,38]. Comparable to previous articles, informed consent was not obtained as the data were retrospective [22,23]. All patient information remained confidential and anonymised. The French law allows approval of certain public institutions, such as large university hospitals, to self-declare authorisation and access to confidential information when using electronic data for research purposes. The French Data Protection Authority have granted access to the PMSI data between 2008 and 2015, for the purpose of assessing the prevalence and incidence of cardiovascular diseases and their complications to the University of Tours in France. Procedures for data collection and management were approved by the Commission Nationale de l’Informatique et des Libertés (CNIL), the independent National Ethical Committee protecting human rights in France, which ensures that all information is kept confidential and anonymous (authorisation number 1897139).
2.3. Statistical Analysis
Baseline characteristics of patients were given as patient number (N) and percentages (%), or mean ± standard deviation, and included CHA2DS2VASc scores with average year follow ups using mean, median and interquartile range. Predictors for new-onset AF were calculated during follow up for the whole population and for patients with dental periapical abscess, which were assessed using univariate and multivariable Cox regression models. For patients with no previous dental periapical abscess, data were censored in case of dental periapical abscess during follow-up at the date of this event. Incidence and hazard ratio predictions, with confidence interval (95% CI) and p value (<0.05) for new-onset atrial fibrillation were calculated in patients with dental periapical abscess during follow up, using different CHA2DS2VASc scores. The specificity and sensitivity for CHA2DS2VASc scores as a predictor for new-onset AF and dental periapical abscess were examined further by plotting the Area Under the Curve-Receiver Operating Characteristic (AUC-ROC). All comparisons with p < 0.05 were considered statistically significant. All analyses were performed using Enterprise Guide 7.1, (SAS Institute Inc., SAS Campus Drive, Cary, NC, USA), USA and STATA version 12.0 (Stata Corp, College Station, TX, USA).
3. Results
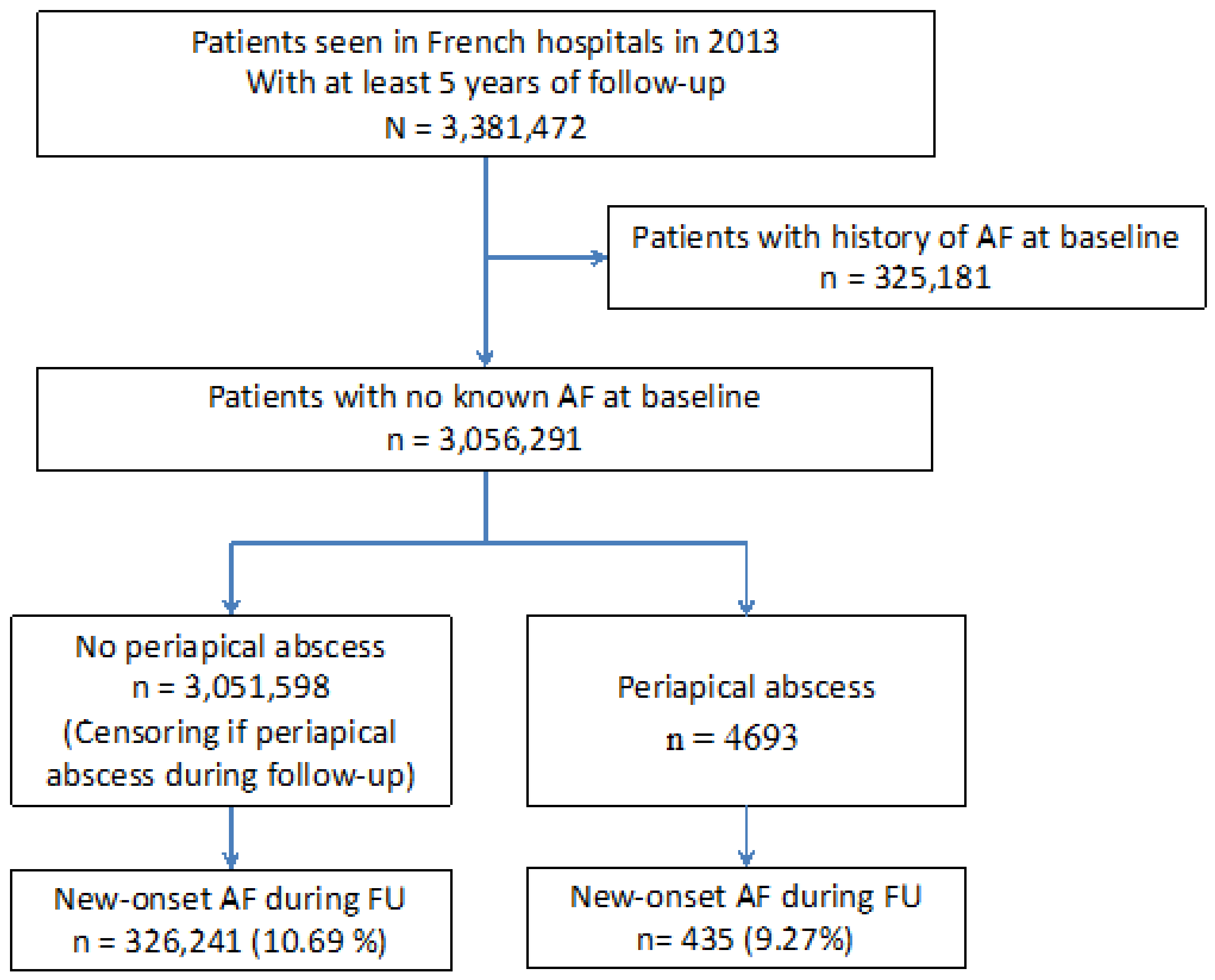
In total, 3,381,472 patients were identified in French hospitals during 2013 that attended emergency services and required hospital admission. The study population included at least five years of follow-up, unless deceased, and excluded patients with history of previous AF. A flowchart of study enrolment is shown in Figure 1. Of patients at baseline, 4693 were identified with a history of dental periapical abscess requiring hospitalisation. During a mean follow up period of 4.8 ± 1.7 years (median 5.5, IQR 5.1–5.8 years), there were 435 (9.27%) patients with a history of dental periapical abscess that were diagnosed with new-onset AF, compared to 326,241 (10.69%) without dental periapical abscess that developed AF.
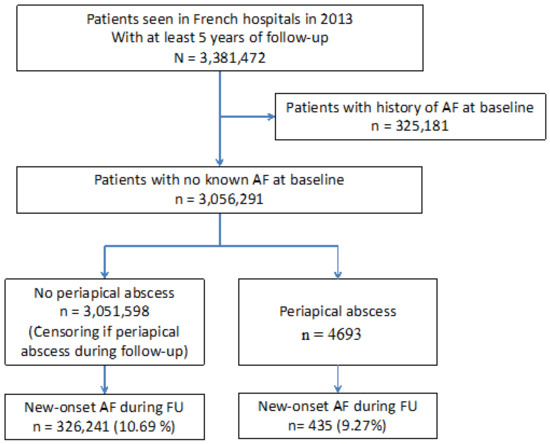
Figure 1.
Flow chart of the study patients.
Baseline characteristics of patients are shown in Table 1. Patients with a history of a dental periapical abscess were older (p value < 0.0001) and were more often male (p value < 0.0001). The prevalence of comorbidities for patients with dental periapical abscess was higher, including hypertension, diabetes mellitus, heart failure, obesity, alcohol related diagnosis, chronic kidney disease, and lung disease (p value < 0.0001, respectively). As shown in Table 2, CHA2DS2VASc scores were higher for those with periapical abscess at baseline (p value < 0.01).

Table 1.
Baseline characteristics of patients seen in French hospitals in 2013 at least 5 years of follow-up (mean follow-up 4.8 ± 1.7 years, median 5.5, IQR 5.1–5.8 years).

Table 2.
CHA2DS2VASc score at baseline in patients seen in French hospitals in 2013 at least 5 years of follow-up (mean follow-up 4.8 ± 1.7 years, median 5.5, IQR 5.1–5.8 years).
The multivariable analysis represented in Table 3 found periapical abscess to be an independent predictor for new-onset AF, hazard ratio (HR) 1.11 (95% confidence interval (CI), 1.01–1.22). Other significant predictors for new-onset AF included, among others, older age, male sex, hypertension, diabetes mellitus, heart failure, aortic stenosis, obesity, and smoking (p value < 0.0001, respectively). For patients with a dental periapical abscess, there were positive associations for new-onset AF with older age, male sex, hypertension, aortic regurgitation, dilated cardiomyopathy, previous pacemaker or defibrillator and inflammatory disease (all p value < 0.05) using multivariable analysis, see Table 4.

Table 3.
Predictors of new-onset atrial fibrillation during follow-up in the whole population of patients seen in French hospitals in 2013 with at least 5 years of follow-up (mean follow-up 4.8 ± 1.7 years, median 5.5, IQR 5.1–5.8 years).

Table 4.
Predictors of new-onset atrial fibrillation during follow-up in patients with periapical abscess seen in French hospitals in 2013 with at least 5 years of follow-up (mean follow-up 4.8 ± 1.7 years, median 5.5, IQR 5.1–5.8 years).
The incidence (per 100 person-years) of new-onset AF generally increased with higher CHA2DS2VASc scores for patients with a history of dental periapical abscess, see Table 5. The HRs for new-onset AF in patients with dental periapical abscess using different CHA2DS2VASc scores (in comparison to patients with a score of zero) are shown in Table 6. As expected, patients with a CHA2DS2VASc score 8 had the highest HR of 34.2.0 (95% CI, 8.3–140.8).

Table 5.
Incidence (per 100 person-years) of new-onset atrial fibrillation with different CHA2DS2VASc scores in patients with periapical abscess seen in French hospitals in 2013 with at least 5 years of follow-up (mean follow-up 4.8 ± 1.7 years, median 5.5, IQR 5.1–5.8 years).

Table 6.
Hazard ratio of new-onset atrial fibrillation in patients with acute dental periapical abscess using different CHA2DS2VASc scores (in comparison to the patients with score of 0).
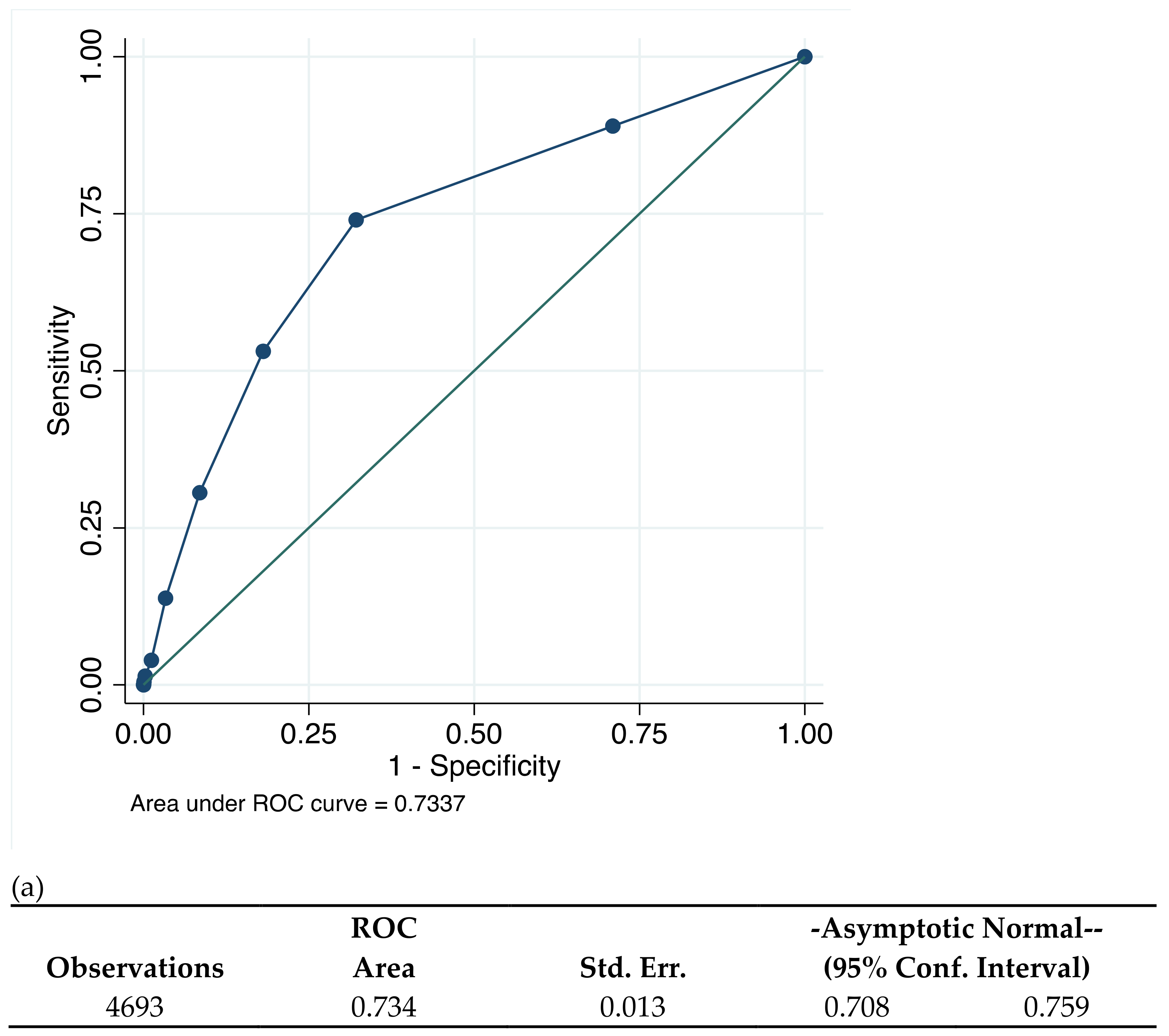
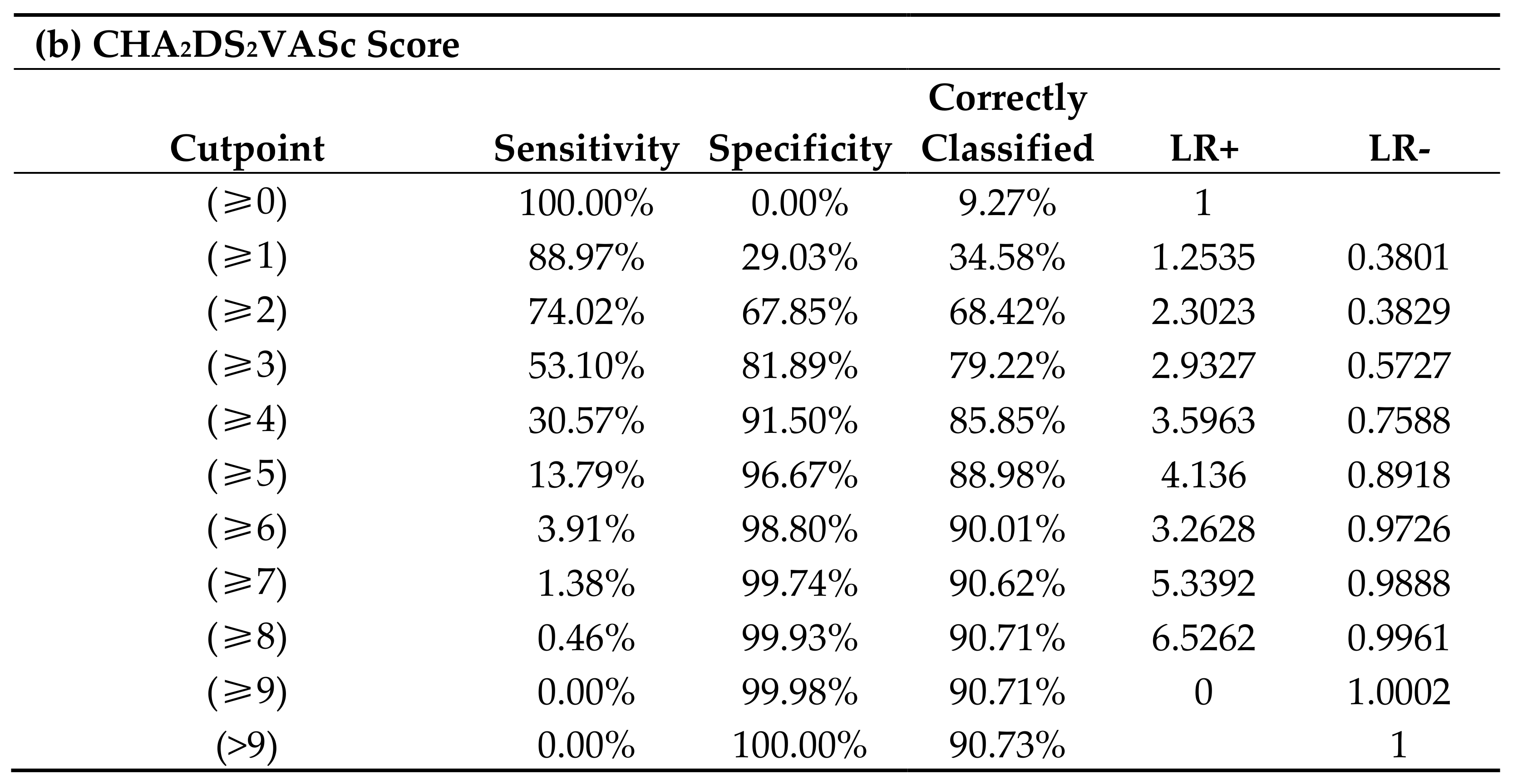
Figure 2 illustrates that the CHA2DS2VASc scores had an intermediate predictive value for incident AF amongst patients with a history of dental periapical abscess, with AUC 0.73 (95% CI, 0.71–0.76). This AUC correctly predicted 79.2% of patients for new-onset AF with CHA2DS2VASc score ≥ 3 with a specificity of patients at 53.1% and sensitivity 81.9%. CHA2DS2VASc scores ≥ 2 were correctly classified at 68.4%, with sensitivity of 74.0% and specificity of 67.9%.
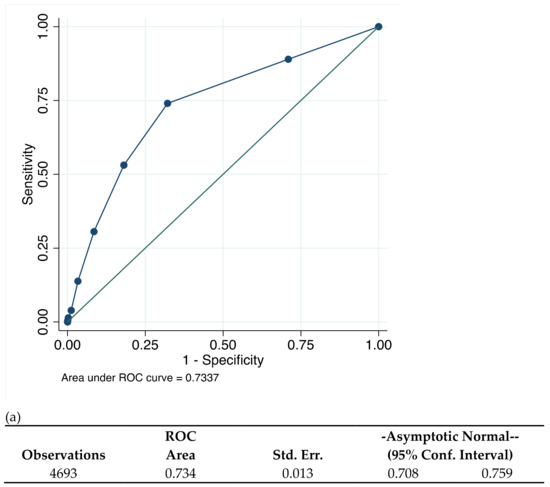
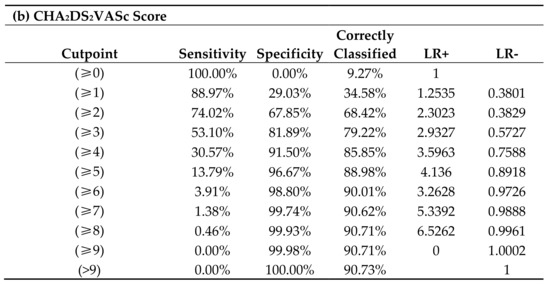
Figure 2.
(a) CHA2DS2VASc score and prediction of new-onset AF and (b) correctly classified individual by CHA2DS2VASc scores.
4. Discussion
In this study to investigate the occurrence of new-onset AF in patients who were hospitalised because of a dental periapical abscess, our principal findings are as follows: (i) dental periapical abscess acted as an independent predictor for new onset AF; (ii) the CHA2DS2VASc score in patients with acute dental periapical abscess has significant predictive value for incident AF; and (iii) other associations of new-onset AF during follow-up in patients with dental periapical abscess were older age, male sex, hypertension, aortic regurgitation, dilated cardiomyopathy, previous pacemaker or defibrillator and inflammatory disease.
To our knowledge, this is the first study to investigate patients with acute dental infections and hospitalisation, as a population-based cohort study for new-onset AF, although as discussed previously, a recent systematic review by Hassan et al. has summarised that there is a need for more research in this area [27]. This is important given that hospitalisations because of dental abscesses are not uncommon—for example, a retrospective analysis in the United States found that over 61,000 patients had been hospitalised for this reason over an 8 year period, and a total of 66 people had died [39].
Although outwith the main empirical focus of our paper, it is useful to consider biological plausibility and the evidence for possible mechanisms linking acute dental infections to AF. Dental periapical abscess represents an immune response from infected pulp tissue mainly due to Streptococcus, Prevotella and Fusobacterium species [40]. The presence of different Gram-positive and Gram-negative oral bacteria induce conflict between microbial pathogens and host immune system, resulting in severe pain and destruction of the surrounding dental tissues [41,42]. The presence of different Gram-positive and Gram-negative oral bacteria in the bloodstream can induce cytokines interleukin-1 (IL-1 β), tumour necrosis factor (TNF-α) and metalloproteinases (MMPs), leading to a dysregulated acute response, and in some instances leading to sepsis, a common life-threatening complication when the body’s immune system responds to infection and injures its own tissues and organs [43,44,45]. It is also known that proinflammatory markers (IL-1, TNF-α) and MMPs associated with acute dental infections and cellular remodelling are expressed during occurrence of AF [46,47,48]. The mechanisms that are expressed during dental periapical abscess may be consequential to the heart, even if the infection is resolved. Other articles have shown that some types of severe infection, such as sepsis, are associated with new-onset AF, although it is not clear in this study how many patients with hospitalised dental periapical abscess have also suffered with sepsis, therefore more research is needed in this area to understand the impact [49,50].
The CHA2DS2VASc score is a validated clinical tool used to predict the risk of stroke and thromboembolism for patients with confirmed diagnosis of AF [51]. Given that the CHA2DS2VASc is a cluster of common cardiovascular risk factors, the score also has been reported to predict adverse events for patients without AF, such as mortality, stroke, cardiovascular events, and subsequent diagnosis of AF [37,52,53]. Prior studies that have investigated oral diseases and AF have never previously evaluated the CHA2DS2VASc score as a prediction tool for new-onset AF. Our results suggest that CHA2DS2VASc score may have modest predictive value for predicting AF, as in the general population without oral sepsis. Nonetheless, the CHA2DS2VASc score was not proposed to predict AF, but to risk stratify for stroke, which may suggest a weakness for the AUC score and design.
4.1. Clinical Implications
With an ageing population demographic predicted to involve 25% being over 60 by 2050, more of whom are likely to retain their teeth into old age; and with AF being more prevalent with increased age, implications of links between poor oral health and CVD and AF are of concern to the dental profession and the health system in general [54,55]. Moreover, since the COVID-19 pandemic has led to suspension of routine dental services in many parts of the world since restoring teeth involves aerosol generating procedures, increased numbers of people are attending services with oral and dental infections [56]. Interventions through improving oral health or signposting and screening for AF and CVD may help improve patient care, prevent severe cardiovascular complication, and reduce health care expenditure across the system. Finally, investigating different biomarkers for both acute and chronic oral diseases and their relationship with AF may serve as a unique model for understanding different inflammatory pathways and their relationship with AF and AF-related complications.
4.2. Limitations
We investigated dental infection using a hospital database, and although this enables us to monitor large amounts of data, a major limitation is its retrospective design, and it does not imply cause or effect. As in previous studies, our study defined diagnosis of new-onset AF during hospitalisation, rather than using detailed investigations or outpatient appointments, meaning it could underestimate the true incidence of AF. Unadjusted incidence rate of AF was lower in patients with dental periapical abscess, but these patients were markedly younger. We thus used multivariable analysis to account for differences in age and prevalent comorbidities associated with AF for the study population. Although we found dental abscess to be an independent risk factor using multivariable analysis, there may have been some confounders missing from the dataset. Nonetheless, our data accounted for more potential confounders (such as smoking, obesity and alcohol related diagnosis) which have been a limitation for other prior studies investigating oral diseases and AF [22,23,24].
Another possible limitation is that even though we accounted for selection-bias using a nationwide database with the French population, different codes may be more common in different countries and that the data presented may not be generalisable. A cross sectional study in England found dental problems accounted for 0.7% of all attendees in emergency departments and “dental unspecified”, “dental abscess” and “toothache” and were the most common codes used [30]. Currie et al. [30] went on to explain that the true number of patients attending with acute dental periapical abscess may have been underrepresented due to non-dental healthcare staff using codes “dental unspecified” and general abscess (rather than dental). It was not possible to check the categorisation of “periapical abscess” with dental imaging to confirm diagnosis, but we attempted to rectify this problem by excluding other codes used apart from “dental periapical abscess”, which we felt healthcare staff would not have coded unless confident of the diagnosis for the patient. We only included patients that were hospitalised, meaning they were likely to be managed under healthcare staff with specialty knowledge of diagnosing and treating the patient. Dental periapical abscess was included as a primary and secondary diagnosis because even as an additional finding, it would still mean the dental periapical abscess was also a problem if a hospital needed to investigate and diagnose it clinically.
5. Conclusions
Our results from this population-based cohort study demonstrate an increased risk of new onset AF for individuals hospitalised with dental periapical abscess. Careful follow up of patients with severe, acute dental infections is needed for incident AF, as well as investigations of possible mechanisms linking these conditions.
Author Contributions
Conceptualization, A.O.H., G.Y.H.L., L.F., R.V.H.; methodology, A.O.H., G.Y.H.L., A.B. (Arnaud Bisson), J.H., A.B. (Alexandre Bodin), L.F., R.V.H.; software, A.B. (Arnaud Bisson), J.H., A.B. (Alexandre Bodin), L.F.; validation, A.O.H., G.Y.H.L., A.B. (Arnaud Bisson), J.H., A.B. (Alexandre Bodin), L.F., R.V.H.; formal analysis, A.O.H., G.Y.H.L., A.B. (Arnaud Bisson), J.H., A.B. (Alexandre Bodin), L.F., R.V.H. investigation, A.O.H., G.Y.H.L., A.B. (Arnaud Bisson), J.H., A.B. (Alexandre Bodin), L.F., R.V.H.; data curation A.B. (Arnaud Bisson), J.H., A.B. (Alexandre Bodin), L.F.; writing—original draft preparation, A.O.H., G.Y.H.L., A.B. (Arnaud Bisson), J.H., A.B. (Alexandre Bodin), L.F., R.V.H.; writing—review and editing, G.Y.H.L., L.F., R.V.H.; supervision, G.Y.H.L., L.F., R.V.H.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Commission Nationale de l’Informatique et des Libertés (CNIL), the independent National Ethical Committee protecting human rights in France, (authorisation number 1897139).
Informed Consent Statement
Patient consent was waived as the data were retrospective, all patient information remained confidential and anonymised. The French law allows approval of certain public institutions, such as large university hospitals, to self-declare authorisation and access to confidential information when using electronic data for research purposes.
Data Availability Statement
The French Data Protection Authority have granted access to the PMSI data between 2008 and 2015, for the purpose of assessing the prevalence and incidence of cardiovascular diseases and their complications to the University of Tours in France.
Acknowledgments
The population for analysis was taken from French hospitals using the PMSI database.
Conflicts of Interest
The authors state that they have no conflict of interest.
References
- Adderley, N.J.; Ryan, R.; Nirantharakumar, K.; Marshall, T. Prevalence and treatment of atrial fibrillation in UK general practice from 2000 to 2016. Heart 2019, 105, 27–33. [Google Scholar] [CrossRef]
- Turakhia, M.P.; Shafrin, J.; Bognar, K.; Trocio, J.; Abdulsattar, Y.; Wiederkehr, D.; Goldman, D.P. Estimated prevalence of undiagnosed atrial fibrillation in the United States. PLoS ONE 2018, 13, e0195088. [Google Scholar] [CrossRef]
- Lloyd-Jones, D.; Adams, R.J.; Brown, T.M.; Carnethon, M.; Dai, S.; De Simone, G.; Ferguson, T.B.; Ford, E.; Furie, K.; Gillespie, C.; et al. Executive summary: Heart disease and stroke statistics—2010 update: A report from the American Heart Association. Circulation 2010, 121, 948–954. [Google Scholar] [PubMed]
- Watson, R.D.; Gibbs, C.R.; Lip, G.Y. ABC of heart failure. Clinical features and complications. BMJ 2000, 320, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Allan, V.; Honarbakhsh, S.; Casas, J.P.; Wallace, J.; Hunter, R.; Schilling, R.; Perel, P.; Morley, K.; Banerjee, A.; Hemingway, H. Are cardiovascular risk factors also associated with the incidence of atrial fibrillation? A systematic review and field synopsis of 23 factors in 32 population-based cohorts of 20 million participants. Thromb. Haemost. 2017, 117, 837–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidal, F.; Fontes, T.V.; Marques, T.V.; Gonçalves, L.S. Association between apical periodontitis lesions and plasmatic levels of C-reactive protein, interleukin 6 and fibrinogen in hypertensive patients. Int. Endod. J. 2016, 49, 1107–1115. [Google Scholar] [CrossRef]
- Psychari, S.N.; Apostolou, T.S.; Sinos, L.; Hamodraka, E.; Liakos, G.; Kremastinos, D.T. Relation of elevated C-reactive protein and interleukin-6 levels to left atrial size and duration of episodes in patients with atrial fibrillation. Am. J. Cardiol. 2005, 95, 764–767. [Google Scholar] [CrossRef]
- Kassebaum, N.J.; Bernabe, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.; Marcenes, W. Global burden of severe periodontitis in 1990–2010: A systematic review and meta-regression. J. Dent. Res. 2014, 93, 1045–1053. [Google Scholar] [CrossRef]
- Jin, L.J.; Lamster, I.B.; Greenspan, J.S.; Pitts, N.B.; Scully, C.; Warnakulasuriya, S. Global burden of oral diseases: Emerging concepts, management and interplay with systemic health. Oral Dis. 2016, 22, 609–619. [Google Scholar] [CrossRef]
- Murray, C.J.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2197–2223. [Google Scholar] [CrossRef]
- Chung, M.K.; Martin, D.O.; Sprecher, D.; Wazni, O.; Kanderian, A.; Carnes, C.A.; Bauer, J.A.; Tchou, P.J.; Niebauer, M.J.; Natale, A.; et al. C-reactive protein elevation in patients with atrial arrhythmias: Inflammatory mechanisms and persistence of atrial fibrillation. Circulation 2001, 104, 2886–2891. [Google Scholar] [CrossRef] [Green Version]
- Zhou, P.; Waresi, M.; Zhao, Y.; Lin, H.C.; Wu, B.; Xiong, N.; Li, H.; Huang, Q.; Luo, X.; Li, J. Increased serum interleukin-6 level as a predictive biomarker for atrial fibrillation: A systematic review and meta-analysis. Rev. Port. Cardiol. 2020, 39, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.B.; Xia, W.H.; Ren, J.; Yu, B.B.; Tong, X.Z.; Chen, Y.B.; Chen, S.; Feng, L.; Dai, J.; Tao, J.; et al. Effect of Intensive Periodontal Therapy on Blood Pressure and Endothelial Microparticles in Patients with Prehypertension and Periodontitis: A Randomized Controlled Trial. J. Periodontol. 2017, 88, 711–722. [Google Scholar] [CrossRef]
- Demmer, R.T.; Trinquart, L.; Zuk, A.; Fu, B.C.; Blomkvist, J.; Michalowicz, B.S.; Ravaud, P.; Desvarieux, M. The influence of anti-infective periodontal treatment on C-reactive protein: A systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2013, 8, e77441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanz, M.; Marco Del Castillo, A.; Jepsen, S.; Gonzalez-Juanatey, J.R.; D’Aiuto, F.; Bouchard, P.; Chapple, I.; Dietrich, T.; Gotsman, I.; Graziani, F.; et al. Periodontitis and cardiovascular diseases: Consensus report. J. Clin. Periodontol. 2020, 47, 268–288. [Google Scholar] [CrossRef]
- Nair, P.N. Pathogenesis of apical periodontitis and the causes of endodontic failures. Crit. Rev. Oral Biol. Med. 2004, 15, 348–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiburcio-Machado, C.S.; Michelon, C.; Zanatta, F.B.; Gomes, M.S.; Marin, J.A.; Bier, C.A. The global prevalence of apical periodontitis: A systematic review and meta-analysis. Int. Endod. J. 2021, 54, 712–735. [Google Scholar] [CrossRef]
- Berlin-Broner, Y.; Febbraio, M.; Levin, L. Association between apical periodontitis and cardiovascular diseases: A systematic review of the literature. Int. Endod. J. 2017, 50, 847–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messing, M.; Souza, L.C.; Cavalla, F.; Kookal, K.K.; Rizzo, G.; Walji, M.; Silva, R.; Letra, A. Investigating Potential Correlations between Endodontic Pathology and Cardiovascular Diseases Using Epidemiological and Genetic Approaches. J. Endod. 2019, 45, 104–110. [Google Scholar] [CrossRef]
- Chauhan, N.; Mittal, S.; Tewari, S.; Sen, J.; Laller, K. Association of Apical Periodontitis with Cardiovascular Disease via Noninvasive Assessment of Endothelial Function and Subclinical Atherosclerosis. J. Endod. 2019, 45, 681–690. [Google Scholar] [CrossRef]
- O’Neal, W.T.; Efird, J.T.; Yeboah, J.; Nazarian, S.; Alonso, A.; Heckbert, S.R.; Soliman, E.Z. Brachial flow-mediated dilation and incident atrial fibrillation: The multi-ethnic study of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2717–2720. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.J.; Liu, C.J.; Chao, T.F.; Wang, K.L.; Chen, T.J.; Chou, P.; Wang, F.D.; Lin, S.J.; Chiang, C.E. Dental scaling and atrial fibrillation: A nationwide cohort study. Int. J. Cardiol. 2013, 168, 2300–2303. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.Y.; Lin, C.H.; Chen, Y.M.; Chen, H.H. Risk of Atrial Fibrillation or Flutter Associated with Periodontitis: A Nationwide, Population-Based, Cohort Study. PLoS ONE 2016, 11, e0165601. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.; Woo, H.G.; Park, J.; Lee, J.S.; Song, T.J. Improved oral hygiene care is associated with decreased risk of occurrence for atrial fibrillation and heart failure: A nationwide population-based cohort study. Eur. J. Prev. Cardiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Redd, K.; Trivedi, T.; Moss, K.; Alonso, A.; Soliman, E.Z.; Magnani, J.W.; Chen, L.Y.; Gottesman, R.F.; Rosamond, W.; et al. Periodontal Disease, Atrial Fibrillation and Stroke. Am. Heart J. 2021, 235. [Google Scholar] [CrossRef] [PubMed]
- Kasperska-Zajac, A.; Grzanka, A.; Kowalczyk, J.; Wyszyńska-Chłap, M.; Lisowska, G.; Kasperski, J.; Jarząb, J.; Misiołek, M.; Kalarus, Z. Refractory chronic spontaneous urticaria and permanent atrial fibrillation associated with dental infection: Mere coincidence or something more to it? Int. J. Immunopathol. Pharmacol. 2016, 29, 112–120. [Google Scholar] [CrossRef] [Green Version]
- Hassan, A.; Lip, G.Y.H.; Harris, R.V. Atrial fibrillation and cardiac arrhythmia associated with acute dental infection: A systematic literature review and case report. Int. J. Clin. Pract. 2020, 75, e13875. [Google Scholar] [CrossRef]
- Lewis, M.A.; Meechan, C.; MacFarlane, T.W.; Lamey, P.J.; Kay, E. Presentation and antimicrobial treatment of acute orofacial infections in general dental practice. Br. Dent. J. 1989, 166, 41–45. [Google Scholar] [CrossRef]
- Matthews, D.C.; Sutherland, S.; Basrani, B. Emergency management of acute apical abscesses in the permanent dentition: A systematic review of the literature. J. Can. Dent. Assoc. 2003, 69, 660. [Google Scholar] [PubMed]
- Currie, C.C.; Stone, S.J.; Connolly, J.; Durham, J. Dental pain in the medical emergency department: A cross-sectional study. J. Oral Rehabil. 2017, 44, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Gift, H.C.; Reisine, S.T.; Larach, D.C. The social impact of dental problems and visits. Am. J. Public Health 1992, 82, 1663–1668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fauchier, L.; Clementy, N.; Pelade, C.; Collignon, C.; Nicolle, E.; Lip, G.Y. Patients with Ischemic Stroke and Incident Atrial Fibrillation: A Nationwide Cohort Study. Stroke 2015, 46, 2432–2437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fetter, R.B.; Shin, Y.; Freeman, J.L.; Averill, R.F.; Thompson, J.D. Case mix definition by diagnosis-related groups. Med. Care 1980, 18 (Suppl. 2), 1–53. [Google Scholar]
- Weiner, M.G. POINT: Is ICD-10 Diagnosis Coding Important in the Era of Big Data? Yes. Chest 2018, 153, 1093–1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakehashi, S.; Stanley, H.R.; Fitzgerald, R.J. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg. Oral Med. Oral Pathol. 1965, 20, 340–349. [Google Scholar] [CrossRef]
- Jensen, P.N.; Johnson, K.; Floyd, J.; Heckbert, S.R.; Carnahan, R.; Dublin, S. A systematic review of validated methods for identifying atrial fibrillation using administrative data. Pharmacoepidemiol. Drug Saf. 2012, 21, 141–147. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.G.; Bisson, A.; Bodin, A.; Herbert, J.; Grammatico-Guillon, L.; Joung, B.; Wang, Y.T.; Lip, G.Y.H.; Fauchier, L. C2HEST Score and Prediction of Incident Atrial Fibrillation in Poststroke Patients a French Nationwide Study. J. Am. Heart Assoc. 2019, 8, e012546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Is My Study Research? Available online: http://www.hra-decisiontools.org.uk/research/ (accessed on 27 May 2021).
- Shah, A.C.; Leong, K.K.; Lee, M.K.; Allareddy, V. Outcomes of hospitalizations attributed to periapical abscess from 2000 to 2008: A longitudinal trend analysis. J. Endod. 2013, 39, 1104–1110. [Google Scholar] [CrossRef]
- De Sousa, E.L.; Ferraz, C.C.; Gomes, B.P.; Pinheiro, E.T.; Teixeira, F.B.; de Souza-Filho, F.J. Bacteriological study of root canals associated with periapical abscesses. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2003, 96, 332–339. [Google Scholar] [CrossRef]
- Kragsbjerg, P.; Söderquist, B.; Holmberg, H.; Vikerfors, T.; Danielsson, D. Production of tumor necrosis factor-alpha and interleukin-6 in whole blood stimulated by live Gram-negative and Gram-positive bacteria. Clin. Microbiol. Infect. 1998, 4, 129–134. [Google Scholar] [CrossRef] [Green Version]
- Rechenberg, D.K.; Galicia, J.C.; Peters, O.A. Biological Markers for Pulpal Inflammation: A Systematic Review. PLoS ONE 2016, 11, e0167289. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.J.; Du, Y.; Cai, W.K.; Kuang, R.; Chang, T.; Zhang, Z.; Yang, Y.X.; Sun, C.; Li, Z.Y.; Kuang, F. Toll-like receptor 4 signaling in neurons of trigeminal ganglion contributes to nociception induced by acute pulpitis in rats. Sci. Rep. 2015, 5, 12549. [Google Scholar] [CrossRef] [Green Version]
- Paula-Silva, F.W.; da Silva, L.A.; Kapila, Y.L. Matrix metalloproteinase expression in teeth with apical periodontitis is differentially modulated by the modality of root canal treatment. J. Endod. 2010, 36, 231–237. [Google Scholar] [CrossRef] [Green Version]
- Hamacher, J.; Lucas, R.; Lijnen, H.R.; Buschke, S.; Dunant, Y.; Wendel, A.; Grau, G.E.; Suter, P.M.; Ricou, B. Tumor necrosis factor-alpha and angiostatin are mediators of endothelial cytotoxicity in bronchoalveolar lavages of patients with acute respiratory distress syndrome. Am. J. Respir. Crit Care Med. 2002, 166, 651–656. [Google Scholar] [CrossRef]
- Gungor, B.; Ekmekci, A.; Arman, A.; Ozcan, K.S.; Ucer, E.; Alper, A.T.; Calik, N.; Yilmaz, H.; Tezel, T.; Coker, A.; et al. Assessment of interleukin-1 gene cluster polymorphisms in lone atrial fibrillation: New insight into the role of inflammation in atrial fibrillation. Pacing Clin. Electrophysiol. 2013, 36, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Xue, Y.M.; Zhan, X.Z.; Liao, H.T.; Guo, H.M.; Wu, S.L. Role of tumor necrosis factor-alpha in the pathogenesis of atrial fibrillation. Chin. Med. J. 2011, 124, 1976–1982. [Google Scholar] [PubMed]
- Ravassa, S.; Ballesteros, G.; López, B.; Ramos, P.; Bragard, J.; González, A.; Moreno, M.U.; Querejeta, R.; Vives, E.; García-Bolao, I.; et al. Combination of Circulating Type I Collagen-Related Biomarkers Is Associated with Atrial Fibrillation. J. Am. Coll. Cardiol. 2019, 73, 1398–1410. [Google Scholar] [CrossRef] [PubMed]
- Walkey, A.J.; Hammill, B.G.; Curtis, L.H.; Benjamin, E.J. Long-term outcomes following development of new-onset atrial fibrillation during sepsis. Chest 2014, 146, 1187–1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuipers, S.; Klein Klouwenberg, P.M.; Cremer, O.L. Incidence, risk factors and outcomes of new-onset atrial fibrillation in patients with sepsis: A systematic review. Crit Care 2014, 18, 688. [Google Scholar] [CrossRef] [Green Version]
- Lip, G.Y.; Nieuwlaat, R.; Pisters, R.; Lane, D.A.; Crijns, H.J. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The euro heart survey on atrial fibrillation. Chest 2010, 137, 263–272. [Google Scholar] [CrossRef]
- Mitchell, L.B.; Southern, D.A.; Galbraith, D.; Ghali, W.A.; Knudtson, M.; Wilton, S.B.; APPROACH Investigators. Prediction of stroke or TIA in patients without atrial fibrillation using CHADS2 and CHA2DS2-VASc scores. Heart 2014, 100, 1524–1530. [Google Scholar] [CrossRef] [Green Version]
- Poçi, D.; Hartford, M.; Karlsson, T.; Herlitz, J.; Edvardsson, N.; Caidahl, K. Role of the CHADS2 score in acute coronary syndromes: Risk of subsequent death or stroke in patients with and without atrial fibrillation. Chest 2012, 141, 1431–1440. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, T.; Webb, I.; Stenhouse, L.; Pattni, A.; Ready, D.; Wanyonyi, K.L.; White, S.; Gallagher, J.E. Evidence summary: The relationship between oral and cardiovascular disease. Br. Dent. J. 2017, 222, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Müller, F.; Shimazaki, Y.; Kahabuka, F.; Schimmel, M. Oral health for an ageing population: The importance of a natural dentition in older adults. Int. Dent. J. 2017, 67 (Suppl. 2), 7–13. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Zhou, Y.; Liu, X.; Tan, J. The impact of the COVID-19 epidemic on the utilization of emergency dental services. J. Dent. Sci. 2020, 15, 564–567. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).