Comparison of the Effect of Oral Versus Intravenous Bisphosphonate Administration on Osteoclastogenesis in Advanced-Stage Medication-Related Osteonecrosis of the Jaw Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection and Specimen Harvesting
2.2. Histochemistry
2.3. Immunohistochemistry
2.4. TRAP Staining
2.5. Quantitative and Qualitative Analysis
2.6. Statistical Analysis
3. Results
3.1. Characteristics of the Included Subjects
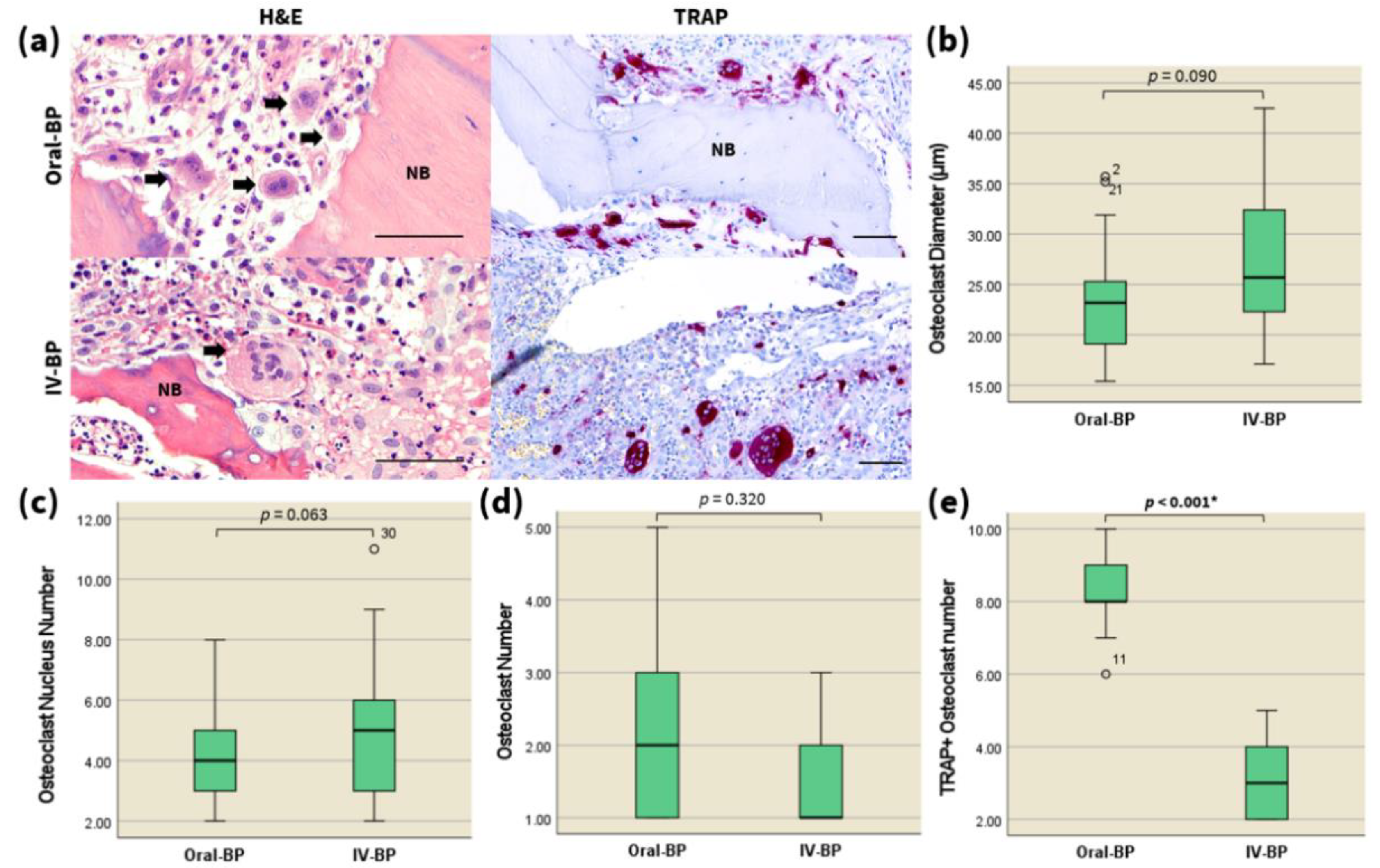
3.2. Quantitative and Qualitative Analysis of Osteoclasts
3.3. TRAP
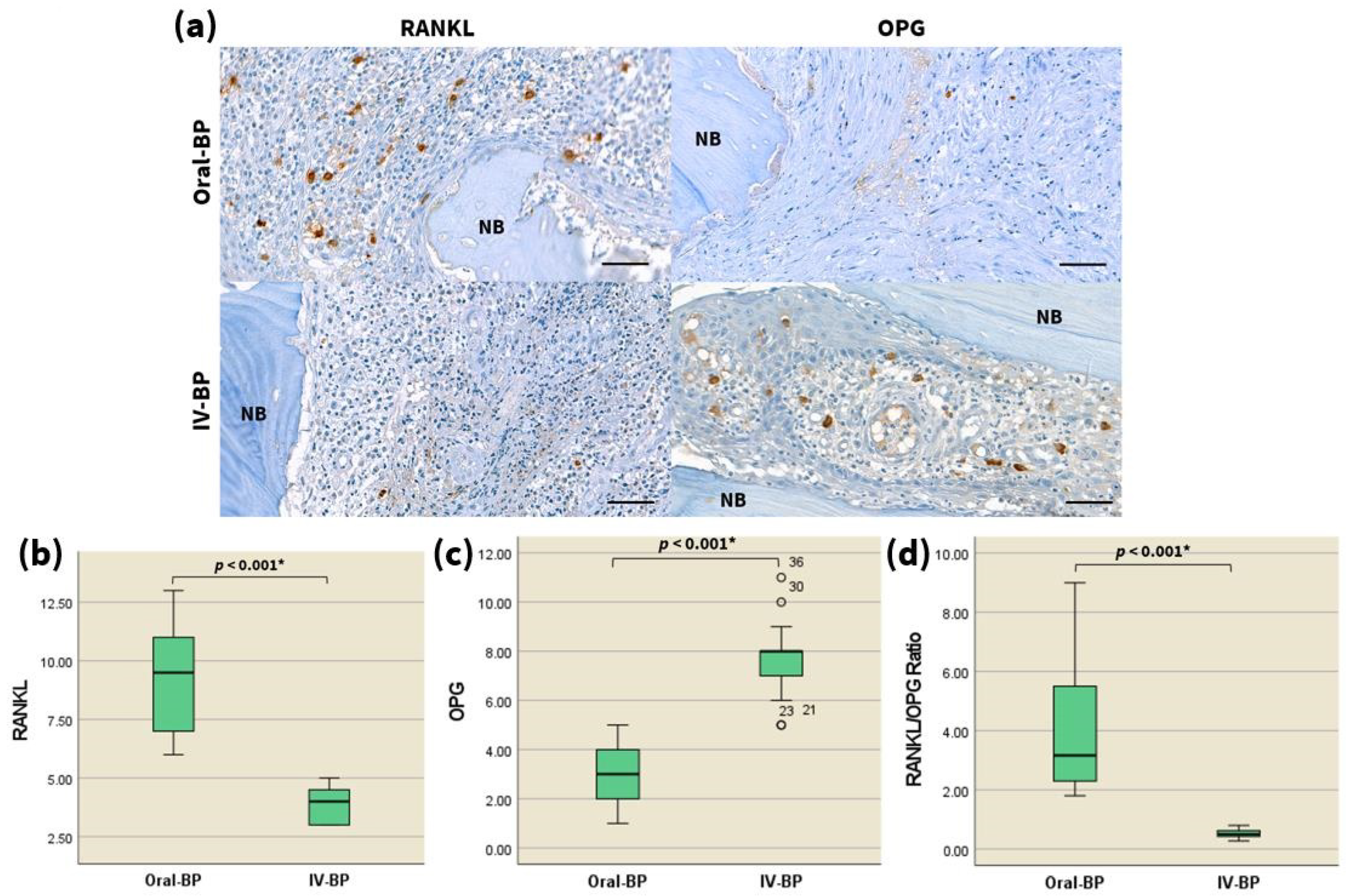
3.4. RANKL/OPG
3.5. Kcnn4
4. Discussion
4.1. Anomalies of Osteoclasts in MRONJ Specimens
4.2. Differences in Expression of Osteoclast-Related Markers—RANKL/OPG
4.3. Differences in Expression of Kcnn4
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Moraschini, V.; de Almeida, D.C.F.; Figueredo, C.M.; Calasans-Maia, M.D. Association between biomarkers and medication-related osteonecrosis of the jaws: A systematic review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 127, 504–515. [Google Scholar] [CrossRef]
- Gross, C.; Weber, M.; Creutzburg, K.; Mobius, P.; Preidl, R.; Amann, K.; Wehrhan, F. Osteoclast profile of medication-related osteonecrosis of the jaw secondary to bisphosphonate therapy: A comparison with osteoradionecrosis and osteomyelitis. J. Transl. Med. 2017, 15, 128. [Google Scholar] [CrossRef]
- Marx, R.E. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: A growing epidemic. J. Oral Maxillofac. Surg. 2003, 61, 1115–1117. [Google Scholar] [CrossRef]
- Gong, X.; Yu, W.; Zhao, H.; Su, J.; Sheng, Q. Skeletal Site-Specific Effects of Zoledronate on In Vivo Bone Remodeling and In Vitro BMSCs Osteogenic Activity. Sci. Rep. 2017, 7, 36129. [Google Scholar] [CrossRef] [Green Version]
- Kikuiri, T.; Kim, I.; Yamaza, T.; Akiyama, K.; Zhang, Q.; Li, Y.; Chen, C.; Chen, W.; Wang, S.; Le, A.D.; et al. Cell-based immunotherapy with mesenchymal stem cells cures bisphosphonate-related osteonecrosis of the jaw-like disease in mice. J. Bone Miner. Res. 2010, 25, 1668–1679. [Google Scholar] [CrossRef] [Green Version]
- Giudice, A.; Barone, S.; Diodati, F.; Antonelli, A.; Nocini, R.; Cristofaro, M.G. Can Surgical Management Improve Resolution of Medication-Related Osteonecrosis of the Jaw at Early Stages? A Prospective Cohort Study. J. Oral Maxillofac. Surg. 2020, 78, 1986–1999. [Google Scholar] [CrossRef]
- Khan, A.A.; Morrison, A.; Hanley, D.A.; Felsenberg, D.; McCauley, L.K.; O’Ryan, F.; Reid, I.R.; Ruggiero, S.L.; Taguchi, A.; Tetradis, S.; et al. Diagnosis and management of osteonecrosis of the jaw: A systematic review and international consensus. J. Bone Miner. Res. 2015, 30, 3–23. [Google Scholar] [CrossRef]
- Giudice, A.; Antonelli, A.; Muraca, D.; Fortunato, L. Usefulness of advanced-platelet rich fibrin (A-PRF) and injectable-platelet rich fibrin (i-PRF) in the management of a massive medication-related osteonecrosis of the jaw (MRONJ): A 5-years follow-up case report. Indian J. Dent. Res. 2020, 31, 813–818. [Google Scholar] [CrossRef]
- Aljohani, S.; Fliefel, R.; Ihbe, J.; Kühnisch, J.; Ehrenfeld, M.; Otto, S. What is the effect of anti-resorptive drugs (ARDs) on the development of medication-related osteonecrosis of the jaw (MRONJ) in osteoporosis patients: A systematic review. J. Craniomaxillofac. Surg. 2017, 45, 1493–1502. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.W.; Lee, C.; Kim, T.; Yagita, H.; Wu, H.; Park, S.; Yang, P.; Liu, H.; Shi, S.; Shin, K.H.; et al. Impaired bone resorption and woven bone formation are associated with development of osteonecrosis of the jaw-like lesions by bisphosphonate and anti-receptor activator of NF-kappaB ligand antibody in mice. Am. J. Pathol. 2014, 184, 3084–3093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagaoka, Y.; Kajiya, H.; Ozeki, S.; Ikebe, T.; Okabe, K. Mevalonates restore zoledronic acid-induced osteoclastogenesis inhibition. J. Dent. Res. 2015, 94, 594–601. [Google Scholar] [CrossRef]
- Shintani, T.; Hayashido, Y.; Mukasa, H.; Akagi, E.; Hoshino, M.; Ishida, Y.; Hamana, T.; Okamoto, K.; Kanda, T.; Koizumi, K.; et al. Comparison of the prognosis of bisphosphonate-related osteonecrosis of the jaw caused by oral and intravenous bisphosphonates. Int. J. Oral Maxillofac. Surg. 2015, 44, 840–844. [Google Scholar] [CrossRef]
- Rodan, G.A.; Fleisch, H.A. Bisphosphonates: Mechanisms of action. J. Clin. Investig. 1996, 97, 2692–2696. [Google Scholar] [CrossRef]
- Rogers, M.J.; Gordon, S.; Benford, H.L.; Coxon, F.P.; Luckman, S.P.; Monkkonen, J.; Frith, J.C. Cellular and molecular mechanisms of action of bisphosphonates. Cancer 2000, 88, 2961–2978. [Google Scholar] [CrossRef]
- Wu, S.N.; Huang, Y.M.; Liao, Y.K. Effects of ibandronate sodium, a nitrogen-containing bisphosphonate, on intermediate-conductance calcium-activated potassium channels in osteoclast precursor cells (RAW 264.7). J. Membr. Biol. 2015, 248, 103–115. [Google Scholar] [CrossRef]
- Halasy-Nagy, J.M.; Rodan, G.A.; Reszka, A.A. Inhibition of bone resorption by alendronate and risedronate does not require osteoclast apoptosis. Bone 2001, 29, 553–559. [Google Scholar] [CrossRef]
- Grossinger, E.M.; Kang, M.; Bouchareychas, L.; Sarin, R.; Haudenschild, D.R.; Borodinsky, L.N.; Adamopoulos, I.E. Ca(2+)-Dependent Regulation of NFATc1 via KCa3.1 in Inflammatory Osteoclastogenesis. J. Immunol. 2018, 200, 749–757. [Google Scholar] [CrossRef] [Green Version]
- Anderson, D.M.; Maraskovsky, E.; Billingsley, W.L.; Dougall, W.C.; Tometsko, M.E.; Roux, E.R.; Teepe, M.C.; DuBose, R.F.; Cosman, D.; Galibert, L. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature 1997, 390, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Yavropoulou, M.P.; Yovos, J.G. Osteoclastogenesis-current knowledge and future perspectives. J. Musculoskelet. Neuronal Interact. 2008, 8, 204–216. [Google Scholar] [PubMed]
- Hanada, R.; Hanada, T.; Sigl, V.; Schramek, D.; Penninger, J.M. RANKL/RANK-beyond bones. J. Mol. Med. 2011, 89, 647–656. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, N. Signaling Pathways in Osteoclast Differentiation. Chonnam Med. J. 2016, 52, 12–17. [Google Scholar] [CrossRef] [Green Version]
- Maisani, M.; Pezzoli, D.; Chassande, O.; Mantovani, D. Cellularizing hydrogel-based scaffolds to repair bone tissue: How to create a physiologically relevant micro-environment? J. Tissue Eng. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.-S.; Kim, J.-J.; Kim, H.-W.; Lewis, M.P.; Wall, I. Impact of mechanical stretch on the cell behaviors of bone and surrounding tissues. J. Tissue Eng. 2016, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyce, B.F.; Xing, L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys. 2008, 473, 139–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyce, B.F.; Xing, L. The RANKL/RANK/OPG pathway. Curr. Osteoporos. Rep. 2007, 5, 98–104. [Google Scholar] [CrossRef]
- Perez-Sayans, M.; Somoza-Martin, J.M.; Barros-Angueira, F.; Rey, J.M.; Garcia-Garcia, A. RANK/RANKL/OPG role in distraction osteogenesis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 109, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Cha, I.H.; Kim, S.J.; Kim, M.R. Biomarkers for Bisphosphonate-Related Osteonecrosis of the Jaw. Clin. Implant. Dent. Relat. Res. 2016, 18, 281–291. [Google Scholar] [CrossRef]
- Kang, H.; Kerloc’h, A.; Rotival, M.; Xu, X.; Zhang, Q.; D’Souza, Z.; Kim, M.; Scholz, J.C.; Ko, J.H.; Srivastava, P.K.; et al. Kcnn4 is a regulator of macrophage multinucleation in bone homeostasis and inflammatory disease. Cell Rep. 2014, 8, 1210–1224. [Google Scholar] [CrossRef] [Green Version]
- Wen, J.; Lin, B.; Lin, L.; Chen, Y.; Wang, O. KCNN4 is a diagnostic and prognostic biomarker that promotes papillary thyroid cancer progression. Aging 2020, 12, 16437–16456. [Google Scholar] [CrossRef]
- Li, Q.T.; Feng, Y.M.; Ke, Z.H.; Qiu, M.J.; He, X.X.; Wang, M.M.; Li, Y.N.; Xu, J.; Shi, L.L.; Xiong, Z.F. KCNN4 promotes invasion and metastasis through the MAPK/ERK pathway in hepatocellular carcinoma. J. Investig. Med. 2020, 68, 68–74. [Google Scholar] [CrossRef]
- Lam, J.; Wulff, H. The Lymphocyte Potassium Channels Kv1.3 and KCa3.1 as Targets for Immunosuppression. Drug Dev. Res. 2011, 72, 573–584. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.; Zhu, L.; Yang, J.; Hu, L.; Gu, J.; Xing, X.; Sun, Y.; Zhang, Z. Integrated expression profiling of potassium channels identifys KCNN4 as a prognostic biomarker of pancreatic cancer. Biochem. Biophys. Res. Commun. 2017, 494, 113–119. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, X.; Yin, Q.; Yi, J.; Shen, W.; Zhao, L.; Zhu, Z.; Liu, J. Inhibition of SK4 Potassium Channels Suppresses Cell Proliferation, Migration and the Epithelial-Mesenchymal Transition in Triple-Negative Breast Cancer Cells. PLoS ONE 2016, 11, e0154471. [Google Scholar] [CrossRef]
- Du, Y.; Song, W.; Chen, J.; Chen, H.; Xuan, Z.; Zhao, L.; Chen, J.; Jin, C.; Zhou, M.; Tuo, B.; et al. The potassium channel KCa3.1 promotes cell proliferation by activating SKP2 and metastasis through the EMT pathway in hepatocellular carcinoma. Int. J. Cancer 2019, 145, 503–516. [Google Scholar] [CrossRef]
- Rabjerg, M.; Olivan-Viguera, A.; Hansen, L.K.; Jensen, L.; Sevelsted-Moller, L.; Walter, S.; Jensen, B.L.; Marcussen, N.; Kohler, R. High expression of KCa3.1 in patients with clear cell renal carcinoma predicts high metastatic risk and poor survival. PLoS ONE 2015, 10, e0122992. [Google Scholar] [CrossRef] [Green Version]
- Bulk, E.; Ay, A.S.; Hammadi, M.; Ouadid-Ahidouch, H.; Schelhaas, S.; Hascher, A.; Rohde, C.; Thoennissen, N.H.; Wiewrodt, R.; Schmidt, E.; et al. Epigenetic dysregulation of KCa 3.1 channels induces poor prognosis in lung cancer. Int. J. Cancer 2015, 137, 1306–1317. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, Y.; Chen, L.; Zhu, J. Effects of Intermediate-Conductance Ca(2+)-Activated K(+) Channels on Human Endometrial Carcinoma Cells. Cell Biochem. Biophys. 2015, 72, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Lallet-Daher, H.; Roudbaraki, M.; Bavencoffe, A.; Mariot, P.; Gackiere, F.; Bidaux, G.; Urbain, R.; Gosset, P.; Delcourt, P.; Fleurisse, L.; et al. Intermediate-conductance Ca2+-activated K+ channels (IKCa1) regulate human prostate cancer cell proliferation through a close control of calcium entry. Oncogene 2009, 28, 1792–1806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruggiero, S.L.; Dodson, T.B.; Fantasia, J.; Goodday, R.; Aghaloo, T.; Mehrotra, B.; O’Ryan, F. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw-2014 update. J. Oral Maxillofac. Surg. 2014, 72, 1938–1956. [Google Scholar] [CrossRef] [PubMed]
- Kellinsalmi, M.; Mönkkönen, H.; Mönkkönen, J.; Leskelä, H.V.; Parikka, V.; Hämäläinen, M.; Lehenkari, P. In vitro comparison of clodronate, pamidronate and zoledronic acid effects on rat osteoclasts and human stem cell-derived osteoblasts. Basic Clin. Pharmacol. Toxicol. 2005, 97, 382–391. [Google Scholar] [CrossRef]
- Giudice, A.; Antonelli, A.; Chiarella, E.; Baudi, F.; Barni, T.; Di Vito, A. The Case of Medication-Related Osteonecrosis of the Jaw Addressed from a Pathogenic Point of View. Innovative Therapeutic Strategies: Focus on the Most Recent Discoveries on Oral Mesenchymal Stem Cell-Derived Exosomes. Pharmaceuticals 2020, 13, 423. [Google Scholar] [CrossRef]
- Di Vito, A.; Chiarella, E.; Baudi, F.; Scardamaglia, P.; Antonelli, A.; Giudice, D.; Barni, T.; Fortunato, L.; Giudice, A. Dose-Dependent Effects of Zoledronic Acid on Human Periodontal Ligament Stem Cells: An In Vitro Pilot Study. Cell Transplant. 2020, 29, 963689720948497. [Google Scholar] [CrossRef]
- Cheng, T.L.; Murphy, C.M.; Ravarian, R.; Dehghani, F.; Little, D.G.; Schindeler, A. Bisphosphonate-adsorbed ceramic nanoparticles increase bone formation in an injectable carrier for bone tissue engineering. J. Tissue Eng. 2015, 6. [Google Scholar] [CrossRef]
- Mavrokokki, T.; Cheng, A.; Stein, B.; Goss, A. Nature and frequency of bisphosphonate-associated osteonecrosis of the jaws in Australia. J. Oral Maxillofac. Surg. 2007, 65, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Kuroshima, S.; Sasaki, M.; Nakajima, K.; Tamaki, S.; Hayano, H.; Sawase, T. Prevalence of bisphosphonate-related osteonecrosis of the jaw-like lesions is increased in a chemotherapeutic dose-dependent manner in mice. Bone 2018, 112, 177–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.H.; Seo, W.G.; Koo, C.H.; Lee, J.H. Evaluation of the predisposing factors and involved outcome of surgical treatment in bisphosphonate-related osteonecrosis of the jaw cases including bone biopsies. J. Korean Assoc. Oral Maxillofac. Surg. 2016, 42, 193–204. [Google Scholar] [CrossRef] [Green Version]
- Khojasteh, A.; Dehghan, M.M.; Nazeman, P. Immediate implant placement following 1-year treatment with oral versus intravenous bisphosphonates: A histomorphometric canine study on peri-implant bone. Clin. Oral Investig. 2019, 23, 1803–1809. [Google Scholar] [CrossRef]
- Marx, R.E.; Cillo, J.E., Jr.; Ulloa, J.J. Oral bisphosphonate-induced osteonecrosis: Risk factors, prediction of risk using serum CTX testing, prevention, and treatment. J. Oral Maxillofac. Surg. 2007, 65, 2397–2410. [Google Scholar] [CrossRef] [Green Version]
- Marx, R.E. Reconstruction of defects caused by bisphosphonate-induced osteonecrosis of the jaws. J. Oral Maxillofac. Surg. 2009, 67, 107–119. [Google Scholar] [CrossRef]
- Carlson, E.R.; Basile, J.D. The role of surgical resection in the management of bisphosphonate-related osteonecrosis of the jaws. J. Oral Maxillofac. Surg. 2009, 67, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Park, J.S.; Righesso, L.; Pabst, A.M.; Al-Nawas, B.; Kwon, Y.D.; Walter, C. Effects of an oral bisphosphonate and three intravenous bisphosphonates on several cell types in vitro. Clin. Oral Investig. 2018, 22, 2527–2534. [Google Scholar] [CrossRef] [PubMed]
- Heijckmann, A.C.; Juttmann, J.R.; Wolffenbuttel, B.H. Intravenous pamidronate compared with oral alendronate for the treatment of postmenopausal osteoporosis. Neth. J. Med. 2002, 60, 315–319. [Google Scholar] [PubMed]
- Ezra, A.; Golomb, G. Administration routes and delivery systems of bisphosphonates for the treatment of bone resorption. Adv. Drug Deliv. Rev. 2000, 42, 175–195. [Google Scholar] [CrossRef]
- Delmas, P.D.; Adami, S.; Strugala, C.; Stakkestad, J.A.; Reginster, J.Y.; Felsenberg, D.; Christiansen, C.; Civitelli, R.; Drezner, M.K.; Recker, R.R.; et al. Intravenous ibandronate injections in postmenopausal women with osteoporosis: One-year results from the dosing intravenous administration study. Arthritis Rheum. 2006, 54, 1838–1846. [Google Scholar] [CrossRef] [PubMed]
- Adami, S.; Felsenberg, D.; Christiansen, C.; Robinson, J.; Lorenc, R.S.; Mahoney, P.; Coutant, K.; Schimmer, R.C.; Delmas, P.D. Efficacy and safety of ibandronate given by intravenous injection once every 3 months. Bone 2004, 34, 881–889. [Google Scholar] [CrossRef]
- Ringe, J.D.; Dorst, A.; Faber, H.; Ibach, K.; Sorenson, F. Intermittent intravenous ibandronate injections reduce vertebral fracture risk in corticosteroid-induced osteoporosis: Results from a long-term comparative study. Osteoporos Int. 2003, 14, 801–807. [Google Scholar] [CrossRef]
- Thiébaud, D.; Burckhardt, P.; Kriegbaum, H.; Huss, H.; Mulder, H.; Juttmann, J.R.; Schöter, K.H. Three Monthly Intravenous Injections of Ibandronate in the Treatment of Postmenopausal Osteoporosis. Am. J. Med. 1997, 103, 298–307. [Google Scholar] [CrossRef]
- Vis, M.; Bultink, I.E.; Dijkmans, B.A.; Lems, W.F. The effect of intravenous pamidronate versus oral alendronate on bone mineral density in patients with osteoporosis. Osteoporos Int. 2005, 16, 1432–1435. [Google Scholar] [CrossRef]
- Roux, C.; Reid, D.M.; Devogelaer, J.P.; Saag, K.; Lau, C.S.; Reginster, J.Y.; Papanastasiou, P.; Bucci-Rechtweg, C.; Su, G.; Sambrook, P.N. Post hoc analysis of a single IV infusion of zoledronic acid versus daily oral risedronate on lumbar spine bone mineral density in different subgroups with glucocorticoid-induced osteoporosis. Osteoporos Int. 2012, 23, 1083–1090. [Google Scholar] [CrossRef]
- Reid, D.M.; Devogelaer, J.P.; Saag, K.; Roux, C.; Lau, C.S.; Reginster, J.Y.; Papanastasiou, P.; Ferreira, A.; Hartl, F.; Fashola, T.; et al. Zoledronic acid and risedronate in the prevention and treatment of glucocorticoid-induced osteoporosis (HORIZON): A multicentre, double-blind, double-dummy, randomised controlled trial. Lancet 2009, 373, 1253–1263. [Google Scholar] [CrossRef]
- Greenspan, S.L.; Perera, S.; Ferchak, M.A.; Nace, D.A.; Resnick, N.M. Efficacy and safety of single-dose zoledronic acid for osteoporosis in frail elderly women: A randomized clinical trial. JAMA Intern. Med. 2015, 175, 913–921. [Google Scholar] [CrossRef] [Green Version]
- Reid, I.R.; Brown, J.P.; Burckhardt, P.; Horowitz, Z.; Richardson, P.; Trechsel, U.; Widmer, A.; Devogelaer, J.-P.; Kaufman, J.-M.; Jaeger, P.; et al. Intravenous Zoledronic Acid in Postmenopausal Women with Low Bone Mineral Density. N. Engl. J. Med. 2002, 346, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Berenson, J.R.; Rosen, L.S.; Howell, A.; Porter, L.; Coleman, R.E.; Morley, W.; Dreicer, R.; Kuross, S.A.; Lipton, A.; Seaman, J.J. Zoledronic acid reduces skeletal-related events in patients with osteolytic metastases. Cancer 2001, 91, 1191–1200. [Google Scholar] [CrossRef]
- Young, R.J.; Coleman, R.E. Zoledronic acid to prevent and treat cancer metastasis: New prospects for an old drug. Future Oncol. 2013, 9, 633–643. [Google Scholar] [CrossRef]
- Lewiecki, E.M.; Miller, P.D. Renal safety of intravenous bisphosphonates in the treatment of osteoporosis. Expert Opin. Drug Saf. 2007, 6, 663–672. [Google Scholar] [CrossRef]
- Weinstein, R.S.; Roberson, P.K.; Manolagas, S.C. Giant Osteoclast Formation and Long-Term Oral Bisphosphonate Therapy. N. Engl. J. Med. 2009, 360, 53–62. [Google Scholar] [CrossRef]
- Mac-Way, F.; Trombetti, A.; Noel, C.; Lafage-Proust, M.-H. Giant osteoclasts in patients under bisphosphonates. BMC Clin. Pathol. 2014, 14, 31. [Google Scholar] [CrossRef] [Green Version]
- de Matos, F.R.; de Moraes, M.; das Neves Silva, E.B.; Galvao, H.C.; de Almeida Freitas, R. Immunohistochemical detection of receptor activator nuclear kappaB ligand and osteoprotegerin in odontogenic cysts and tumors. J. Oral Maxillofac. Surg. 2013, 71, 1886–1892. [Google Scholar] [CrossRef] [PubMed]
- Simonet, W.S.; Lacey, D.L.; Dunstan, C.R.; Kelley, M.; Chang, M.S.; Lüthy, R.; Nguyen, H.Q.; Wooden, S.; Bennett, L.; Boone, T.; et al. Osteoprotegerin: A Novel Secreted Protein Involved in the Regulation of Bone Density. Cell 1997, 89, 309–319. [Google Scholar] [CrossRef] [Green Version]
- Cankaya, M.; Cizmeci Senel, F.; Kadioglu Duman, M.; Muci, E.; Dayisoylu, E.H.; Balaban, F. The effects of chronic zoledronate usage on the jaw and long bones evaluated using RANKL and osteoprotegerin levels in an animal model. Int. J. Oral Maxillofac. Surg. 2013, 42, 1134–1139. [Google Scholar] [CrossRef] [PubMed]
- Koch, F.P.; Merkel, C.; Ziebart, T.; Smeets, R.; Walter, C.; Al-Nawas, B. Influence of bisphosphonates on the osteoblast RANKL and OPG gene expression in vitro. Clin. Oral Investig. 2012, 16, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Martini, G.; Gennari, L.; Merlotti, D.; Salvadori, S.; Franci, M.B.; Campagna, S.; Avanzati, A.; De Paola, V.; Valleggi, F.; Nuti, R. Serum OPG and RANKL levels before and after intravenous bisphosphonate treatment in Paget’s disease of bone. Bone 2007, 40, 457–463. [Google Scholar] [CrossRef] [PubMed]

| Group | Age | Gender | BP Type | Dose | BP Duration (Months) |
|---|---|---|---|---|---|
| Oral-BP | 73 | F | Ibandronate | 150 mg/month | 107 |
| Oral-BP | 84 | M | Alendronate | 70 mg/week | 2 |
| Oral-BP | 73 | F | Alendronate | 35 mg/week | 7 |
| Oral-BP | 79 | F | Ibandronate | 150 mg/month | 20 |
| Oral-BP | 80 | F | Risedronate | 35 mg/week | 7 |
| Oral-BP | 78 | F | Alendronate | 70 mg/week | 20 |
| Oral-BP | 81 | F | Ibandronate | 150 mg/month | 20 |
| Oral-BP | 80 | F | Ibandronate | 150 mg/month | 5 |
| Oral-BP | 72 | F | Risedronate | 35 mg/week | 5 |
| Oral-BP | 77 | F | Alendronate | 70 mg/week | 3 |
| IV-BP | 84 | F | Ibandronate Hydrate | 3 mg/3 months | 1.5 |
| IV-BP | 75 | F | Zoledronate Hydrate | 5 mg/year | 3 |
| IV-BP | 79 | M | Ibandronate Hydrate | 3 mg/3 months | 1 |
| IV-BP | 81 | F | Ibandronate Hydrate | 3 mg/3 months | 2 |
| IV-BP | 79 | F | Ibandronate Hydrate | 3 mg/3 months | 4 |
| IV-BP | 73 | F | Ibandronate Hydrate | 3 mg/3 months | 3 |
| IV-BP | 74 | F | Zoledronate Hydrate | 5 mg/year | 3 |
| IV-BP | 84 | F | Zoledronate Hydrate | 5 mg/year | 2 |
| IV-BP | 80 | F | Zoledronate Hydrate | 5 mg/year | 3 |
| IV-BP | 76 | F | Ibandronate Hydrate | 3 mg/3 months | 3 |
| Group | Minimum | Maximum | Average | Median | IQR | SD | p-Value 1 | |
|---|---|---|---|---|---|---|---|---|
| H&E Staining | 0.09 | |||||||
| Osteoclast diameter (μm) | Oral-BP | 2 | 8 | 4.04 | 4 | 2 | 0.296 | |
| IV-BP | 2 | 11 | 5.26 | 5 | 3 | 0.496 | ||
| Nuclearity of osteoclasts (nuclei/osteoclast) | Oral-BP | 15.4 | 35.7 | 23.285 | 23.2 | 6.4 | 1.046 | 0.063 |
| IV-BP | 17.1 | 42.5 | 27.083 | 25.7 | 10.6 | 1.461 | ||
| Osteoclasts per ROI | Oral-BP | 1 | 5 | 2.17 | 2 | 2 | 0.366 | 0.32 |
| IV-BP | 1 | 3 | 1.62 | 1 | 1 | 0.213 | ||
| TRAP staining | <0.001 2 | |||||||
| TRAP+ osteoclasts per ROI | Oral-BP | 6 | 10 | 8.25 | 8. | 1 | 0.204 | |
| IV-BP | 2 | 5 | 3.15 | 3 | 2 | 0.221 | ||
| Immunohistochemistry | <0.001 2 | |||||||
| RANKL+ cells per ROI | Oral-BP | 6 | 13 | 9.25 | 9.5 | 4 | 0.502 | |
| IV-BP | 3 | 5 | 3.88 | 4 | 2 | 0.202 | ||
| OPG+ cells per ROI | Oral-BP | 1 | 5 | 2.9 | 3 | 2 | 0.307 | <0.001 2 |
| IV-BP | 5 | 11 | 7.65 | 8 | 2 | 0.373 | ||
| RANKL/OPG ratio | Oral-BP | 1.8 | 9 | 3.899 | 3.167 | 3.35 | 0.438 | <0.001 2 |
| IV-BP | 0.27 | 0.8 | 0.523 | 0.5 | 0.2 | 0.04 | ||
| Kcnn4+ cells per ROI | Oral-BP | 3 | 10 | 6 | 6 | 2 | 0.447 | <0.001 2 |
| IV-BP | 0 | 4 | 1.9 | 2 | 2 | 0.261 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-W.; Lee, M.-W.; Lee, J.-H.; Kim, M.-Y. Comparison of the Effect of Oral Versus Intravenous Bisphosphonate Administration on Osteoclastogenesis in Advanced-Stage Medication-Related Osteonecrosis of the Jaw Patients. J. Clin. Med. 2021, 10, 2988. https://doi.org/10.3390/jcm10132988
Kim H-W, Lee M-W, Lee J-H, Kim M-Y. Comparison of the Effect of Oral Versus Intravenous Bisphosphonate Administration on Osteoclastogenesis in Advanced-Stage Medication-Related Osteonecrosis of the Jaw Patients. Journal of Clinical Medicine. 2021; 10(13):2988. https://doi.org/10.3390/jcm10132988
Chicago/Turabian StyleKim, Hye-Won, Min-Woo Lee, Jung-Hwan Lee, and Moon-Young Kim. 2021. "Comparison of the Effect of Oral Versus Intravenous Bisphosphonate Administration on Osteoclastogenesis in Advanced-Stage Medication-Related Osteonecrosis of the Jaw Patients" Journal of Clinical Medicine 10, no. 13: 2988. https://doi.org/10.3390/jcm10132988
APA StyleKim, H.-W., Lee, M.-W., Lee, J.-H., & Kim, M.-Y. (2021). Comparison of the Effect of Oral Versus Intravenous Bisphosphonate Administration on Osteoclastogenesis in Advanced-Stage Medication-Related Osteonecrosis of the Jaw Patients. Journal of Clinical Medicine, 10(13), 2988. https://doi.org/10.3390/jcm10132988







