Alloreactive Immune Response Associated to Human Mesenchymal Stromal Cells Treatment: A Systematic Review
Abstract
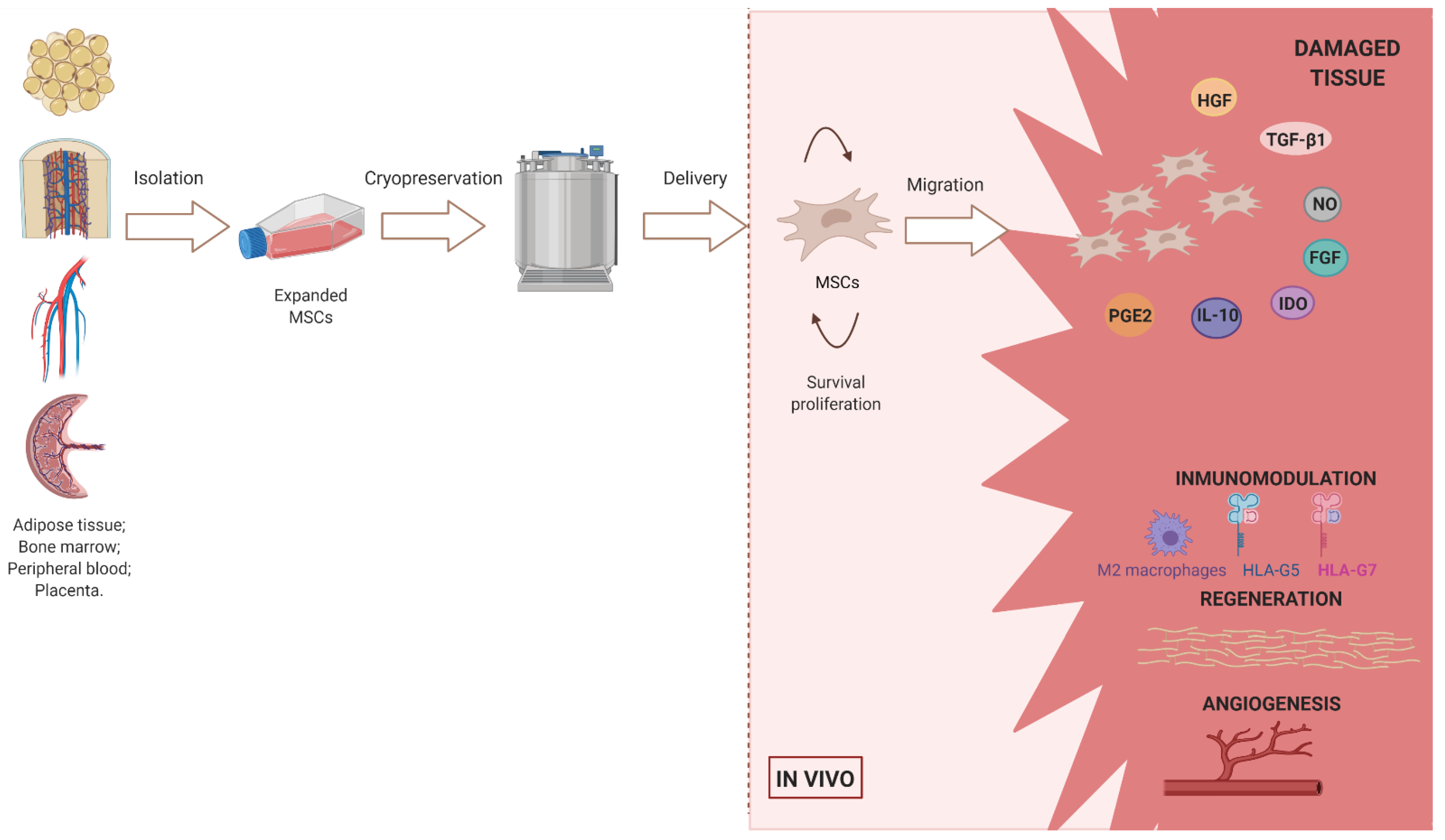
:1. Introduction
2. Materials and Methods
2.1. Research Questions
- −
- What is the prevalence of alloantibodies’ development in patients treated with allo-MSCs?
- −
- Does the route of administration of allo-MSCs influence the development of alloantibodies?
- −
- Does the tissue of origin of the MSCs influence the development of alloantibodies?
- −
- Is the total dose of allo-MSCs associated with increased alloantibodies response?
- −
- Are repeated doses of treatment associated with higher frequency of alloantibodies or impact of treatment?
- −
- Does the presensitization status of patients influence the development of alloantibodies?
- −
- How relevant is alloantibodies’ development to the safety of the treatment?
2.2. Search Strategy
2.3. Eligibility and Exclusion Criteria
2.4. Study Selection
2.5. Variables
2.6. Risk of Bias Assessment
3. Results
3.1. Study Identification
3.2. Bias Assessment
3.3. Outcomes
| First Author, Publish Year | Country | Identifier | Study Design F/U (mo) | Age Mean, (SD) | Disease | n | Co | Tx | Cell Dose (×106) | Injections | Route of Delivery | MSC Origin | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hare, 2012 [54] | United States | NCT01087996 | RCT 13 | Allo-MSCs 62.8 (10.5) | ICM | 30 | No | Cohort A | Auto-MSC (n = 5) Allo-MSC (n = 5) | 20 | 1 | TESI | BM |
| Cohort B | Auto-MSC (n = 5) Allo- MSC (n = 5) | 100 | |||||||||||
| Auto-MSCs 63.7 (9.3) | |||||||||||||
| Cohort C | Auto-MSC (n = 5) Allo- MSC (n = 5) | 200 | |||||||||||
| Skyler, 2015 [58] | United States | NCT01576328 | RCT 24 | Co 58.7 (7.3) | Type 2 Diabetes | 61 | 16 | Cohort A | Allo-MSC (n = 15) | 0.3/kg | 1 | IV | BM |
| Cohort A 57.7 (8.2) | Cohort B | Allo-MSC (n = 15) | 1/kg | ||||||||||
| Cohort B 55.3 (11.4) | Cohort C | Allo-MSC (n = 15) | 2/kg | ||||||||||
| Cohort C 57.2 (6.6) | |||||||||||||
| Perin, 2015 [55] | Australia | NCT00721045 | RCT 12 | Co 62.7 (11.2) | Chronic HF | 60 | 15 | Cohort A | Allo-MSC (n = 15) | 25 | 1 | TESI | BM |
| Cohort A 60.1 (8.8) | Cohort B | Allo-MSC (n = 15) | 75 | ||||||||||
| Cohort B 63.9 (11.5) | Cohort C | Allo-MSC (n = 15) | 150 | ||||||||||
| Cohort C 62.7 (10.8) | |||||||||||||
| Vega, 2015 [48] | Spain | NCT01586312 | RCT 12 | Co 57.5 (9.5) | OA | 30 | 15 | Cohort A | Allo-MSC (n = 15) | 40 | 1 | IA | BM |
| Cohort A 56.6 (9.6) | |||||||||||||
| Panés, 2016 [59] | Austria, Belgium, France, Germany, Israel, Italy, Netherlands, Spain | NCT01541579 | RCT 24 | Co 39.0 (13.1) | CD | 113 | 60 | Cohort A | Allo-MSC (n = 63) | 120 | 1 | IL | AT |
| Cohort A 37.6 (13.1) | |||||||||||||
| Packham, 2016 [53] | Mesoblast, Australia | NCT01843387 | RCT 15 | Co 74.8 (7.9) | DN | 30 | 10 | Cohort A | Allo-MSC (n = 10) | 150 | 1 | IV | BM |
| Cohort A 70.5 (7.4) | |||||||||||||
| Cohort B 64.8 (10.1) | Cohort B | Allo-MSC (n = 10) | 300 | ||||||||||
| Florea, 2017 [46] | United States | NCT02013674 | RCT 12 | Cohort A 66.8 (12.2) | ICM | 30 | No | Cohort A | Allo-MSC (n = 15) | 20 | 10 | TESI | BM |
| Cohort B 65.6 (9.4) | Cohort B | Allo-MSC (n = 15) | 100 | ||||||||||
| Tompkins, 2017 [52] | United States | NCT02065245 | RCT 12 | Co 75.3 (6.8) | FS | 30 | 10 | Cohort A | Allo-MSC (n = 10) | 100 | 1 | IV | BM |
| Cohort A 75.0 (7.4) | Cohort B | Allo-MSC (n = 10) | 200 | ||||||||||
| Cohort B 76.3 (8.4) | |||||||||||||
| Álvaro-Gracia, 2017 [57] | Spain | NCT01663116 | RCT 6 | Co58.43 (14.25) | RA | 53 | 7 | Cohort A | Allo-MSC (n = 20) | 1/kg | 3 | IV | AT |
| Cohort A 54.15 (7.79) | Cohort B | Allo-MSC (n = 20) | 2/kg | ||||||||||
| Cohort B 57.40 (11.01) | Cohort C | Allo-MSC (n = 6) | 4/kg | ||||||||||
| Cohort C 50.33 (15.62) | |||||||||||||
| Hare, 2017 [47] | United States | NCT01392625 | RCT 12 | Cohort A 54.4 (11.5) | NDC | 37 | No | Cohort A | Auto-MSC (n = 18) | 100 | 10 | TESI | BM |
| Cohort B 57.4 (11.0) | Cohort B | Allo-MSC (n = 19) | |||||||||||
| Bartolucci, 2017 [56] | Chile | NCT01739777 | RCT 12 | Co 57.2 (11.6) | HF | 30 | 15 | Cohort A | Allo-MSC (n = 15) | 1/kg | 1 | IV | UC |
| Cohort A 57.3 (10.1) | |||||||||||||
| Noriega, 2017 [51] | Spain | NCT01860417 | RCT 12 | 38 (2) | DDD | 24 | 12 | Cohort A | Allo-MSC (n = 12) | 25/disc | 1 | ID | BM |
| Wang, 2017 [49] | Australia | NCT01088191 | RCT 24 | Co 26.0 (3.6) | OA | 17 | 6 | Cohort A | Allo-MSC (n = 11) | 75 | 1 | IA | BM |
| Cohort A 26.9 (10.3) | |||||||||||||
- -
- What is the prevalence of alloantibodies development in patients treated with allo-MSCs?
- -
- Does the route of administration of allo-MSCs influence the development of alloantibodies?
- -
- Does the tissue of origin of the MSCs influence the development of alloantibodies?
- -
- Is the total dose of allo-MSCs associated with increased alloantibodies response?
- -
- Are repeated doses of treatment associated with higher frequency of alloantibodies or impact of treatment?
- -
- Does the presensitization status of patients influence the development of alloantibodies?
- -
- How relevant is alloantibodies development to the safety of the treatment?
| Research Question | Answer |
|---|---|
| What is the prevalence of alloantibodies’ development in patients treated with allo-MSCs? | 10.67% of patients developed alloantibodies |
| Does the route of administration of allo-MSCs influence the development of alloantibodies? | IL injection produced the highest production of alloantibodies ID injection did not produce any alloantibodies |
| Does the tissue of origin of the MSCs influence the development of alloantibodies? | MSC-AT produced the highest production of alloantibodies MSC-UC did not produce any alloantibodies |
| Is the total dose of allo-MSCs associated with increased alloantibodies response? | Higher doses of allo-MSCs are generally associated with increased development of alloantibodies |
| Are repeated doses of treatment associated with higher frequency of alloantibodies or impact of treatment? | No correlation was established between the development of alloantibodies and the number of doses of allo-MSCs |
| Does the presensitization status of patients influence the development of alloantibodies? | Presensitized patients generally showed the highest frequency of alloantibodies |
| How relevant is alloantibodies development to the safety of the treatment? | No correlation was established between the development of alloantibodies and the safety of treatment with allo-MSCs |
| First Author, Publish Year | Nº Patients | Disease | Adverse Events (AEs) and Severe Adverse Events (SAEs) | Efficacy | Alloantibodies | Correlation Antibodies + Adverse Events | ||
|---|---|---|---|---|---|---|---|---|
| Principal Endpoints | Results | Measurement | Results | |||||
| Hare, 2012 [54] | 30 | ICM | 30 days, one patient in each group was hospitalized for heart failure (TE-SAE rate 6.7%) The 1-year incidence of SAEs was 33.3% in the allo-MSC and 53.3% in the auto-MSC (p = 0.46). At 1 year, there were no ventricular arrhythmia SAEs observed among allo-MSC recipients compared with four patients (26.7%) in the auto-MSC group (p = 0.10). | 6 MWT, exercise peak VO2, MLHFQ, LV volumes, EF, EED | Auto-MSC but not allo-MSC therapy was associated with an improvement in the 6 MWT and the MLHFQ score, but neither improved exerciseVO2 max. Allo-MSCs and auto-MSCs reduced mean EED by −33.21% and sphericity index but did not increase EFAllo-MSCs reduced LV end-diastolic volumes. Low-dose concentration MSCs produced greatest reductions in LV volumes and increased EF. | cPRA | More than 30% of patients tested showed sensitization to HLA antigens at baseline. A majority of the sensitized patients (87.5%) demonstrated sensitization at all time points with minimal variation of antibody levels. Two patients in the allo-MSC group showed sensitization only at the 6-month time point. Of these, one patient developed low-level HLA class I antibodies to HLA antigen specificities not expressed by the donor MSC. The other sensitized patient showed low-level donor-specific HLA class I antibodies. | Not found |
| Skyler, 2015 [58] | 61 | Type 2 Diabetes | 27 AEs Week 12: No SAEs, serious hypoglycemia AEs, or discontinuations due to AEs were found. | HbA1 c | Week 12: The HbA1c target of 7% was achieved in 8 of 45 subjects treated with 2M-MSCs versus 0 of 15 subjects treated with placebo | DSA | No subjects developed antibodies specific to the donor HLA | Not found |
| Perin, 2015 [55] | 60 | Chronic HF | The incidence of AEs was similar across all groups. Three AEs associated to a procedure in the 150 M. Two episodes of sustained ventricular tachycardia in treated patients (one patient in the 25 M and one in the 75 M). MACE was seen in 15 patients: 10 of 45 (22%) MSC-treated and 5 of 15 (33%) control patients. Two cardiac deaths in the MSC groups (4.4%), both in the M and three in the control group (20%). | LVESV | Compared with the control group, the 150 M-MSC group showed improvement in LVESV with a statistically significant decrease at 6 months (p = 0.015) and a non significant decrease at 12 months. There were no consistent differences between the treated and control groups in myocardial perfusion | DSA | 11% of the 45 M treated patients developed DSA This response was transient in three patients (two 25 M-treated patients and one 150 M-treated patient) but persisted for ≤12 months in two patients in the 150 M group. | Not found |
| Vega, 2015 [48] | 30 | OA | 48 AEs (25 in the control group and 23 in treated patients). No SAEs occurred during treatment | VAS, Lequesne index, WOMAC index PCI | Values of all evaluation scales improved with the cell treatment. Improvement of patients was medium to large (effect size, 0.58 to 1.12 for the different algofunctional indices), whereas improvement was small (0.19 to 0.48) after control treatment. The PCI decreased in both groups, but the decrease was not statistically significant in the control, whereas it reached significance (p < 0.05) in the experimental group at the 12-month follow up. Pain was significantly reduced by 6 and 12 months after MSC treatment. In the treated patients, the slope of the line (efficiency of treatment) was 0.69, whereas in the control series, the slope was only 0.28. 77% of the patients were satisfied or very satisfied with the treatment, whereas in the control group, this percentage fell to 38%. | cPRA | Specific anti-HLA antibodies targeted to alleles present in the donor were found in only 2 of the 13 patients assessed during the trial. In these patients, the reactivity decreased with time. | Not found |
| Panés, 2016 [59] | 212 | CD | 17% of patients in the MSC-treated group versus 29% in the placebo group experienced TE-AEs, the most common of which were anal abscess and proctalgia. TE-SAEs reported were 5% in the MSC group vs. 7% in the placebo group. | Clinical remission Response Time to relapse PDAI | 50% clinical remission in MSC-treated patients (53 of 107) versus 34% remission in placebo-treated patients (36 of 105). Time to remission was significantly shorter in MSC-treated patients (6–7 weeks) than in the placebo group (14 weeks). The improvement in PDAI with MSCs was significantly greater than with placebo at week 6 (change from baseline treatment difference −1.0, 95% CI −1.7 to −0.3), week 12 (−1.2, −2.0 to −0.4) and week 18 (−1.2, −2.0 to −0.3), but not at week 24 (−0.8, −1.8 to 0.2). | DSA | 16% MSC-treated patients and 15% placebo-treated patients were sensitized 34% of MSC-treated patients and none of the placebo-treated patients generated DSA. Presensitized patients were prone to a sustained humoral response longer. | Not found |
| Packham, 2016 [53] | 30 | 12 weeks | Seven (70%) of TE-AEs were reported in the placebo group, Eight (80.0%) in the 150 M group Nine (90.0%) in the 300 M group. The most commonly were edema peripheral, lower respiratory tract infection, urinary tract, infection, cataract, and anemia. No acute allergic or immunologic adverse events were reported. TE-SAEs were Two (20.0%) in the placebo group, four (40.0%) in the 150 M, one (10.0%) in the 300 M. | eGFR mGFR | The adjusted least squares mean differences from placebo in changes from baseline in the treated-groups were 4.4 ± 2.16 and 1.6 ± 2.15 mL/min/1.73 m2 for eGFR and 4.1 ± 2.75 and 3.9 ± 2.75 for mGFR for the 150 M and 300 M groups, respectively. | cPRA | Two treated-patient developed DSA. One placebo-treated patient was sensitized. | Not found |
| Florea, 2017 [46] | 30 | 12 months | The incidence of AE was 10 (66.7%) in the 20 M group and 13 (86.7%) in the 100 M group. The incidence of SAE was seven (46.7%) in the 20 M group and five (33.3%) in the 100 M group. MACE rate was 20.0% in the 20 M group and 13.3% in the 100 M group. Worsening heart failure rehospitalization was 20.0% in the 20 M group and 7.1% in the 100 M group. One case of death in the 100 M group | Scar size EF | Scar size was reduced to a similar degree in both groups: 20 M by −6.4 g (interquartile range, −13.5 to −3.4 g; p = 0.001) and 100 M by −6.1 g (interquartile range, −8.1 to −4.6 g; p = 0.0002), the EF improved only with 100 M by 3.7 U (interquartile range, 1.1 to 6.1; p = 0.04 | cPRA | Only one patient in the 20 M treated group had no- to low-cPRA response. | Not found |
| Tompkins, 2017 [52] | 30 | AF | 10 AEs (four in MSC-treated patients and six AEs in placebo-treated patients). One SAE (death) in MSC-treated group was reported (non-treatment related) | Physical performance: 6 MWT, SPPB score, FEV1. Immune biomarkers | 6 MWT increased in the 100 M-group from baseline to 6 months (345.9 ± 103.4 to 410.7 ± 155.4 m, p = 0.011) SPPB total score was significantly improved in the 100 M-group from baseline to 6 months (median 10.5, IQR 9.0, 12.0 to 12.0, IQR 11.0, 12.0; p = 0.031) FEV1 improved in the 100 M-group from baseline to 6 months (2.5 ± 0.66 to 2.6 ± 0.77 L/min, p = 0.025) Serum TNF-α levels decreased in the 100 M-group (p = 0.03). B cell intracellular TNF-α improved in both the 100 M- (p < 0.0001) and 200 M-groups (p = 0.002) as well as between groups compared to placebo (p = 0.003 and p = 0.039, respectively) | cPRA | Three patients had a mild/moderate increase in donor specific antibodies (one mild in the 100 M- and two moderates in the 200 M-group). There were no clinically significant immune reactions reported. | Not found |
| Álvaro-Gracia, 2017 [57] | 53 | 24 weeks | 141 AEs (most frequent were fever (17%) and infections (15%)). 85% of 1 M-MSC group, 75% of 2 M-MSC group, 100% of 4 M-MSC group and 57% of the placebo group experienced at least one AE. Five SAEs in 1 M-MSC group: lacunar infarction, diarrhea, tendon rupture, rheumatoid nodule and arthritis Two SAEs in 2 M-MSC group: sciatica and rheumatoid arthritis One SAE in placebo: asthenia | Proportion of ACR20, ACR50, ACR70. | ACR20 responses for cohorts A, B, C and placebo were 45%, 20%, 33% and 29%, respectively, at month 1; 25%, 30%, 17% and 14%, respectively, at month 2; and 25%, 15%, 17% and 0%, respectively, at month 3. ACR50 responses tended to be generally greater in patients treated whereas ACR70 responses were very low. | DSA | 19% of patients generated DSA without apparent clinical consequences. 43% of treated patients presented baseline anti-HLA-I antibodies (presensitized) Presensitized patients showed higher frequency of DSA (30% vs. 11%). | Not found |
| Hare, 2017 [47] | 37 | NDC | The 12-month post-TESI AE incidence was 66.7% in allo-MSC and 87.5% in auto-MSC patients. The 12-month post-TESI SAE incidence was 28.2% in allo-MSC and 63.5% in auto-MSC patients. | LV structure and function, QOL, 6 MWT, FEV1, endothelial function EPC-CFU FMB | EF increased significantly in the allo-MSCs group by 8.0 percentage points (p = 0.004), but not in the auto-MSCs cohort (p = 0.116) at 12 months. Functional capacity and QOL showed greater improvement with allo- compared with auto-MSCs use. The 6 MWT distance significantly increased in patients receiving allo-MSCs by 37.0 m (p = 0.04) at 12 months compared with baseline, but did not significantly change in the auto-MSCs group (p = 0.71). FEV1 improved in allo-MSCs patients by 3.7% (p = 0.2423) compared with a decrease of 3.8% (p = 0.16) among the auto-MSCs group at 12 months. EPC-CFU significantly increased with allo-MSCs (p = 0.0107) as did the percentage of FMD at 3 months (p = 0.09). | cPRA | One allo-MSC patient developed an elevated (>80%) donor-specific cPRA level | Not found |
| Bartolucci, 2017 [56] | 30 | HF | There were no acute AEs associated with the infusion Of allo-MSCs or placebo 28 clinically relevant events were reported and the most frequent were non sustained ventricular tachycardia (seven in the placebo group and seven in the treated group) | LVEF, NYHA functional classification, ventilatory efficiency (VE/VCO2 slope) | Only the MSC- treated group exhibited significant improvements in LVEF at 3, 6 and 12 months of follow-up assessed both through transthoracic echocardiography (p = 0.0167 versus baseline) and cardiac MRI (p = 0.025 versus baseline). Echocardiographic LVEF change from baseline to month 12 differed significantly between groups (+7.07 ± 6.22% versus +1.85 ± 5.60%; p = 0.028). MSC- treated patients displayed improvements of NHYA functional class (p = 0.0167 versus baseline) MSC-treated exhibited an improvement in VE/VCO2 at 12 months (−1.89 ± 3.19; p = 0.023 versus baseline) | DSA | None of the patients tested presented alloantibodies to the MSCs | Not found |
| Noriega, 2017 [51] | 24 | DDD | Occasional mild pain reactions | Pain and disability indexes VAS ODI SF-12 life quality questionnaire Pfirrmann grading | MSC-treated patients displayed a quick and significant improvement in algofunctional indices vs. the controls. This improvement seemed restricted to a group of responders that included 40% of the cohort. Degeneration improved in the MSC treated patients and worsened in the controls. | cPRA | Specific antibodies were not detected in any of the nine patients tested. | Not found |
| Wang, 2017 [49] | 17 | OA | 133 AEs Most frequent were musculoskeletal and connective tissue disorders (11 in MSC group and six in control group) Two SAEs were reported in MSC group (fracture of the humerus and infective bursitis). These were not considered to be treatment related. | KOOS SF-36v2 scores Joint space width tibial cartilage volume bone area | Compared with the control group, MSC-treated patients showed greater improvements in KOOS pain, symptom, activities of daily living and SF-36 bodily pain scores (p < 0.05). 26 weeks: The MSC group had reduced medial and lateral tibiofemoral joint space narrowing (p < 0.05), less tibial bone expansion (0.5% vs. 4.0%, p = 0.02), and a trend towards reduced tibial cartilage volume loss (0.7% vs. −4.0%, p = 0.10) than the controls. | cPRA | Increases in anti-HLA (class I) PRA >10% were observed at week 4 in the cell group that decreased to baseline levels by week 104 | Not found |
4. Discussion
- -
- What is the prevalence of alloantibodies development in patients treated with allo-MSCs?
- -
- Does the presensitization status of patients influence the development of alloantibodies?
- -
- Does allo-MSC treatment influence the safety of the treatment?
- -
- What information do non-randomized studies give us?
4.1. Limitations
4.2. Recommendations for Futures Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACR | American College of Rheumatology |
| AEs | Adverse Events |
| Allo-MSCs | Allogeneic Mesenchymal Stromal Cells |
| Auto-MSCs | Autologous Mesenchymal Stromal Cells |
| APC AT | Antigen Presentig Cell Adipose Tissue |
| ARDS | Acute Respiratory Distress Syndrome |
| ATMP | Advanced Therapy Medicinal Product |
| BM | Bone Marrow |
| CD | Crohn’s Disease |
| CDC | Complement Dependent Cytotoxicity |
| cPRA | Calculated Panel Reactive Antibody |
| DDD | Degenerative Disc Disease |
| DN | Diabetic Neuropathy |
| DSA | Donor Specific Antibodies |
| EBV | Epstein Barr Virus |
| EED | Early Enhancement Defect |
| EF | Ejection Fraction |
| eGFR | Estimated Glomerular Filtration Rate |
| ELISPOT | Enzyme-linked Immunospot |
| EMA | European Medicine Agency |
| EPC-CFU | Endothelial Progenitor Cell Colony Forming Unit |
| FEV1 | Forced Expiratory Volume in 1 Second |
| FMD | Flow-Mediated Vasodilation |
| FGF | Fibroblast Growth Factor |
| FS | Frailty Syndrome |
| GVHD | Graft Versus Host Disease |
| HF | Heart Failure |
| HGF | Hepatocyte Growth Factor |
| HLA | Human Leucocyte Antigen |
| IA | Intra-articular |
| IBMIR | Instantaneous Blood-Mediated Inflammatory Reaction |
| ICM | Ischemic Cardiomyopathy |
| ID | Intradiscal |
| IDO | Indolamine-pyrrole 2,3-dioxygenase |
| IL-10 | Interleukin 10 |
| IL | Intralesional |
| ISCT | International Society of Cell and Gene Therapy |
| IV | Intravenous |
| KOOS | Knee Injury and Osteoarthritis Outcome Score |
| LV | Left Ventricular |
| LVEF | Left Ventricular Ejection Fraction |
| LVESV | Left Ventricular End Systolic Volume |
| MACE | Major Adverse Cardiac Events |
| mGFR | Measured Glomerular Filtration Rate |
| MHC | Major Histocompatibilty Complex |
| MLHFQ | Minnesota Living with Heart Failure Questionnaire |
| MLR | Mixed Lymphocyte Reaction |
| MSC | Mesenchymal Stromal Cells |
| NDC | Non-Ischemic Dilated Cardiomyopathy |
| NK | Natural Killer |
| NO | Nitric Oxide |
| NYHA | New York Heart Association Class |
| OA | Osteoarthritis |
| ODI | Oswestry Disability Index |
| PCI | Poor Cartilage Index |
| PDAI | Perianal disease activity index |
| PGE2 | Prostaglandin E2 |
| QOL | Quality of Life |
| RA RTC | Rheumatoid Arthritis Randomized Clinical Trial |
| SAEs | Severe Adverse Events |
| SF-12 | Short Form- 12 |
| SPPB | Short Physical Performance Battery |
| TE-AEs | Treatment-Emergent Adverse Events |
| TE-SAEs | Treatment-Emergent Severe Adverse Events |
| TESI | Transendocardial Stem Cell Injection |
| TGF-β1 | Transforming Growth Factor β1 |
| UC | Umbilical Cord |
| VAS | Visual Analogue Scale |
| 6MWT | 6-Minute Walk Test |
References
- Viswanathan, S.; Shi, Y.; Galipeau, J.; Krampera, M.; Leblanc, K.; Martin, I.; Nolta, J.; Phinney, D.G.; Sensebe, L. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy 2019, 21, 1019–1024. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Kot, M.; Baj-Krzyworzeka, M.; Szatanek, R.; Musiał-Wysocka, A.; Suda-Szczurek, M.; Majka, M. The Importance of HLA Assessment in “Off-the-Shelf” Allogeneic Mesenchymal Stem Cells Based-Therapies. Int. J. Mol. Sci. 2019, 20, 5680. [Google Scholar] [CrossRef] [Green Version]
- Quiñones-Vico, M.I.; Sanabria-de la Torre, R.; Sánchez-Díaz, M.; Sierra-Sánchez, Á.; Montero-Vílchez, T.; Fernández-González, A.; Arias-Santiago, S. The Role of Exosomes derived from Mesenchymal Stromal Cells in Dermatology. Front. Cell Dev. Biol. 2021, 9, 632. [Google Scholar] [CrossRef]
- Merimi, M.; Lewalle, P.; Meuleman, N.; Agha, D.M.; El-Kehdy, H.; Bouhtit, F.; Ayoub, S.; Burny, A.; Fahmi, H.; Lagneaux, L.; et al. Mesenchymal Stem/Stromal Cell Therapeutic Features: The Bridge between the Bench and the Clinic. J. Clin. Med. 2021, 10, 905. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, M.; Wang, F.; Heng, B.C.; Zhou, J.; Cai, Z.; Liu, H. Understanding the Immunological Mechanisms of Mesenchymal Stem Cells in Allogeneic Transplantation: From the Aspect of Major Histocompatibility Complex Class i. Stem Cells Dev. 2019, 28, 1141–1150. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, B.; Shi, H.; Qian, H.; Xu, W. MSC-exosome: A novel cell-free therapy for cutaneous regeneration. Cytotherapy 2018, 20, 291–301. [Google Scholar] [CrossRef]
- Casado-Díaz, A.; Quesada-Gómez, J.M.; Dorado, G. Extracellular Vesicles Derived From Mesenchymal Stem Cells (MSC) in Regenerative Medicine: Applications in Skin Wound Healing. Front. Bioeng. Biotechnol. 2020, 8, 1–19. [Google Scholar] [CrossRef] [Green Version]
- European Medicines Agency. Advanced Therapy Medicinal Products: Overview. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/advanced-therapy-medicinal-products-overview (accessed on 17 March 2021).
- Rendra, E.; Scaccia, E.; Biebac, K. Recent advances in understanding mesenchymal stromal cells. F1000Research 2020, 9, 1–9. [Google Scholar] [CrossRef]
- Le Blanc, K.; Tammik, C.; Rosendahl, K.; Zetterberg, E.; Ringdén, O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp. Hematol. 2003, 31, 890–896. [Google Scholar] [CrossRef]
- Le Blanc, K.; Mougiakakos, D. Multipotent mesenchymal stromal cells and the innate immune system. Nat. Rev. Immunol. 2012, 12, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Du, L. The role of secreted factors in stem cells-mediated immune regulation. Cell. Immunol. 2018, 326, 24–32. [Google Scholar] [CrossRef] [PubMed]
- De Vasconcellos Machado, C.; da Silva Telles, P.D.; Nascimento, I.L.O. Immunological characteristics of mesenchymal stem cells. Rev. Bras. Hematol. Hemoter. 2013, 35, 62–67. [Google Scholar] [CrossRef]
- Ankrum, J.A.; Ong, J.F.; Karp, J.M. Mesenchymal stem cells: Immune evasive, not immune privileged. Nat. Biotechnol. 2014, 32, 252–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zangi, L.; Margalit, R.; Reich-Zeliger, S.; Bachar-Lustig, E.; Beilhack, A.; Negrin, R.; Reisner, Y. Direct imaging of immune rejection and memory induction by allogeneic mesenchymal stromal cells. Stem Cells 2009, 27, 2865–2874. [Google Scholar] [CrossRef]
- Nauta, A.J.; Westerhuis, G.; Kruisselbrink, A.B.; Lurvink, E.G.A.; Willemze, R.; Fibbe, W.E. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood 2006, 108, 2114–2120. [Google Scholar] [CrossRef]
- Poncelet, A.J.; Vercruysse, J.; Saliez, A.; Gianello, P. Although pig allogeneic mesenchymal stem cells are not immunogenic in vitro, intracardiac injection elicits an immune response in vivo. Transplantation 2007, 83, 783–790. [Google Scholar] [CrossRef]
- Schu, S.; Nosov, M.; O’Flynn, L.; Shaw, G.; Treacy, O.; Barry, F.; Murphy, M.; O’Brien, T.; Ritter, T. Immunogenicity of allogeneic mesenchymal stem cells. J. Cell. Mol. Med. 2012, 16, 2094–2103. [Google Scholar] [CrossRef] [PubMed]
- Beggs, K.J.; Lyubimov, A.; Borneman, J.N.; Bartholomew, A.; Moseley, A.; Dodds, R.; Archambault, M.P.; Smith, A.K.; McIntosh, K.R. Immunologic consequences of multiple, high-dose administration of allogeneic mesenchymal stem cells to baboons. Cell Transplant. 2006, 15, 711–721. [Google Scholar] [CrossRef]
- Barnhoorn, M.C.; Van Halteren, A.G.S.; Van Pel, M.; Molendijk, I.; Struijk, A.C.; Jansen, P.M.; Verspaget, H.W.; Dijkstra, G.; Oosten, L.E.M.; Van Der Meulen-De Jong, A.E. Lymphoproliferative Disease in the Rectum 4 Years after Local Mesenchymal Stromal Cell Therapy for Refractory Perianal Crohn’s Fistulas: A Case Report. J. Crohn’s Colitis 2019, 13, 807–811. [Google Scholar] [CrossRef]
- Camp, D.M.; Loeffler, D.A.; Farrah, D.M.; Borneman, J.N.; LeWitt, P.A. Cellular immune response to intrastriatally implanted allogeneic bone marrow stromal cells in a rat model of Parkinson’s disease. J. Neuroinflamm. 2009, 6. [Google Scholar] [CrossRef] [Green Version]
- Joswig, A.J.; Mitchell, A.; Cummings, K.J.; Levine, G.J.; Gregory, C.A.; Smith, R.; Watts, A.E. Repeated intra-articular injection of allogeneic mesenchymal stem cells causes an adverse response compared to autologous cells in the equine model. Stem Cell Res. Ther. 2017, 8, 42. [Google Scholar] [CrossRef] [Green Version]
- Berglund, A.K.; Schnabel, L.V. Allogeneic major histocompatibility complex-mismatched equine bone marrow-derived mesenchymal stem cells are targeted for death by cytotoxic anti-major histocompatibility complex antibodies. Equine Vet. J. 2017, 49, 539–544. [Google Scholar] [CrossRef]
- Isakova, I.A.; Lanclos, C.; Bruhn, J.; Kuroda, M.J.; Baker, K.C.; Krishnappa, V.; Phinney, D.G. Allo-Reactivity of Mesenchymal Stem Cells in Rhesus Macaques Is Dose and Haplotype Dependent and Limits Durable Cell Engraftment In Vivo. PLoS ONE 2014, 9, e87238. [Google Scholar] [CrossRef]
- Barrachina, L.; Cequier, A.; Romero, A.; Vitoria, A.; Zaragoza, P.; Vázquez, F.J.; Rodellar, C. Allo-antibody production after intraarticular administration of mesenchymal stem cells (MSCs) in an equine osteoarthritis model: Effect of repeated administration, MSC inflammatory stimulation, and equine leukocyte antigen (ELA) compatibility. Stem Cell Res. Ther. 2020, 11. [Google Scholar] [CrossRef]
- Badillo, A.T.; Beggs, K.J.; Javazon, E.H.; Tebbets, J.C.; Flake, A.W. Murine Bone Marrow Stromal Progenitor Cells Elicit an In Vivo Cellular and Humoral Alloimmune Response. Biol. Blood Marrow Transplant. 2007, 13, 412–422. [Google Scholar] [CrossRef] [Green Version]
- Haynes, M.K.; Hume, E.L.; Smith, J.B. Phenotypic characterization of inflammatory cells from osteoarthritic synovium and synovial fluids. Clin. Immunol. 2002, 105, 315–325. [Google Scholar] [CrossRef]
- Da Silva Meirelles, L.; Fontes, A.M.; Covas, D.T.; Caplan, A.I. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009, 20, 419–427. [Google Scholar] [CrossRef]
- Zhou, X.; Jin, N.; Wang, F.; Chen, B. Mesenchymal stem cells: A promising way in therapies of graft-versus-host disease. Cancer Cell Int. 2020, 20, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Jurado, M.; De La Mata, C.; Ruiz-García, A.; López-Fernández, E.; Espinosa, O.; Remigia, M.J.; Moratalla, L.; Goterris, R.; García-Martín, P.; Ruiz-Cabello, F.; et al. Adipose tissue-derived mesenchymal stromal cells as part of therapy for chronic graft-versus-host disease: A phase I/II study. Cytotherapy 2017, 19, 927–936. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Q.; Wang, W.; Lin, F.; Wang, S.; Zhao, J. Mesenchymal stem cell therapy for ischemic stroke: A look into treatment mechanism and therapeutic potential. J. Neurol. 2020, 1–13. [Google Scholar] [CrossRef]
- Singh, V.K.; Mishra, A.; Bark, S.; Mani, A.; Subbian, S.; Hunter, R.L.; Jagannath, C.; Khan, A. Human mesenchymal stem cell based intracellular dormancy model of Mycobacterium tuberculosis. Microbes Infect. 2020, 22, 423–431. [Google Scholar] [CrossRef]
- Doyle, E.C.; Wragg, N.M.; Wilson, S.L. Intraarticular injection of bone marrow-derived mesenchymal stem cells enhances regeneration in knee osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 3827–3842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, W.; Li, H.Y.; Yang, Q.; Chen, G.; Lin, S.; Liao, C.; Zhou, T. Administration of mesenchymal stem cells in diabetic kidney disease: A systematic review and meta-analysis. Stem Cell Res. Ther. 2021, 12, 1–21. [Google Scholar] [CrossRef]
- Aali, E.; Madjd, Z.; Tekiyehmaroof, N.; Sharifi, A.M. Control of Hyperglycemia Using Differentiated and Undifferentiated Mesenchymal Stem Cells in Rats with Type 1 Diabetes. Cells Tissues Organs 2020, 209, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Rodríguez, M.; Viciana, P.; Rivas-Jeremías, I.; Álvarez-Ríos, A.I.; Ruiz-García, A.; Espinosa-Ibáñez, O.; Arias-Santiago, S.; Martínez-Atienza, J.; Mata, R.; Fernández-López, O.; et al. Mesenchymal stromal cells in Human Immunodeficiency Virus-infected patients with discordant immune response: Early results of a phase I/II clinical trial. Stem Cells Transl. Med. 2020, 1–8. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://www.clinicaltrials.gov/ct2/results?cond=&term=mesenchymal+stem+cells&cntry=&state=&city=&dist= (accessed on 17 March 2021).
- Sierra-Sánchez, Á.; Montero-Vilchez, T.; Quiñones-Vico, M.I.; Sanchez-Diaz, M.; Arias-Santiago, S. Current Advanced Therapies Based on Human Mesenchymal Stem Cells for Skin Diseases. Front. Cell Dev. Biol. 2021, 9. [Google Scholar] [CrossRef]
- Wilson, J.G.; Liu, K.D.; Zhuo, H.; Caballero, L.; McMillan, M.; Fang, X.; Cosgrove, K.; Vojnik, R.; Calfee, C.S.; Lee, J.-W.; et al. Mesenchymal Stem (Stromal) Cells for Treatment of ARDS: A Phase 1 Clinical Trial. Lancet Respir. Med. 2015, 3, 24–32. [Google Scholar] [CrossRef] [Green Version]
- Qu, W.; Wang, Z.; Hare, J.M.; Bu, G.; Mallea, J.M.; Pascual, J.M.; Caplan, A.I.; Kurtzberg, J.; Zubair, A.C.; Kubrova, E.; et al. Cell-based therapy to reduce mortality from COVID-19: Systematic review and meta-analysis of human studies on acute respiratory distress syndrome. Stem Cells Transl. Med. 2020, 9, 1007–1022. [Google Scholar] [CrossRef]
- Qin, H.; Zhao, A. Mesenchymal stem cell therapy for acute respiratory distress syndrome: From basic to clinics. Protein Cell 2020, 11, 707–722. [Google Scholar] [CrossRef]
- Khoury, M.; Cuenca, J.; Cruz, F.F.; Figueroa, F.E.; Rocco, P.R.M.; Weiss, D.J. Current status of cell-based therapies for respiratory virus infections: Applicability to COVID-19. Eur. Respir. J. 2020, 55. [Google Scholar] [CrossRef] [Green Version]
- Lohan, P.; Treacy, O.; Griffin, M.D.; Ritter, T.; Ryan, A.E. Anti-donor immune responses elicited by allogeneic mesenchymal stem cells and their extracellular vesicles: Are we still learning? Front. Immunol. 2017, 8, 1626. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Florea, V.; Rieger, A.C.; DiFede, D.L.; El-Khorazaty, J.; Natsumeda, M.; Banerjee, M.N.; Tompkins, B.A.; Khan, A.; Schulman, I.H.; Landin, A.M.; et al. Dose comparison study of allogeneic mesenchymal stem cells in patients with ischemic cardiomyopathy (The TRIDENT study). Circ. Res. 2017, 121, 1279–1290. [Google Scholar] [CrossRef]
- Hare, J.M.; DiFede, D.L.; Rieger, A.C.; Florea, V.; Landin, A.M.; El-Khorazaty, J.; Khan, A.; Mushtaq, M.; Lowery, M.H.; Byrnes, J.J.; et al. Randomized Comparison of Allogeneic Versus Autologous Mesenchymal Stem Cells for Nonischemic Dilated Cardiomyopathy: POSEIDON-DCM Trial. J. Am. Coll. Cardiol. 2017, 69, 526–537. [Google Scholar] [CrossRef]
- Vega, A.; Martín-Ferrero, M.A.; Del Canto, F.; Alberca, M.; García, V.; Munar, A.; Orozco, L.; Soler, R.; Fuertes, J.J.; Huguet, M.; et al. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: A randomized controlled trial. Transplantation 2015, 99, 1681–1690. [Google Scholar] [CrossRef]
- Wang, Y.; Shimmin, A.; Ghosh, P.; Marks, P.; Linklater, J.; Connell, D.; Hall, S.; Skerrett, D.; Itescu, S.; Cicuttini, F.M. Safety, tolerability, clinical, and joint structural outcomes of a single intra-articular injection of allogeneic mesenchymal precursor cells in patients following anterior cruciate ligament reconstruction: A controlled double-blind randomised trial. Arthritis Res. Ther. 2017, 19, 1–12. [Google Scholar] [CrossRef] [Green Version]
- García-Sancho, J.; Sánchez, A.; Vega, A.; Noriega, D.C.; Nocito, M. Influence of HLA Matching on the Efficacy of Allogeneic Mesenchymal Stromal Cell Therapies for Osteoarthritis and Degenerative Disc Disease. Transplant. Direct 2017, 3, e205. [Google Scholar] [CrossRef]
- Noriega, D.C.; Ardura, F.; Hernández-Ramajo, R.; Martín-Ferrero, M.Á.; Sánchez-Lite, I.; Toribio, B.; Alberca, M.; Garciá, V.; Moraleda, J.M.; Sánchez, A.; et al. Intervertebral Disc Repair by Allogeneic Mesenchymal Bone Marrow Cells: A Randomized Controlled Trial. Transplantation 2017, 101, 1945–1951. [Google Scholar] [CrossRef]
- Tompkins, B.A.; Difede, D.L.; Khan, A.; Landin, A.M.; Schulman, I.H.; Pujol, M.V.; Heldman, A.W.; Miki, R.; Goldschmidt-Clermont, P.J.; Goldstein, B.J.; et al. Allogeneic Mesenchymal Stem Cells Ameliorate Aging Frailty: A Phase II Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2017, 72, 1513–1521. [Google Scholar] [CrossRef]
- Packham, D.K.; Fraser, I.R.; Kerr, P.G.; Segal, K.R. Allogeneic Mesenchymal Precursor Cells (MPC) in Diabetic Nephropathy: A Randomized, Placebo-controlled, Dose Escalation Study. EBioMedicine 2016, 12, 263–269. [Google Scholar] [CrossRef] [Green Version]
- Hare, J.M.; Fishman, J.E.; Gerstenblith, G.; DiFede Velazquez, D.L.; Zambrano, J.P.; Suncion, V.Y.; Tracy, M.; Ghersin, E.; Johnston, P.V.; Brinker, J.A.; et al. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: The POSEIDON randomized trial. JAMA J. Am. Med. Assoc. 2012, 308, 2369–2379. [Google Scholar] [CrossRef]
- Perin, E.C.; Borow, K.M.; Silva, G.V.; DeMaria, A.N.; Marroquin, O.C.; Huang, P.P.; Traverse, J.H.; Krum, H.; Skerrett, D.; Zheng, Y.; et al. A Phase II dose-escalation study of allogeneic mesenchymal precursor cells in patients with ischemic or nonischemic heart failure. Circ. Res. 2015, 117, 576–584. [Google Scholar] [CrossRef] [Green Version]
- Bartolucci, J.; Verdugo, F.J.; González, P.L.; Larrea, R.E.; Abarzua, E.; Goset, C.; Rojo, P.; Palma, I.; Lamich, R.; Pedreros, P.A.; et al. Safety and efficacy of the intravenous infusion of umbilical cord mesenchymal stem cells in patients with heart failure: A phase 1/2 randomized controlled trial (RIMECARD trial [Randomized clinical trial of intravenous infusion umbilical cord mesenchymal Stem Cells on Cardiopathy]). Circ. Res. 2017, 121, 1192–1204. [Google Scholar] [CrossRef]
- Álvaro-Gracia, J.M.; Jover, J.A.; García-Vicuña, R.; Carreño, L.; Alonso, A.; Marsal, S.; Blanco, F.; Martínez-Taboada, V.M.; Taylor, P.; Martín-Martín, C.; et al. Intravenous administration of expanded allogeneic adipose-derived mesenchymal stem cells in refractory rheumatoid arthritis (Cx611): Results of a multicentre, dose escalation, randomised, singleblind, placebo-controlled phase Ib/IIa clinical trial. Ann. Rheum. Dis. 2017, 76, 196–202. [Google Scholar] [CrossRef]
- Skyler, J.S.; Fonseca, V.A.; Segal, K.R.; Rosenstock, J. Allogeneic mesenchymal precursor cells in type 2 diabetes: A randomized, placebo-controlled, dose-escalation safety and tolerability pilot study. Diabetes Care 2015, 38, 1742–1749. [Google Scholar] [CrossRef] [Green Version]
- Panés, J.; García-Olmo, D.; Van Assche, G.; Colombel, J.F.; Reinisch, W.; Baumgart, D.C.; Dignass, A.; Nachury, M.; Ferrante, M.; Kazemi-Shirazi, L.; et al. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: A phase 3 randomised, double-blind controlled trial. Lancet 2016, 388, 1281–1290. [Google Scholar] [CrossRef]
- Clé, D.V.; Santana-Lemos, B.; Tellechea, M.F.; Prata, K.L.; Orellana, M.D.; Covas, D.T.; Calado, R.T. Intravenous infusion of allogeneic mesenchymal stromal cells in refractory or relapsed aplastic anemia. Cytotherapy 2015, 17, 1696–1705. [Google Scholar] [CrossRef]
- Giri, J.; Galipeau, J. Mesenchymal stromal cell therapeutic potency is dependent upon viability, route of delivery, and immune match. Blood Adv. 2020, 4, 1987–1997. [Google Scholar] [CrossRef]
- Ringdén, O.; Uzunel, M.; Rasmusson, I.; Remberger, M.; Sundberg, B.; Lönnies, H.; Marschall, H.U.; Dlugosz, A.; Szakos, A.; Hassan, Z.; et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation 2006, 81, 1390–1397. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Lou, R.; Huang, F.; Peng, Y.; Jiang, Z.; Huang, K.; Wu, X.; Zhang, Y.; Fan, Z.; Zhou, H.; et al. Immunomodulation effects of mesenchymal stromal cells on acute graft-versus-host disease after hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 2015, 21, 97–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaipe, H.; Carlson, L.M.; Erkers, T.; Nava, S.; Mollden, P.; Gustafsson, B.; Qian, H.; Li, X.; Hashimoto, T.; Sadeghi, B.; et al. Immunogenicity of decidual stromal cells in an epidermolysis bullosa patient and in allogeneic hematopoietic stem cell transplantation patients. Stem Cells Dev. 2015, 24, 1471–1482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.M.; Dawson, G.; Franz, L.; Howard, J.; McLaughlin, C.; Kistler, B.; Waters-Pick, B.; Meadows, N.; Troy, J.; Kurtzberg, J. Infusion of human umbilical cord tissue mesenchymal stromal cells in children with autism spectrum disorder. Stem Cells Transl. Med. 2020, 9, 1137–1146. [Google Scholar] [CrossRef]
- Wilson, A.; Hodgson-Garms, M.; Frith, J.E.; Genever, P. Multiplicity of mesenchymal stromal cells: Finding the right route to therapy. Front. Immunol. 2019, 10, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Nemeth, K. Mesenchymal stem cell therapy for immune-modulation: The donor, the recipient, and the drugs in-between. Exp. Dermatol. 2014, 23, 625–628. [Google Scholar] [CrossRef] [Green Version]
- Abdelrazik, H.; Spaggiari, G.M.; Chiossone, L.; Moretta, L. Mesenchymal stem cells expanded in human platelet lysate display a decreased inhibitory capacity on T- and NK-cell proliferation and function. Eur. J. Immunol. 2011, 41, 3281–3290. [Google Scholar] [CrossRef]
- Samuelsson, H.; Ringden, O.; Lonnies, H.; Le Blanc, K. Optimizing in vitro conditions for immunomodulation and expansion of mesenchymal stromal cells. Cytotherapy 2009, 11, 129–136. [Google Scholar] [CrossRef]
- El-Sayed, M.; El-Feky, M.A.; El-Amir, M.I.; Hasan, A.S.; Tag-Adeen, M.; Urata, Y.; Goto, S.; Luo, L.; Yan, C.; Li, T.S. Immunomodulatory effect of mesenchymal stem cells: Cell origin and cell quality variations. Mol. Biol. Rep. 2019, 46, 1157–1165. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanabria-de la Torre, R.; Quiñones-Vico, M.I.; Fernández-González, A.; Sánchez-Díaz, M.; Montero-Vílchez, T.; Sierra-Sánchez, Á.; Arias-Santiago, S. Alloreactive Immune Response Associated to Human Mesenchymal Stromal Cells Treatment: A Systematic Review. J. Clin. Med. 2021, 10, 2991. https://doi.org/10.3390/jcm10132991
Sanabria-de la Torre R, Quiñones-Vico MI, Fernández-González A, Sánchez-Díaz M, Montero-Vílchez T, Sierra-Sánchez Á, Arias-Santiago S. Alloreactive Immune Response Associated to Human Mesenchymal Stromal Cells Treatment: A Systematic Review. Journal of Clinical Medicine. 2021; 10(13):2991. https://doi.org/10.3390/jcm10132991
Chicago/Turabian StyleSanabria-de la Torre, Raquel, María I. Quiñones-Vico, Ana Fernández-González, Manuel Sánchez-Díaz, Trinidad Montero-Vílchez, Álvaro Sierra-Sánchez, and Salvador Arias-Santiago. 2021. "Alloreactive Immune Response Associated to Human Mesenchymal Stromal Cells Treatment: A Systematic Review" Journal of Clinical Medicine 10, no. 13: 2991. https://doi.org/10.3390/jcm10132991
APA StyleSanabria-de la Torre, R., Quiñones-Vico, M. I., Fernández-González, A., Sánchez-Díaz, M., Montero-Vílchez, T., Sierra-Sánchez, Á., & Arias-Santiago, S. (2021). Alloreactive Immune Response Associated to Human Mesenchymal Stromal Cells Treatment: A Systematic Review. Journal of Clinical Medicine, 10(13), 2991. https://doi.org/10.3390/jcm10132991












