Is TGF-β1 a Biomarker of Huntington’s Disease Progression?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Classification and Neurological Examination in HD Patients
- Preclinical stage—0 points
- Very early disease stage 1–13 points
- Early disease stage 14–37 points
- Intermediate disease stage 38–67 points
- Advanced disease stage >67 points
2.2. Assessment of Cognitive Functions and Mood Disorders in HD Patients
- Mini-Mental State Examination (MMSE)
- Montreal Cognitive Assessment (MoCA)
- Clock Drawing Test (CDT)
- Symbol Digital Modality Test (SDMT), assessing visual-spatial attention based on matching digits to a sequence of symbols in 90 s as in a formula consisting of 9 characters combined with digits
- Verbal fluency tests determining verbal fluency (VF) and phonemic fluency (Total Fluency, TF)
- Trail Making Test (TMT) part one (connecting points numbered from 1 to 25 in sequential order) and two (connecting points in order while alternating between numbers and letters, i.e., 1-A-2-B-3-C-etc.)
- Stroop test (ST) parts one, two and three. In the first part, a sheet of paper is given to the examined person, which contains squares filled with colored ink. The task is to recognize as many consecutive colors as possible within 45 s. In the second part, the examined person receives a sheet with one hundred words, divided into ten lines with the names of the colors—the task is to read as many words as possible within 45 s. In the last part of the test, there are words that represent colors written in a different ink color than the word they actually mean—the task is to recognize the ink color with which the word is written.
2.3. Biochemical Assessment
2.4. Statistical Analysis
3. Results
3.1. Basic Results of the Patients
3.2. Neurological Examination of HD Patients
3.3. Assessment of Cognitive Functions in HD Patients
3.4. Plasma Levels of TGF-β1 in HD Patients vs. Control Group
3.4.1. Basic Results
3.4.2. Correlations between Plasma Levels of TGF-β1 and the Results of Neurological Examination and Cognitive Function Tests
3.4.3. Results Summary
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pringsheim, T.; Wiltshire, K.; Day, L.; Dykeman, J.; Steeves, T.; Jette, N. The incidence and prevalence of Huntington’s disease: A systematic review and meta-analysis. Mov. Disord. 2012, 27, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Warby, S.C.; Montpetit, A.; Hayden, A.R.; Carroll, J.B.; Butland, S.L.; Visscher, H.; Collins, J.A.; Semaka, A.; Hudson, T.J.; Hayden, M.R. CAG expansion in the Huntington disease gene is associated with a specific and targetable predisposing haplogroup. Am. J. Hum. Genet. 2009, 84, 351–366. [Google Scholar] [CrossRef] [Green Version]
- Wolters, E.C.; van Laar, T.; Berendse, H.W. Parkinsonism and Related Disorders, 2nd ed.; VU University Press: Amsterdam, The Netherland, 2008. [Google Scholar]
- McColgan, P.; Tabrizi, S.J. Huntington’s disease: A clinical review. Eur. J. Neurol. 2018, 25, 24–34. [Google Scholar] [CrossRef]
- Ross, C.A.; Shoulson, I. Huntington disease: Pathogenesis, biomarkers, and approaches to experimental therapeutics. Parkinsonism Relat. Disord. 2009, 15, S135–S138. [Google Scholar] [CrossRef]
- Jimenez-Sanchez, M.; Licitra, F.; Underwood, B.R.; Rubinsztein, D.C. Huntington’s Disease: Mechanisms of Pathogenesis and Therapeutic Strategies. Cold Spring Harb. Perspect. Med. 2017, 7, a024240. [Google Scholar] [CrossRef] [Green Version]
- Mestre, T.A.; Sampaio, C. Huntington Disease: Linking Pathogenesis to the Development of Experimental Therapeutics. Curr. Neurol. Neurosci. Rep. 2017, 17, 18. [Google Scholar] [CrossRef]
- Kashima, R.; Hata, A. The role of TGF-β superfamily signaling in neurological disorders. Acta Biochim. Biophys. Sin (Shanghai) 2018, 50, 106–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ring, K.L.; An, M.C.; Zhang, N.; O’Brien, R.N.; Ramos, E.M.; Gao, F.; Atwood, R.; Bailus, B.J.; Melov, S.; Mooney, S.D.; et al. Genomic Analysis Reveals Disruption of Striatal Neuronal Development and Therapeutic Targets in Human Huntington’s Disease Neural Stem Cells. Stem Cell Rep. 2015, 5, 1023–1038. [Google Scholar] [CrossRef] [Green Version]
- Flanders, K.C.; Ren, R.F.; Lippa, C.F. Transforming growth factor-betas in neurodegenerative disease. Prog. Neurobiol. 1998, 54, 71–85. [Google Scholar] [CrossRef]
- Dennler, S.; Goumans, M.J.; ten Dijke, P. Transforming growth factor beta signal transduction. J. Leukoc. Biol. 2002, 71, 731–740. [Google Scholar]
- Annes, J.P.; Munger, J.S.; Rifkin, D.B. Making sense of latent TGFbeta activation. J. Cell Sci. 2003, 116, 217–224. [Google Scholar] [CrossRef] [Green Version]
- Clark, D.A.; Coker, R. Transforming growth factor-beta (TGF-beta). Int. J. Biochem. Cell Biol. 1998, 30, 293–298. [Google Scholar] [CrossRef]
- Battaglia, G.; Cannella, M.; Riozzi, B.; Orobello, S.; Maat-Schieman, M.L.; Aronica, E.; Busceti, C.L.; Ciarmiello, A.; Alberti, S.; Amico, E.; et al. Early defect of transforming growth factor β1 formation in Huntington’s disease. J. Cell Mol. Med. 2011, 15, 555–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scarpa, J.R.; Jiang, P.; Losic, B.; Readhead, B.; Gao, V.D.; Dudley, J.T.; Vitaterna, M.H.; Turek, F.W.; Kasarskis, A. Systems Genetic Analyses Highlight a TGFβ-FOXO3 Dependent Striatal Astrocyte Network Conserved across Species and Associated with Stress, Sleep, and Huntington’s Disease. PLoS Genet. 2016, 12, e1006137. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.H.; Wu, Y.R.; Chen, Y.C.; Chen, C.M. Plasma inflammatory biomarkers for Huntington’s disease patients and mouse model. Brain Behav. Immun. 2015, 44, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Di Pardo, A.; Alberti, S.; Maglione, V.; Amico, E.; Cortes, E.P.; Elifani, F.; Battaglia, G.; Busceti, C.L.; Nicoletti, F.; Vonsattel, J.P.; et al. Changes of peripheral TGF-β1 depend on monocytes-derived macrophages in Huntington disease. Mol. Brain 2013, 6, 55. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Wei, J.; Sun, J. Roles of TGF-β signaling pathway in tumor microenvirionment and cancer therapy. Int. Immunopharmacol. 2020, 89, 107101. [Google Scholar] [CrossRef]
- Seoane, J.; Gomis, R.R. TGF-β Family Signaling in Tumor Suppression and Cancer Progression. Cold Spring Harb. Perspect. Biol. 2017, 9, a022277. [Google Scholar] [CrossRef] [Green Version]
- Mangiarini, L.; Sathasivam, K.; Seller, M.; Cozens, B.; Harper, A.; Hetherington, C.; Lawton, M.; Trottier, Y.; Lehrach, H.; Davies, S.W.; et al. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell 1996, 87, 493–506. [Google Scholar] [CrossRef] [Green Version]
- Slow, E.J.; van Raamsdonk, J.; Rogers, D.; Coleman, S.H.; Graham, R.K.; Deng, Y.; Oh, R.; Bissada, N.; Hossain, S.M.; Yang, Y.Z.; et al. Selective striatal neuronal loss in a YAC128 mouse model of Huntington disease. Hum. Mol. Genet. 2003, 12, 1555–1567. [Google Scholar] [CrossRef]
- Squitieri, F.; Ciarmiello, A.; Di Donato, S.; Frati, L. The search for cerebral biomarkers of Huntington’s disease: A review of genetic models of age at onset prediction. Eur. J. Neurol. 2006, 13, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Harris, G.J.; Codori, A.M.; Lewis, R.F.; Schmidt, E.; Bedi, A.; Brandt, J. Reduced basal ganglia blood flow and volume in pre-symptomatic, gene-tested persons at-risk for Huntington’s disease. Brain 1999, 122, 1667–1678. [Google Scholar] [CrossRef] [PubMed]
- Reilmann, R.; Bohlen, S.; Klopstock, T.; Bender, A.; Weindl, A.; Saemann, P.; Auer, D.P.; Ringelstein, E.B.; Lange, H.W. Tongue force analysis assesses motor phenotype in premanifest and symptomatic Huntington’s disease. Mov. Disord. 2010, 25, 2195–2202. [Google Scholar] [CrossRef]
- Bechtel, N.; Scahill, R.I.; Rosas, H.D.; Acharya, T.; van den Bogaard, S.J.; Jauffret, C.; Say, M.J.; Sturrock, A.; Johnson, H.; Onorato, C.E.; et al. Tapping linked to function and structure in premanifest and symptomatic Huntington disease. Neurology 2010, 75, 2150–2160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbergenova, Y.; Littleton, J.T. Pathogenic Huntington Alters BMP Signaling and Synaptic Growth through Local Disruptions of Endosomal Compartments. J. Neurosci. 2017, 37, 3425–3439. [Google Scholar] [CrossRef] [Green Version]
- Naphade, S.; Embusch, A.; Madushani, K.L.; Ring, K.L.; Ellerby, L.M. Altered Expression of Matrix Metalloproteinases and Their Endogenous Inhibitors in a Human Isogenic Stem Cell Model of Huntington’s Disease. Front. Neurosci. 2018, 11, 736. [Google Scholar] [CrossRef] [Green Version]
- Wachs, F.P.; Winner, B.; Couillard-Despres, S.; Schiller, T.; Aigner, R.; Winkler, J.; Bogdahn, U.; Aigner, L. Transforming growth factor-beta1 is a negative modulator of adult neurogenesis. J. Neuropathol. Exp. Neurol. 2006, 65, 358–370. [Google Scholar] [CrossRef] [Green Version]
- Kandasamy, M.; Reilmann, R.; Winkler, J.; Bogdahn, U.; Aigner, L. Transforming Growth Factor-Beta Signaling in the Neural Stem Cell Niche: A Therapeutic Target for Huntington’s Disease. Neurol. Res. Int. 2011, 2011, 124256. [Google Scholar] [CrossRef] [Green Version]
- Tourjman, V.; Kouassi, É.; Koué, M.È.; Rocchetti, M.; Fortin-Fournier, S.; Fusar-Poli, P.; Potvin, S. Antipsychotics’ effects on blood levels of cytokines in schizophrenia: A meta-analysis. Schizophr. Res. 2013, 151, 43–47. [Google Scholar] [CrossRef]

| Variables/ HD Stages | All HD | Preclinical | Very Early | Early | Intermediate | Advanced | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ± SD | Range | ± SD | Range | ± SD | Range | ± SD | Range | ± SD | Range | ± SD | Range | |
| Age | 46.87 ± 13.70 | 20–73 | 40.33 ± 1.52 | 19–42 | 32.83 ± 10.93 | 20–55 | 54.84 ± 9.73 | 33–70 | 47.28 ± 12.75 | 22–66 | 54.20 ± 16.33 | 35–69 |
| Age of symptom onset | 40.05 ± 18.54 | 19–68 | - | - | 30.33 ± 1.07 | 20–50 | 49.00 ± 9.26 | 33–65 | 35.33 ± 11.20 | 30–52 | 40.25 ± 3.36 | 39–60 |
| Disease duration | 9.53 ± 6.71 | 0–25 | - | - | 6.60 ± 8.26 | 1–25 | 6.16 ± 4.26 | 1–19 | 9.75 ± 6.22 | 2–26 | 11.85 ± 5.35 | 4–21 |
| CAG repeats small allele | 18.03 ± 2.84 | 12–30 | 17.50 ± 0.83 | 13–27 | 17.77 ± 3.07 | 12–23 | 18.36 ± 4.16 | 14–30 | 18.25 ± 3.36 | 15–23 | 17.26 ± 1.90 | 15–22 |
| CAG repeats large allele | 44.40 ± 4.28 | 40–63 | 41.83 ± 1.16 | 40–60 | 45.11 ± 3.95 | 40–52 | 43.21 ± 3.10 | 40–50 | 45.12 ± 1.01 | 40–63 | 45.53 ± 4.27 | 41–54 |
| Motor UHDRS | 40.39 ± 28.84 | 0–102 | - | - | 4.66 ± 2.70 | 1–9 | 26.38 ± 6.03 | 14–35 | 52.95 ± 7.92 | 39–67 | 76.80 ± 2.77 | 74–80 |
| Years of study | 13.51 ± 3.21 | 8–27 | 18.00 ± 3.68 | 13–27 | 15.00 ± 1.78 | 12–18 | 14.23 ± 2.68 | 11–17 | 13.00 ± 2.79 | 8–19 | 12.40 ± 3.36 | 8–17 |
| MMSE | 23.75 ± 5.92 | 0–30 | 29.87 ± 0.35 | 29–30 | 28.22 ± 1.78 | 25–30 | 27.11 ± 2.26 | 22–30 | 22.62 ± 4.52 | 7–29 | 14.58 ± 4.62 | 8–22 |
| MoCA | 23.98 ± 6.15 | 0–30 | 29.00 ± 1.78 | 25–30 | 26.71 ± 4.64 | 17–30 | 26.92 ± 2.55 | 20–30 | 21.80 ± 5.22 | 7–30 | 15.00 ± 6.32 | 9–28 |
| Beck’s scale | 5.43 ± 6.09 | 0–63 | 3.54 ± 4.43 | 0–12 | 6.54 ± 8.04 | 0–26 | 3.09 ± 4.01 | 0–12 | 5.70 ± 5.38 | 0–18 | 5.90 ± 6.47 | 0–16 |
| Clock drawing test | 8.1 ± 2.66 | 0–10 | 10 ± 0 | 10–10 | 9.63 ± 1.20 | 6–10 | 9.15 ± 1.77 | 5–10 | 6.40 ± 6.34 | 3–10 | 4.18 ± 2.71 | 0–8 |
| Functional assessment | 17.95 ± 6.21 | 4–25 | 25 ± 0 | 25 | 24.75 ± 9.33 | 22–25 | 21.61 ± 3.47 | 15–25 | 16.28 ± 5.94 | 8–25 | 11.20 ± 5.49 | 4–19 |
| Independence scale | 74.76 ± 16.22 | 45–100 | 100 ± 0 | 100 | 90.00 ± 6.35 | 89–100 | 86.15 ± 9.38 | 70–100 | 67.61 ± 13.65 | 45–90 | 60.00 ± 7.90 | 50–70 |
| Variables/HD Stages | All HD | Very Early | Early | Intermediate | Advanced |
|---|---|---|---|---|---|
| ± SD | |||||
| Vertical pursuit | 1.13 ± 1.06 | 0.25 ± 0.45 | 0.56 ± 0.58 | 1.60 ± 1.24 | 2.20 ± 1.00 |
| Horizontal pursuit | 1.14 ± 1.09 | 0.16 ± 0.38 | 0.65 ± 0.57 | 1.60 ± 0.50 | 2.20 ± 1.00 |
| Vertical saccades initiation | 1.70 ± 1.29 | 0.25 ± 0.45 | 1.04 ± 0.47 | 2.36 ± 2.80 | 3.25 ± 0.55 |
| Horizontal saccades initiation | 1.67 ± 1.25 | 0.33 ± 0.65 | 1.04 ± 0.47 | 2.27 ± 1.14 | 3.20 ± 0.61 |
| Speed of horizontal saccades | 1.54 ± 1.18 | 0.25 ± 0.45 | 1.04 ± 0.47 | 2.09 ± 2.39 | 2.90 ± 0.78 |
| Speed of vertical saccades | 1.58 ± 1.2 | 0.25 ± 0.45 | 1.08 ± 0.51 | 2.12 ± 0.25 | 3.00 ± 0.72 |
| Dysarthria | 1.20 ± 1.02 | 0.16 ± 0.38 | 0.82 ± 0.49 | 1.45 ± 0.82 | 2.55 ± 0.82 |
| Tongue protrusion | 1.40 ± 1.36 | 0 ± 0 | 0.52 ± 0.84 | 2.00 ± 0.93 | 3.10 ± 0.55 |
| Bradykinesia (Tapping) of the right upper limb | 1.78 ± 1.42 | 0.25 ± 0.45 | 1.13 ± 0.54 | 2.18 ± 0.82 | 3.85 ± 0.36 |
| Bradykinesia (Tapping) of the left upper limb | 1.91 ± 1.48 | 0.25 ± 0.62 | 1.30 ± 0.82 | 2.45 ± 0.71 | 3.85 ± 0.36 |
| Pronation/supination of the left upper limb | 1.72 ± 1.29 | 0.33 ± 0.49 | 1.26 ± 0.61 | 2.09 ± 0.76 | 3.50 ± 0.68 |
| Pronation/supination of the right upper limb | 1.87 ± 1.33 | 0.33 ± 0.49 | 1.43 ± 1.33 | 2.39 ± 0.78 | 3.55 ± 0.68 |
| Luria’s test | 1.87 ± 1.64 | 0.50 ± 0.90 | 1.60 ± 1.64 | 2.12 ± 0.61 | 3.70 ± 0.57 |
| Left upper limb stiffness | 0.93 ± 1.06 | 0.08 ± 0.28 | 0.60 ± 1.06 | 1.06 ± 0.93 | 2.15 ± 1.08 |
| Right upper limb stiffness | 0.96 ± 1.05 | 0.08 ± 0.28 | 0.60 ± 1.05 | 1.15 ± 0.84 | 2.15 ± 1.08 |
| Bradykinesia | 1.44 ± 1.19 | 0.41 ± 0.66 | 1.21 ± 1.19 | 1.66 ± 0.90 | 2.80 ± 0.83 |
| Torso dystonia | 0.49 ± 0.78 | 0.08 ± 0.28 | 0.26 ± 0.78 | 0.54 ± 0.67 | 1.20 ± 0.95 |
| Right upper limb dystonia | 0.64 ± 1.03 | 0 ± 0 | 0.17 ± 1.03 | 0.63 ± 0.60 | 1.95 ± 1.27 |
| Left upper limb dystonia | 0.69 ± 1.09 | 0 ± 0 | 0.13 ± 1.09 | 0.75 ± 0.92 | 2.05 ± 1.27 |
| Right lower limb dystonia | 0.61 ± 0.99 | 0 ± 0 | 0.17 ± 0.99 | 0.69 ± 0.89 | 1.70 ± 1.21 |
| Left lower limb dystonia | 0.65 ± 1.02 | 0 ± 0 | 0.30 ± 1.02 | 0.72 ± 0.87 | 1.70 ± 1.17 |
| Facial chorea | 1.21 ± 1.09 | 0.08 ± 0.28 | 1.17 ± 1.09 | 1.51 ± 0.95 | 2.15 ± 1.13 |
| Oral-buccal-lingual chorea | 1.29 ± 1.24 | 0 ± 0 | 1.17 ± 1.24 | 1.57 ± 0.71 | 2.50 ± 1.27 |
| Torso chorea | 1.30 ± 1.13 | 0 ± 0 | 1.17 ± 1.13 | 1.81 ± 0.82 | 2.15 ± 1.26 |
| Left upper limb chorea | 1.44 ± 1.53 | 0.16 ± 0.38 | 1.56 ± 1.53 | 2.00 ± 0.93 | 2.00 ± 1.52 |
| Right upper limb chorea | 1.27 ± 1.17 | 0.08 ± 0.28 | 1.04 ± 1.17 | 1.87 ± 0.95 | 2.00 ± 1.52 |
| Left lower limb chorea | 1.38 ± 1.19 | 0 ± 0 | 1.26 ± 1.19 | 1.90 ± 1.06 | 2.30 ± 1.30 |
| Right lower limb chorea | 1.37 ± 1.17 | 0 ± 0 | 1.21 ± 1.17 | 1.87 ± 1.00 | 2.35 ± 1.30 |
| Gait | 1.27 ± 1.04 | 0.08 ± 0.28 | 0.82 ± 1.04 | 1.69 ± 0.96 | 2.55 ± 0.68 |
| Tandem gait | 1.58 ± 1.27 | 0.25 ± 0.45 | 1.04 ± 1.27 | 1.96 ± 0.76 | 3.30 ± 0.57 |
| Retropulsion | 1.11 ± 1.05 | 0 ± 0 | 0.86 ± 1.05 | 1.24 ± 0.86 | 2.50 ± 0.88 |
| Variables/HD Stages | All HD | Preclinical | Very Early | Early | Intermediate | Advanced |
|---|---|---|---|---|---|---|
| ± SD | ||||||
| SDMT Correct answers | 21.85 ± 18.66 | 54.50 ± 10.73 | 43.58 ± 12.88 | 25.80 ± 9.70 | 14.75 ± 8.36 | 1.90 ± 5.56 |
| SDMT Incorrect answers | 0.68 ± 1.39 | 0.37 ± 0.47 | 0.91 ± 1.08 | 0.71 ± 1.76 | 1.03 ± 1.63 | 0.15 ± 0.67 |
| VF Correct answers | 11.55 ± 8.23 | 24.85 ± 2.41 | 19.72 ± 5.23 | 12.50 ± 5.72 | 10.06 ± 6.45 | 3.50 ± 4.85 |
| VF Incorrect answers | 0.03 ± 0.18 | 0 ± 0 | 0 ± 0 | 0.04 ± 0.21 | 0.06 ± 0.25 | 0 ± 0 |
| VF Repetitions | 0.68 ± 1.35 | 0.42 ± 0.78 | 0.45 ± 1.50 | 0.86 ± 1.12 | 0.93 ± 1.81 | 0.35 ± 0.74 |
| TF I Correct words | 6.40 ± 5.68 | 16.12 ± 4.35 | 12.25 ± 5.20 | 6.63 ± 3.53 | 4.63 ± 3.65 | 1.40 ± 2.25 |
| TF II Correct words | 8.31 ± 8.09 | 20.75 ± 4.39 | 13.58 ± 5.10 | 8.68 ± 4.40 | 5.50 ± 3.56 | 14.10 ± 11.66 |
| TF III Correct words | 7.05 ± 6.33 | 18.25 ± 5.77 | 13.66 ± 4.73 | 7.63 ± 4.04 | 5.16 ± 3.53 | 0.80 ± 1.50 |
| TF I–III Average number of correct words for 3 tests | 21.38 ± 18.11 | 55.37 ± 12.33 | 39.5 ± 13.20 | 22.95 ± 10.69 | 15.56 ± 2.14 | 3.90 ± 6.87 |
| TF I–III Average number of repetitions for 3 tests | 0.61 ± 1.08 | 0.50 ± 0.84 | 0.58 ± 0.9 | 1.00 ± 1.15 | 0.70 ± 10.52 | 0.15 ± 0.48 |
| TMT part 1 Filling time | 106.76 ± 78.5 | 27.12 ± 8.37 | 37.16 ± 9.30 | 68.31 ± 25.96 | 122.80 ± 66.12 | 203.47 ± 68.91 |
| TMT part 1 Correct answers | 20.43 ± 9.54 | 25 ± 0 | 25 ± 0 | 25 ± 0 | 23.03 ± 6.360 | 6.26 ± 10.84 |
| TMT part 1 Incorrect answers | 0.31 ± 0.96 | 0 ± 0 | 0 ± 0 | 0.22 ± 0.75 | 0.46 ± 1.04 | 0.52 ± 1.42 |
| TMT part 2 Filling time | 169.86 ± 114.43 | 47.62 ± 10.87 | 19.72 ± 5.23 | 149.90 ± 59.84 | 203.43 ± 56.3 | 252.36 ± 181.81 |
| TMT part 2 Correct answers | 18.89 ± 10.19 | 25 ± 0 | 25 ± 0 | 23.81 ± 4.11 | 20.90 ± 7.94 | 3.57 ± 8.55 |
| TMT part 2 Incorrect answers | 0.90 ± 1.72 | 0.12 ± 0.35 | 0.45 ± 1.50 | 1.36 ± 1.91 | 1.40 ± 2.07 | 0.36 ± 1.38 |
| SCNT Correct answers | 36.53 ± 23.96 | 72.25 ± 13.82 | 63.25 ± 6.81 | 44.72 ± 13.87 | 29.46 ± 14.2 | 7.80 ± 10.94 |
| SCNT Incorrect answers | 0.19 ± 0.59 | 0 ± 0 | 0.08 ± 0.28 | 0.36 ± 0.95 | 0.20 ± 0.48 | 0.15 ± 0.48 |
| SCNT Incorrect answers–corrected | 0.58 ± 1.02 | 0.62 ± 0.91 | 0.58 ± 0.79 | 0.50 ± 0.96 | 0.96 ± 1.320 | 0.10 ± 0.44 |
| SWRT Correct answers | 43.03 ± 29.04 | 89.25 ± 8.24 | 72.66 ± 23.96 | 50.22 ± 10.92 | 36.86 ± 17.60 | 7.80 ± 13.02 |
| SWRT Incorrect answers | 0.05 ± 0.22 | 0 ± 0 | 0 ± 0 | 0.09 ± 0.29 | 0.06 ± 0.25 | 0.05 ± 0.22 |
| SWRT Incorrect answers–corrected | 0.13 ± 0.47 | 0 ± 0 | 0.08 ± 0.28 | 0.22 ± 0.75 | 0.13 ± 0.44 | 0.10 ± 0.30 |
| SIT Correct answers | 20.04 ± 15.5 | 46.25 ± 14.36 | 34.83 ± 8.24 | 23.63 ± 6.98 | 16.03 ± 10.19 | 2.55 ± 5.28 |
| SIT Incorrect answers | 0.52 ± 1.09 | 0 ± 0 | 0.58 ± 0.99 | 0.86 ± 1.24 | 0.72 ± 1.38 | 0.05 ± 0.22 |
| SIT Incorrect answers–corrected | 0.62 ± 1.02 | 0.25 ± 0.46 | 0.75 ± 0.96 | 1.13 ± 1.48 | 0.68 ± 0.89 | 0.05 ± 0.22 |
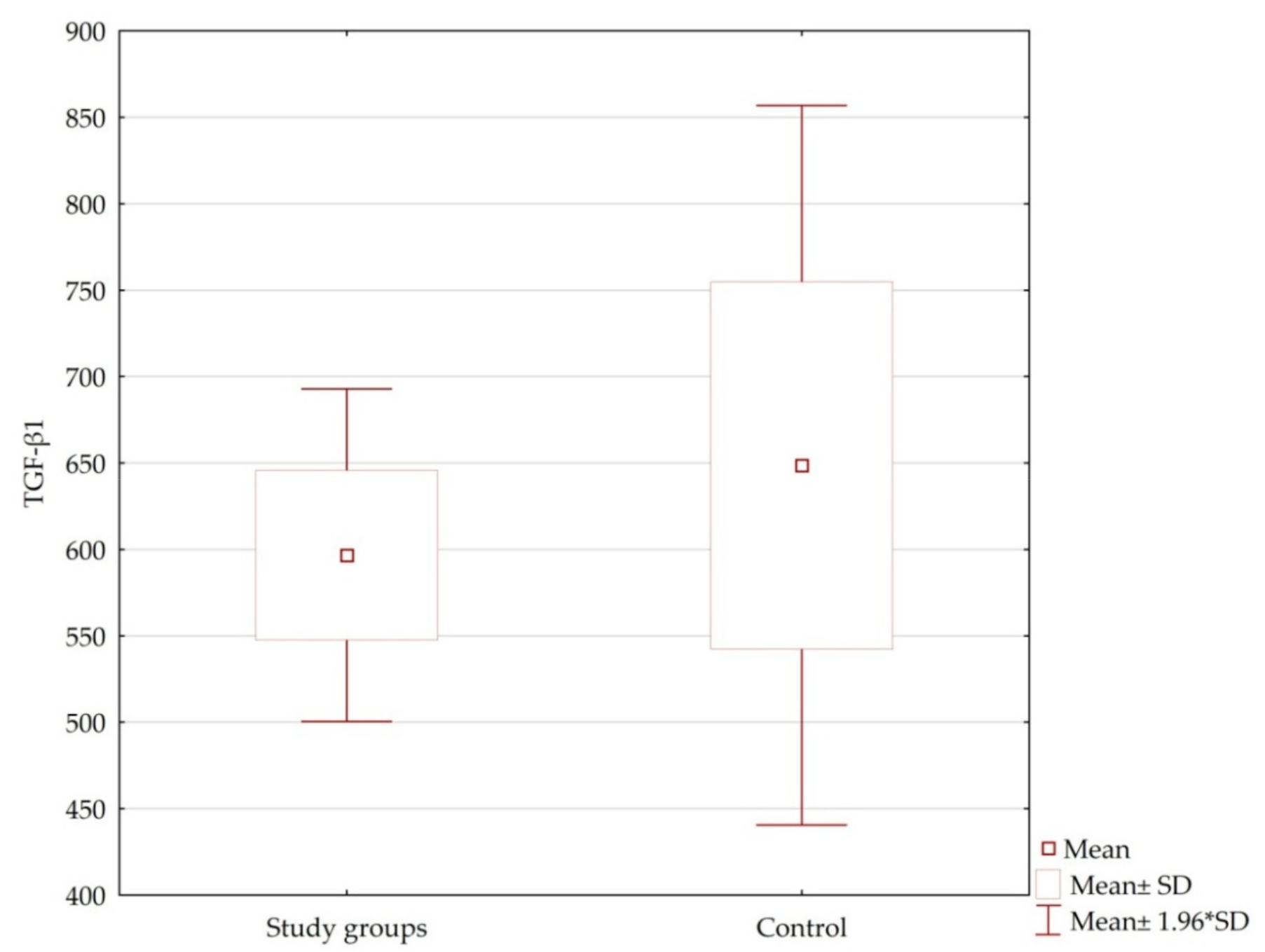
| HD | Number of Measurements | TGF-β1 ± SD | TGF-β1 Average Range |
|---|---|---|---|
| Control group | 39 | 648.69 ± 663.35 | 0–2096.87 |
| All patients | 100 | 611.34 ± 542.79 | 0–2096.87 |
| Preclinical | 12 | 711.31 ± 556.99 | 0–1970.48 |
| Very early | 12 | 602.40 ± 634.34 | 0–2096.87 |
| Early | 23 | 517.93 ± 453.91 | 0–1647.37 |
| Intermediate | 33 | 543.87 ± 482.81 | 0–2096.87 |
| Advanced | 20 | 702.60 ± 423.80 | 94.26–423.80 |
| Correlations/ HD Stages | All HD | Very Early | Early | Intermediate | Advanced | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| R | p | R | p | R | p | R | p | R | p | |
| TGF-β1 and tongue protrusion | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | 0.53 | 0.01 |
| TGF-β1 and right upper limb tapping | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | −0.47 | 0.03 |
| TGF-β1 and left upper limb tapping | n.s. | n.s. | n.s. | n.s. | 0.46 | 0.02 | n.s. | n.s. | −0.47 | 0.03 |
| TGF-β1 and left upper limb pronation/supination | n.s. | n.s. | n.s. | n.s. | 0.47 | 0.02 | n.s. | n.s. | n.s. | n.s. |
| TGF-β1 and right upper limb pronation/supination | n.s. | n.s. | n.s. | n.s. | 0.48 | 0.01 | n.s. | n.s. | n.s. | n.s. |
| TGF-β1 and torso dystonia | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | −0.45 | 0.04 |
| TGF-β1 and right lower limb dystonia | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | 0.36 | 0.03 | n.s. | n.s. |
| TGF-β1 and left lower limb dystonia | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | −0.44 | 0.04 |
| Correlations/ HD Stages | All HD | Preclinical | Very Early | Early | Intermediate | Advanced | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R | p | R | p | R | p | R | p | R | p | R | p | |
| TGF-β1 and the MMSE test | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | 0.52 | 0.03 | n.s. | n.s. | −0.66 | 0.01 |
| TGF-β1 and clock drawing test | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | 0.56 | 0.01 | n.s. | n.s. | n.s. | n.s. |
| TGF-β1 and Beck’s scale | n.s. | n.s. | 0.74 | <0.01 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| TGF-β1 and the independence scale | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | 0.45 | <0.01 | n.s. | n.s. |
| Correlations/ HD Stages | All HD | Preclinical | Very Early | Early | Intermediate | Advanced | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R | p | R | p | R | p | R | p | R | p | R | p | |
| TGF-β1 and VF Repetitions 0–60 s | −0.25 | 0.01 | n.s. | n.s. | n.s. | n.s. | −0.43 | 0.04 | 0.39 | 0.02 | −0.57 | <0.01 |
| TGF-β1 and SWRT Correct answers | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | −0.43 | 0.04 | n.s. | n.s. | n.s. | n.s. |
| TGF-β1 and SWRT Incorrect answers–corrected | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | −0.46 | 0.04 |
| TGF-β1 and TMT part 1 Incorrect selections | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | 0.42 | 0.04 | n.s. | n.s. | n.s. | n.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plinta, K.; Plewka, A.; Wójcik-Pędziwiatr, M.; Zmarzły, N.; Rudziński, M.; Rudzińska-Bar, M. Is TGF-β1 a Biomarker of Huntington’s Disease Progression? J. Clin. Med. 2021, 10, 3001. https://doi.org/10.3390/jcm10133001
Plinta K, Plewka A, Wójcik-Pędziwiatr M, Zmarzły N, Rudziński M, Rudzińska-Bar M. Is TGF-β1 a Biomarker of Huntington’s Disease Progression? Journal of Clinical Medicine. 2021; 10(13):3001. https://doi.org/10.3390/jcm10133001
Chicago/Turabian StylePlinta, Klaudia, Andrzej Plewka, Magdalena Wójcik-Pędziwiatr, Nikola Zmarzły, Marcin Rudziński, and Monika Rudzińska-Bar. 2021. "Is TGF-β1 a Biomarker of Huntington’s Disease Progression?" Journal of Clinical Medicine 10, no. 13: 3001. https://doi.org/10.3390/jcm10133001
APA StylePlinta, K., Plewka, A., Wójcik-Pędziwiatr, M., Zmarzły, N., Rudziński, M., & Rudzińska-Bar, M. (2021). Is TGF-β1 a Biomarker of Huntington’s Disease Progression? Journal of Clinical Medicine, 10(13), 3001. https://doi.org/10.3390/jcm10133001






