Oxidative Stress—A Key Player in the Course of Alcohol-Related Liver Disease
Abstract
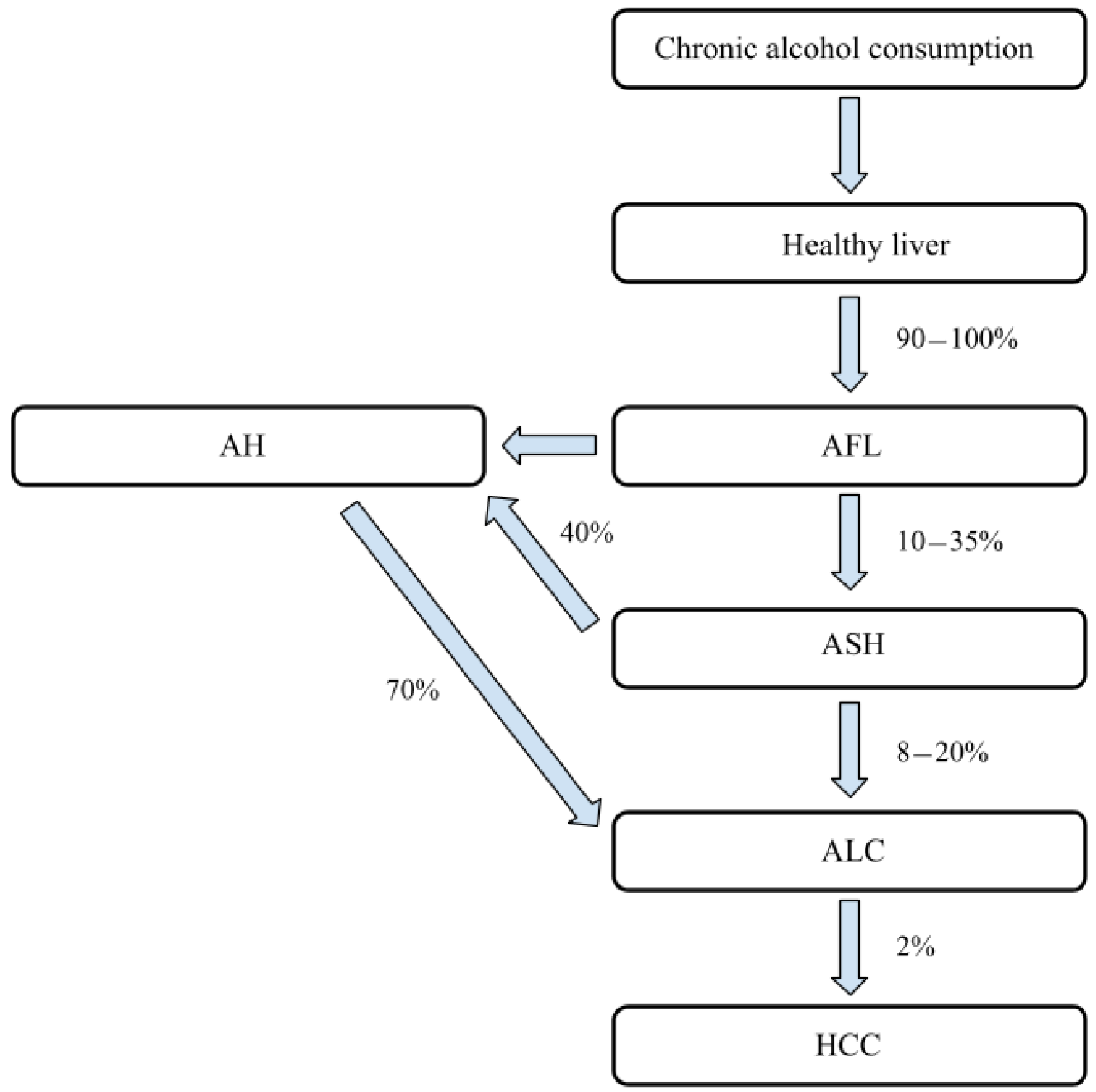
:1. Introduction
2. Alcohol, High Fat Diet and Mitochondria
2.1. Ethanol Metabolism and Oxidative Stress
2.2. Nitrosative Stress
2.3. Lipids, Steatosis and Steatohepatitis in ALD
3. Antioxidants, ALD Exacerbation and Signaling Pathways
3.1. Acute-on-Chronic Liver Failure and Oxidative Stress
3.2. Sirtuin-Related Pathways in ALD Natural History
3.3. Micro-RNA and Oxidative Stress
4. Hepatocyte, PRMT1 and Oxidative Stress
5. Oxidative Stress and Epigenetic Background
6. Redox State in the Liver and Potential Pharmacological Strategies
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Ethics Approval and Consent to Participate
References
- Iracheta-Vellve, A.; Petrasek, J.; Gyongyosi, B.; Satishchandran, A.; Lowe, P.; Kodys, K.; Catalano, D.; Calenda, C.D.; Kurt-Jones, E.A.; Fitzgerald, K.; et al. Endoplasmic Reticulum Stress-induced Hepatocellular Death Pathways Mediate Liver Injury and Fibrosis via Stimulator of Interferon Genes. J. Biol. Chem. 2016, 291, 26794–26805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Association for The Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J. Hepatol. 2018, 69, 406–460. [Google Scholar] [CrossRef] [Green Version]
- Lanthier, N.; Stärkel, P. Treatment of severe alcoholic hepatitis: Past, present and future. Eur. J. Clin. Investig. 2017, 47, 531–539. [Google Scholar] [CrossRef] [Green Version]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Z.; Ye, Y.; Xie, L.; Li, W. Oxidative Stress and Liver Cancer: Etiology and Therapeutic Targets. Oxidative Med. Cell. Longev. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathurin, P.; Bataller, R. Trends in the management and burden of alcoholic liver disease. J. Hepatol. 2015, 62, S38–S46. [Google Scholar] [CrossRef] [Green Version]
- Rawla, P.; Sunkara, T.; Muralidharan, P.; Raj, J.P. Update in global trends and aetiology of hepatocellular carcinoma. Współczesna Onkologia 2018, 22, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Shen, N.T.; Salajegheh, A.; Brown, R.S., Jr. A Call to Standardize Definitions, Data Collection, and Outcome Assessment to Improve Care in Alcohol-Related Liver Disease. J. Hepatol. 2019, 70, 1038–1044. [Google Scholar] [CrossRef]
- Fuster, D.; Samet, J.H. Alcohol Use in Patients with Chronic Liver Disease. N. Engl. J. Med. 2018, 379, 1251–1261. [Google Scholar] [CrossRef]
- Del Campo, J.A.; Gallego, P.; Grande, L. Role of inflammatory response in liver diseases: Therapeutic strategies. World J. Hepatol. 2018, 10, 1–7. [Google Scholar] [CrossRef]
- Galicia-Moreno, M.; Rosique-Oramas, D.; Medina-Avila, Z.; Álvarez-Torres, T.; Falcón, D.; La Tijera, F.H.-D.; Béjar, Y.L.; Cordero-Pérez, P.; Muñoz-Espinosa, L.; Pérez-Hernández, J.L.; et al. Behavior of Oxidative Stress Markers in Alcoholic Liver Cirrhosis Patients. Oxidative Med. Cell. Longev. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Szabo, G.; Kamath, P.S.; Shah, V.H.; Thursz, M.; Mathurin, P.; Addolorato, G.; Bataller, R.; Burra, P.; Castera, L.; Pinto, H.C.; et al. Alcohol-Related Liver Disease: Areas of Consensus, Unmet Needs and Opportunities for Further Study. J. Hepatol. 2019, 69, 2271–2283. [Google Scholar] [CrossRef]
- Seo, W.; Gao, Y.; He, Y.; Sun, J.; Xu, H.; Feng, D.; Park, S.H.; Cho, Y.-E.; Guillot, A.; Ren, T.; et al. ALDH2 deficiency promotes alcohol-associated liver cancer by activating oncogenic pathways via oxidized DNA-enriched extracellular vesicles. J. Hepatol. 2019, 71, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Edenberg, H.J.; McClintick, J.N. Alcohol Dehydrogenases, Aldehyde Dehydrogenases, and Alcohol Use Disorders: A Critical Review. Alcohol. Clin. Exp. Res. 2018, 42, 2281–2297. [Google Scholar] [CrossRef]
- Shearn, C.T.; Fritz, K.; Shearn, A.H.; Saba, L.M.; Mercer, K.E.; Engi, B.; Galligan, J.J.; Zimniak, P.; Orlicky, D.J.; Ronis, M.J.; et al. Deletion of GSTA4-4 results in increased mitochondrial post-translational modification of proteins by reactive aldehydes following chronic ethanol consumption in mice. Redox Biol. 2016, 7, 68–77. [Google Scholar] [CrossRef] [Green Version]
- Galligan, J.J.; Fritz, K.; Backos, D.; Shearn, C.T.; Smathers, R.L.; Jiang, H.; MacLean, K.N.; Reigan, P.R.; Petersen, D.R. Oxidative stress-mediated aldehyde adduction of GRP78 in a mouse model of alcoholic liver disease: Functional independence of ATPase activity and chaperone function. Free Radic. Biol. Med. 2014, 73, 411–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neuman, M.G.; Malnick, S.; Maor, Y.; Nanau, R.M.; Melzer, E.; Ferenci, P.; Seitz, H.K.; Mueller, S.; Mell, H.; Samuel, D.; et al. Alcoholic liver disease: Clinical and translational research. Exp. Mol. Pathol. 2015, 99, 596–610. [Google Scholar] [CrossRef]
- Singal, A.K.; Bataller, R.; Ahn, J.; Kamath, P.S.; Shah, V.H. ACG Clinical Guideline: Alcoholic Liver Disease. Am. J. Gastroenterol. 2018, 113, 175–194. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [Green Version]
- Seitz, H.K.; Bataller, R.; Cortez-Pinto, H.; Gao, B.; Gual, A.; Lackner, C.; Mathurin, P.; Mueller, S.; Szabo, G.; Tsukamoto, H. Alcoholic liver disease. Nat. Rev. Dis. Prim. 2018, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Weiskirchen, R.; Weiskirchen, S.; Tacke, F. Recent advances in understanding liver fibrosis: Bridging basic science and individualized treatment concepts. F1000Research 2018, 7, 921. [Google Scholar] [CrossRef] [Green Version]
- Unsal, V.; Belge-Kurutaş, E. Experimental Hepatic Carcinogenesis: Oxidative Stress and Natural Antioxidants. Open Access Maced. J. Med. Sci. 2017, 5, 686–691. [Google Scholar] [CrossRef] [Green Version]
- Sugimoto, K.; Takei, Y. Pathogenesis of alcoholic liver disease. Hepatol. Res. 2016, 47, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Menk, M.; Graw, J.A.; Poyraz, D.; Möbius, N.; Spies, C.; Von Haefen, C. Chronic Alcohol Consumption Inhibits Autophagy and Promotes Apoptosis in the Liver. Int. J. Med. Sci. 2018, 15, 682–688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Z.Y.; Xiao, Z.-H.; Wang, F.-F. Inhibition of autophagy reverses alcohol-induced hepatic stellate cells activation through activation of Nrf2-Keap1-ARE signaling pathway. Biochimie 2018, 147, 55–62. [Google Scholar] [CrossRef]
- Lu, Y.; Cederbaum, A.I. Autophagy Protects against CYP2E1/Chronic Ethanol-Induced Hepatotoxicity. Biomolecules 2015, 5, 2659–2674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.W.; Zhang, H.; Li, M.; Xiong, X.; Chen, X.; Chen, X.; Dong, X.C.; Yin, X.M. Pharmacological promotion of autophagy alleviates steatosis and injury in alcoholic and non-alcoholic fatty liver conditions in mice. J. Hepatol. 2013, 58, 993–999. [Google Scholar] [CrossRef] [Green Version]
- Han, W.; Fu, X.; Xie, J.; Meng, Z.; Gu, Y.; Wang, X.; Li, L.; Pan, H.; Huang, W. miR-26a enhances autophagy to protect against ethanol-induced acute liver injury. J. Mol. Med. 2015, 93, 1045–1055. [Google Scholar] [CrossRef] [Green Version]
- Mahli, A.; Thasler, W.E.; Patsenker, E.; Müller, S.; Stickel, F.; Müller, M.; Seitz, H.K.; Cederbaum, A.I.; Hellerbrand, C. Identification of cytochrome CYP2E1 as critical mediator of synergistic effects of alcohol and cellular lipid accumulation in hepatocytes in vitro. Oncotarget 2015, 6, 41464–41478. [Google Scholar] [CrossRef] [Green Version]
- Gao, B.; Xu, M.-J.; Bertola, A.; Wang, H.; Zhou, Z.; Liangpunsakul, S. Animal Models of Alcoholic Liver Disease: Pathogenesis and Clinical Relevance. Gene Expr. 2017, 17, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Dastidar, S.G.; Warner, J.B.; Warner, D.R.; McClain, C.J.; Kirpich, I.A. Rodent Models of Alcoholic Liver Disease: Role of Binge Ethanol Administration. Biomolecules 2018, 8, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunn, W.; Shah, V.H. Pathogenesis of Alcoholic Liver Disease. Clin. Liver Dis. 2016, 20, 445–456. [Google Scholar] [CrossRef] [Green Version]
- Kema, V.H.; Mojerla, N.R.; Khan, I.; Mandal, P. Effect of alcohol on adipose tissue: A review on ethanol mediated adipose tissue injury. Adipocyte 2015, 4, 225–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, C. New Insights into the Pathogenesis of Alcohol-Induced ER Stress and Liver Diseases. Int. J. Hepatol. 2014, 2014, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ceni, E.; Mello, T.; Galli, A. Pathogenesis of alcoholic liver disease: Role of oxidative metabolism. World J. Gastroenterol. 2014, 20, 17756–17772. [Google Scholar] [CrossRef] [PubMed]
- Abdelmegeed, M.A.; Ha, S.-K.; Choi, Y.; Akbar, M.; Song, B.-J. Role of CYP2E1 in Mitochondrial Dysfunction and Hepatic Injury by Alcohol and Non-Alcoholic Substances. Curr. Mol. Pharmacol. 2017, 10, 207–225. [Google Scholar] [CrossRef] [Green Version]
- Zeng, T.; Zhang, C.-L.; Zhao, N.; Guan, M.-J.; Xiao, M.; Yang, R.; Zhao, X.-L.; Yu, L.-H.; Zhu, Z.-P.; Xie, K.-Q. Impairment of Akt activity by CYP2E1 mediated oxidative stress is involved in chronic ethanol-induced fatty liver. Redox Biol. 2018, 14, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P.; Avadhani, N.G. Roles of Cytochrome P450 in Metabolism of Ethanol and Carcinogens. Adv. Exp. Med. Biol. 2018, 1032, 15–35. [Google Scholar] [CrossRef]
- Yin, C.; Evason, K.J.; Asahina, K.; Stainier, D.Y. Hepatic stellate cells in liver development, regeneration, and cancer. J. Clin. Investig. 2013, 123, 1902–1910. [Google Scholar] [CrossRef] [Green Version]
- Lan, T.; Kisseleva, T.; Brenner, D.A. Deficiency of NOX1 or NOX4 Prevents Liver Inflammation and Fibrosis in Mice through Inhibition of Hepatic Stellate Cell Activation. PLoS ONE 2015, 10, e0129743. [Google Scholar] [CrossRef]
- Stickel, F.; Datz, C.; Hampe, J.; Bataller, R. Pathophysiology and Management of Alcoholic Liver Disease: Update 2016. Gut Liver 2017, 11, 173–188. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.P.; Sies, H. The Redox Code. Antioxid. Redox Signal. 2015, 23, 734–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enriquez-Cortina, C.; Bello-Monroy, O.; Rosales-Cruz, P.; Souza, V.; Miranda, R.U.; Toledo-Pérez, R.; Luna-López, A.; Simoni-Nieves, A.; Hernández-Pando, R.; Gutiérrez-Ruíz, M.C.; et al. Cholesterol overload in the liver aggravates oxidative stress-mediated DNA damage and accelerates hepatocarcinogenesis. Oncotarget 2017, 8, 104136–104148. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Zhong, W.; Zhang, W.; Zhou, Z. Defect of mitochondrial respiratory chain is a mechanism of ROS overproduction in a rat model of alcoholic liver disease: Role of zinc deficiency. Am. J. Physiol. Liver Physiol. 2016, 310, G205–G214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, B.-J.; Abdelmegeed, M.A.; Henderson, L.E.; Yoo, S.-H.; Wan, J.; Purohit, V.; Hardwick, J.P.; Moon, K.-H. Increased Nitroxidative Stress Promotes Mitochondrial Dysfunction in Alcoholic and Nonalcoholic Fatty Liver Disease. Oxidative Med. Cell. Longev. 2013, 2013, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramadori, P.; Cubero, F.J.; Liedtke, C.; Trautwein, C.; Nevzorova, Y.A. Alcohol and Hepatocellular Carcinoma: Adding Fuel to the Flame. Cancers 2017, 9, 130. [Google Scholar] [CrossRef] [Green Version]
- Sacco, R.; Eggenhoffner, R.; Giacomelli, L. Glutathione in the treatment of liver diseases: Insights from clinical practice. Minerva Gastroenterol. e Dietol. 2016, 62, 316–324. [Google Scholar]
- Wang, Y.; Yu, D.; Tolleson, W.H.; Yu, L.-R.; Green, B.; Zeng, L.; Chen, Y.; Chen, S.; Ren, Z.; Guo, L.; et al. A systematic evaluation of microRNAs in regulating human hepatic CYP2E1. Biochem. Pharmacol. 2017, 138, 174–184. [Google Scholar] [CrossRef] [Green Version]
- Hu, Q.; Wei, J.; Liu, Y.; Fei, X.; Hao, Y.; Pei, D.; Di, D. Discovery and identification of potential biomarkers for alcohol-induced oxidative stress based on cellular metabolomics. Biomed. Chromatogr. 2017, 31, e3907. [Google Scholar] [CrossRef]
- Deng, S.-Y.; Zhang, L.-M.; Ai, Y.-H.; Pan, P.-H.; Zhao, S.-P.; Su, X.-L.; Wu, N.-D.; Tan, H.-Y.; Zhang, L.-N.; Tsung, A. Role of interferon regulatory factor-1 in lipopolysaccharide-induced mitochondrial damage and oxidative stress responses in macrophages. Int. J. Mol. Med. 2017, 40, 1261–1269. [Google Scholar] [CrossRef] [Green Version]
- Ren, T.; Mackowiak, B.; Lin, Y.; Gao, Y.; Niu, J.; Gao, B. Hepatic injury and inflammation alter ethanol metabolism and drinking behavior. Food Chem. Toxicol. 2020, 136, 111070. [Google Scholar] [CrossRef] [PubMed]
- Bertola, A.; Mathews, S.; Ki, S.H.; Wang, H.; Gao, B. Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat. Protoc. 2013, 8, 627–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cederbaum, A.I. Alcohol Metabolism. Clin. Liver Dis. 2012, 16, 667–685. [Google Scholar] [CrossRef] [Green Version]
- Seki, E.; Schwabe, R.F. Hepatic inflammation and fibrosis: Functional links and key pathways. Hepatology 2015, 61, 1066–1079. [Google Scholar] [CrossRef] [PubMed]
- Duarte, S.; Baber, J.; Fujii, T.; Coito, A.J. Matrix metalloproteinases in liver injury, repair and fibrosis. Matrix Biol. 2015, 44–46, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tan, H.-Y.; Wang, N.; Zhang, Z.-J.; Lao, L.; Wong, C.-W.; Feng, Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Y.; Zhang, L.; Forsyth, C.B.; Shaikh, M.; Song, S.; Keshavarzian, A. The Role of miR-212 and iNOS in Alcohol-Induced Intestinal Barrier Dysfunction and Steatohepatitis. Alcohol. Clin. Exp. Res. 2015, 39, 1632–1641. [Google Scholar] [CrossRef] [PubMed]
- Rusyn, I.; Bataller, R. Alcohol and toxicity. J. Hepatol. 2013, 59, 387–388. [Google Scholar] [CrossRef] [Green Version]
- Ezhilarasan, D. Oxidative stress is bane in chronic liver diseases: Clinical and experimental perspective. Arab. J. Gastroenterol. 2018, 19, 56–64. [Google Scholar] [CrossRef]
- Ding, W.X.; Yin, X.M. Cellular Injury in Liver Diseases; Springer: Berlin, Germany, 2017. [Google Scholar]
- Go, K.L.; Lee, S.; Zendejas, I.; Behrns, K.E.; Kim, J.-S. Mitochondrial Dysfunction and Autophagy in Hepatic Ischemia/Reperfusion Injury. BioMed Res. Int. 2015, 2015, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Hann, H.-W.; Hann, R.S.; Wan, S.; Myers, R.E.; Ye, Z.; Xing, J.; Yang, H. Circulating Mitochondrial DNA Content Associated with the Risk of Liver Cirrhosis: A Nested Case–Control Study. Dig. Dis. Sci. 2015, 60, 1707–1715. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Wood, C.R.; Cimen, S.; Venkatachalam, A.B.; Alwayn, I.P.J. Mitochondrial Damage-Associated Molecular Patterns (MTDs) Are Released during Hepatic Ischemia Reperfusion and Induce Inflammatory Responses. PLoS ONE 2015, 10, e0140105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, X.; Hu, Q.; Wu, J.; Wang, G.; Hong, Z.; Ren, J. Mitochondrial DNA in liver inflammation and oxidative stress. Life Sci. 2019, 236, 116464. [Google Scholar] [CrossRef]
- Mansouri, A.; Gattolliat, C.-H.; Asselah, T. Mitochondrial Dysfunction and Signaling in Chronic Liver Diseases. Gastroenterology 2018, 155, 629–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assiri, M.A.; Roy, S.R.; Harris, P.S.; Ali, H.; Liang, Y.; Shearn, C.T.; Orlicky, D.J.; Roede, J.R.; Hirschey, M.D.; Backos, D.S.; et al. Chronic Ethanol Metabolism Inhibits Hepatic Mitochondrial Superoxide Dismutase via Lysine Acetylation. Alcohol. Clin. Exp. Res. 2017, 41, 1705–1714. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Xu, M.-J.; Koritzinsky, E.H.; Zhou, Z.; Wang, W.; Cao, H.; Yuen, P.S.; Ross, R.A.; Star, R.A.; Liangpunsakul, S.; et al. Mitochondrial DNA–enriched microparticles promote acute-on-chronic alcoholic neutrophilia and hepatotoxicity. JCI Insight 2017, 2. [Google Scholar] [CrossRef] [PubMed]
- Louvet, A.; Mathurin, P. Alcoholic liver disease: Mechanisms of injury and targeted treatment. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 231–242. [Google Scholar] [CrossRef]
- Dennis, E.A.; Norris, P.C. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 2015, 15, 511–523. [Google Scholar] [CrossRef] [Green Version]
- Guo, B.; Li, Z. Endoplasmic reticulum stress in hepatic steatosis and inflammatory bowel diseases. Front. Genet. 2014, 5. [Google Scholar] [CrossRef] [Green Version]
- Osna, N.A.; Carter, W.G.; Ganesan, M.; Kirpich, I.A.; McClain, C.J.; Petersen, D.R.; Shearn, C.T.; Tomasi, M.L.; Kharbanda, K.K. Aberrant post-translational protein modifications in the pathogenesis of alcohol-induced liver injury. World J. Gastroenterol. 2016, 22, 6192–6200. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.-B.; Tian, K.; Cao, Y.-W.; Bao, J.-L.; Wang, M.; He, C.; Huanxing, S.; Su, H.; Wan, J.-B. Protective Effect ofPanax notoginsengSaponins on Acute Ethanol-Induced Liver Injury Is Associated with Ameliorating Hepatic Lipid Accumulation and Reducing Ethanol-Mediated Oxidative Stress. J. Agric. Food Chem. 2015, 63, 2413–2422. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Lee, D.E.; Kang, J.H.; Nam, M.J.; Park, J.-W.; Kang, B.S.; Lee, N.-S.; Lee, H.-S.; Kwon, O.-S. New Potential Biomarker Proteins for Alcoholic Liver Disease Identified by a Comparative Proteomics Approach. J. Cell. Biochem. 2017, 118, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Ansari, R.A.; Husain, K.; Rizvi, S.A.A. Role of Transcription Factors in Steatohepatitis and Hypertension after Ethanol: The Epicenter of Metabolism. Biomolecules 2016, 6, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Yang, C.; Thomes, P.G.; Kharbanda, K.K.; Casey, C.A.; McNiven, M.A.; Donohue, T.M.J. Lipophagy and Alcohol-Induced Fatty Liver. Front. Pharmacol. 2019, 10, 495. [Google Scholar] [CrossRef] [Green Version]
- Li, H.-D.; Chen, X.; Yang, Y.; Huang, H.-M.; Zhang, L.; Zhang, X.; Zhang, L.; Huang, C.; Meng, X.-M.; Li, J. Wogonin attenuates inflammation by activating PPAR-γ in alcoholic liver disease. Int. Immunopharmacol. 2017, 50, 95–106. [Google Scholar] [CrossRef]
- Wang, F.; Liu, J.-C.; Zhou, R.-J.; Zhao, X.; Liu, M.; Ye, H.; Xie, M.-L. Apigenin protects against alcohol-induced liver injury in mice by regulating hepatic CYP2E1-mediated oxidative stress and PPARα-mediated lipogenic gene expression. Chem. Biol. Interact. 2017, 275, 171–177. [Google Scholar] [CrossRef]
- Galicia-Moreno, M.; Gutiérrez-Reyes, G. The role of oxidative stress in the development of alcoholic liver disease. Rev. Gastroenterol. México 2014, 79, 135–144. [Google Scholar] [CrossRef] [Green Version]
- Shukla, S.D.; Lim, R.W. Epigenetic Effects of Ethanol on the Liver and Gastrointestinal System. Alcohol Res. Curr. Rev. 2013, 35, 47–55. [Google Scholar]
- Lívero, F.A.; Acco, A. Molecular basis of alcoholic fatty liver disease: From incidence to treatment. Hepatol. Res. 2015, 46, 111–123. [Google Scholar] [CrossRef]
- Xu, J.; Ma, H.-Y.; Liang, S.; Sun, M.; Karin, G.; Koyama, Y.; Hu, R.; Quehenberger, O.; Davidson, N.O.; Dennis, E.A.; et al. The role of human cytochrome P450 2E1 in liver inflammation and fibrosis. Hepatol. Commun. 2017, 1, 1043–1057. [Google Scholar] [CrossRef]
- Luedde, T.; Kaplowitz, N.; Schwabe, R.F. Cell death and cell death responses in liver disease: Mechanisms and clinical relevance. Gastroenterology 2014, 147, 765–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Nassir, F. Role of mitochondria in alcoholic liver disease. World J. Gastroenterol. 2014, 20, 2136–2142. [Google Scholar] [CrossRef] [PubMed]
- Andringa, K.K.; Udoh, U.S.; Landar, A.; Bailey, S.M. Proteomic analysis of 4-hydroxynonenal (4-HNE) modified proteins in liver mitochondria from chronic ethanol-fed rats. Redox Biol. 2014, 2, 1038–1047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, I.H.; Kisseleva, T.; Brenner, D.A. Aging and liver disease. Curr. Opin. Gastroenterol. 2015, 31, 184–191. [Google Scholar] [CrossRef] [Green Version]
- Ramos-Tovar, E.; Muriel, P. Molecular mechanisms that link oxidative stress, inflammation, and fibrosis in the liver. Antioxidants 2020, 9, 1279. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014, 39, 199–218. [Google Scholar] [CrossRef] [PubMed]
- Kansanen, E.; Linna-Kuosmanen, S.; Leinonen, H.; Levonen, A.-L. The Keap1-Nrf2 pathway: Mechanisms of activation and dysregulation in cancer. Redox Biol. 2013, 1, 45–49. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Fu, J.; Zhong, Y.; Li, L.; Chen, C.; Wang, X.; Wang, L.; Hou, Y.; Wang, H.; Zhao, R.; et al. NRF2 mitigates acute alcohol-induced hepatic and pancreatic injury in mice. Food Chem. Toxicol. 2018, 121, 495–503. [Google Scholar] [CrossRef]
- Rodriguez, W.E.; Wahlang, B.; Wang, Y.; Zhang, J.; Vadhanam, M.V.; Joshi-Barve, S.; Bauer, P.; Cannon, R.; Ahmadi, A.R.; Sun, Z.; et al. Phosphodiesterase 4 Inhibition as a Therapeutic Target for Alcoholic Liver Disease: From Bedside to Bench. Hepatology 2019, 70, 1958–1971. [Google Scholar] [CrossRef]
- Wahlang, B.; McClain, C.; Barve, S.; Gobejishvili, L. Role of cAMP and phosphodiesterase signaling in liver health and disease. Cell. Signal. 2018, 49, 105–115. [Google Scholar] [CrossRef]
- Fertig, B.A.; Baillie, G.S. PDE4-Mediated cAMP Signalling. J. Cardiovasc. Dev. Dis. 2018, 5, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klussmann, E. Protein–protein interactions of PDE4 family members—Functions, interactions and therapeutic value. Cell. Signal. 2016, 28, 713–718. [Google Scholar] [CrossRef] [Green Version]
- Shearn, C.T.; Backos, D.; Orlicky, D.J.; Smathers-McCullough, R.L.; Petersen, D.R. Identification of 5′ AMP-activated Kinase as a Target of Reactive Aldehydes during Chronic Ingestion of High Concentrations of Ethanol. J. Biol. Chem. 2014, 289, 15449–15462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, H.; Assiri, M.A.; Shearn, C.T.; Fritz, K.S. Lipid peroxidation derived reactive aldehydes in alcoholic liver disease. Curr. Opin. Toxicol. 2019, 13, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Singh, S.; Matsumoto, A.; Manna, S.K.; Abdelmegeed, M.A.; Golla, S.; Murphy, R.C.; Dong, H.; Song, B.-J.; Gonzalez, F.J.; et al. Chronic Glutathione Depletion Confers Protection against Alcohol-induced Steatosis: Implication for Redox Activation of AMP-activated Protein Kinase Pathway. Sci. Rep. 2016, 6, 29743. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Chu, S.-F.; Xia, C.-Y.; Zhang, Z.; Zhang, S.; Chen, N.-H. Rg1 Attenuates alcoholic hepatic damage through regulating AMP-activated protein kinase and nuclear factor erythroid 2-related factor 2 signal pathways. J. Asian Nat. Prod. Res. 2016, 18, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, C.; Zhang, S.; Liu, S.; Zhao, L.; Luole, Z.; Chen, Y.; Huang, W. Ginsenoside Rg1 inhibits inflammatory responses via modulation of the nuclear factor-κB pathway and inhibition of inflammasome activation in alcoholic hepatitis. Int. J. Mol. Med. 2017, 41, 899–907. [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, Q.; Li, X.; Jiang, M.; Cui, B.-W.; Xia, K.-L.; Wu, Y.-L.; Lian, L.-H.; Nan, J.-X. Amelioration of Alcoholic Liver Steatosis by Dihydroquercetin through the Modulation of AMPK-Dependent Lipogenesis Mediated by P2X7R–NLRP3-Inflammasome Activation. J. Agric. Food Chem. 2018, 66, 4862–4871. [Google Scholar] [CrossRef]
- Arroyo, V.; Moreau, R.; Jalan, R.; Ginès, P. Acute-on-chronic liver failure: A new syndrome that will re-classify cirrhosis. J. Hepatol. 2015, 62, S131–S143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P.; Stärkel, P.; Turner, J.R.; Ho, S.B.; Schnabl, B. Dysbiosis-induced intestinal inflammation activates tumor necrosis factor receptor I and mediates alcoholic liver disease in mice. Hepatology 2015, 61, 883–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, V.; Karvellas, C.J. Acute-on-chronic liver failure: Objective admission and support criteria in the intensive care unit. JHEP Rep. 2019, 1, 44–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.-S.; Ong, M.; Qu, X. Optimal management for alcoholic liver disease: Conventional medications, natural therapy or combination? World J. Gastroenterol. 2016, 22, 8–23. [Google Scholar] [CrossRef]
- Szabo, G.; Petrasek, J. Gut–liver axis and sterile signals in the development of alcoholic liver disease. Alcohol Alcohol. 2017, 52, 414–424. [Google Scholar] [CrossRef]
- Jalan, R.; Perricone, G.; Moreau, R.; Arroyo, V.; Williams, R. Acute-on-Chronic Liver Failure: A New Disease or an Old One Hiding in Plain Sight? Clin. Liver Dis. 2020, 15, S45–S51. [Google Scholar] [CrossRef] [PubMed]
- Qin, K.; Zhang, N.; Zhang, Z.; Nipper, M.; Zhu, Z.; Leighton, J.; Xu, K.; Musi, N.; Wang, P. SIRT6-mediated transcriptional suppression of Txnip is critical for pancreatic beta cell function and survival in mice. Diabetologia 2018, 61, 906–918. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Li, H.; Guo, Q.; Zhang, L.; Zhu, J.; Ji, J. Sirtuin6 inhibits c-triggered inflammation through TLR4 abrogation regulated by ROS and TRPV1/CGRP. J. Cell. Biochem. 2018, 119, 9141–9153. [Google Scholar] [CrossRef]
- Ka, S.; Bang, I.H.; Bae, E.J.; Park, B. Hepatocyte-specific sirtuin 6 deletion predisposes to nonalcoholic steatohepatitis by up-regulation of Bach1, an Nrf2 repressor. FASEB J. 2017, 31, 3999–4010. [Google Scholar] [CrossRef]
- Cui, X.; Yao, L.; Yang, X.; Gao, Y.; Fang, F.; Zhang, J.; Wang, Q.; Chang, Y. SIRT6 regulates metabolic homeostasis in skeletal muscle through activation of AMPK. Am. J. Physiol. Metab. 2017, 313, E493–E505. [Google Scholar] [CrossRef]
- Zhang, P.; Tu, B.; Wang, H.; Cao, Z.; Tang, M.; Zhang, C.; Gu, B.; Li, Z.; Wang, L.; Yang, Y.; et al. Tumor suppressor p53 cooperates with SIRT6 to regulate gluconeogenesis by promoting FoxO1 nuclear exclusion. Proc. Natl. Acad. Sci. USA 2014, 111, 10684–10689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, X.; Wang, G.; Tao, R.; Wu, P.; Kono, T.; Li, K.; Ding, W.-X.; Tong, X.; Tersey, S.A.; Harris, R.A.; et al. Sirtuin 6 regulates glucose-stimulated insulin secretion in mouse pancreatic beta cells. Diabetologia 2015, 59, 151–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasai, S.; Shimizu, S.; Tatara, Y.; Mimura, J.; Itoh, K. Regulation of Nrf2 by Mitochondrial Reactive Oxygen Species in Physiology and Pathology. Biomolecules 2020, 10, 320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Wei, R.; Zhang, L.; Tan, Y.; Qian, C. Sirtuin 6 protects the brain from cerebral ischemia/reperfusion injury through NRF2 activation. Neuroscience 2017, 366, 95–104. [Google Scholar] [CrossRef]
- Xiong, X.; Sun, X.; Wang, Q.; Qian, X.; Zhang, Y.; Pan, X.; Dong, X.C. SIRT6 protects against palmitate-induced pancreatic β-cell dysfunction and apoptosis. J. Endocrinol. 2016, 231, 159–165. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.G.; Huang, M.; Xin, Y.; Zhang, Y.; Zhang, X.; Wang, G.; Liu, S.; Wan, J.; Ahmadi, A.R.; Sun, Z.; et al. The epigenetic regulator SIRT6 protects the liver from alcohol-induced tissue injury by reducing oxidative stress in mice. J. Hepatol. 2019, 71, 960–969. [Google Scholar] [CrossRef]
- Elhanati, S.; Ben-Hamo, R.; Kanfi, Y.; Varvak, A.; Glazz, R.; Lerrer, B.; Efroni, S.; Cohen, H.Y. Reciprocal Regulation between SIRT6 and miR-122 Controls Liver Metabolism and Predicts Hepatocarcinoma Prognosis. Cell Rep. 2016, 14, 234–242. [Google Scholar] [CrossRef] [Green Version]
- Han, J.Y.; Lee, S.; Yang, J.H.; Kim, S.; Sim, J.; Kim, M.G.; Jeong, T.C.; Ku, S.K.; Cho, I.J.; Ki, S.H. Korean Red Ginseng attenuates ethanol-induced steatosis and oxidative stress via AMPK/Sirt1 activation. J. Ginseng. Res. 2015, 39, 105–115. [Google Scholar] [CrossRef] [Green Version]
- Yin, H.; Hu, M.; Liang, X.; Ajmo, J.M.; Li, X.; Bataller, R.; Odena, G.; Stevens, S.M., Jr.; You, M. Deletion of SIRT1 From Hepatocytes in Mice Disrupts Lipin-1 Signaling and Aggravates Alcoholic Fatty Liver. Gastroenterology 2014, 146, 801–811. [Google Scholar] [CrossRef] [Green Version]
- Yin, H.; Liang, X.; Jogasuria, A.; Davidson, N.O.; You, M. miR-217 Regulates Ethanol-Induced Hepatic Inflammation by Disrupting Sirtuin 1–Lipin-1 Signaling. Am. J. Pathol. 2015, 185, 1286–1296. [Google Scholar] [CrossRef] [Green Version]
- Qu, L.; Zhu, Y.; Liu, Y.; Yang, H.; Zhu, C.; Ma, P.; Deng, J.; Fan, D. Protective effects of ginsenoside Rk3 against chronic alcohol-induced liver injury in mice through inhibition of inflammation, oxidative stress, and apoptosis. Food Chem. Toxicol. 2019, 126, 277–284. [Google Scholar] [CrossRef]
- McKillop, I.H.; Schrum, L.W.; Thompson, K.J. Role of alcohol in the development and progression of hepatocellular carcinoma. Hepatic Oncol. 2016, 3, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.P.; Bhopale, K.K.; Amer, S.M.; Wan, J.; Kaphalia, L.; Ansari, G.S.; Kaphalia, B.S. Linking Dysregulated AMPK Signaling and ER Stress in Ethanol-Induced Liver Injury in Hepatic Alcohol Dehydrogenase Deficient Deer Mice. Biomolecules 2019, 9, 560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramirez, T.; Li, Y.-M.; Yin, S.; Xu, M.-J.; Feng, D.; Zhou, Z.; Zang, M.; Mukhopadhyay, P.; Varga, Z.; Pacher, P.; et al. Aging aggravates alcoholic liver injury and fibrosis in mice by downregulating sirtuin 1 expression. J. Hepatol. 2017, 66, 601–609. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Koh, H.; Joo, D.J.; Nedumaran, B.; Jeon, H.; Park, C.; Harris, R.A.; Kim, Y.D. Induction of SIRT1 by melatonin improves alcohol-mediated oxidative liver injury by disrupting the CRBN-YY1-CYP2E1 signaling pathway. J. Pineal Res. 2020, 68, e12638. [Google Scholar] [CrossRef]
- Tu, Y.; Zhu, S.; Wang, J.; Burstein, E.; Jia, D. Natural compounds in the chemoprevention of alcoholic liver disease. Phytother. Res. 2019, 33, 2192–2212. [Google Scholar] [CrossRef] [PubMed]
- Dippold, R.P.; Vadigepalli, R.; Gonye, G.E.; Patra, B.; Hoek, J.B. Chronic ethanol feeding alters miRNA expression dynamics during liver regeneration. Alcohol. Clin. Exp. Res. 2012, 37, E59–E69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambade, A.; Satishchandran, A.; Szabo, G. Alcoholic hepatitis accelerates early hepatobiliary cancer by increasing stemness and miR-122-mediated HIF-1α activation. Sci. Rep. 2016, 6, 21340. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.; Liu, H.; Chen, F.; Li, D.; Zhao, Y. MiR-214 Promotes the Alcohol-Induced Oxidative Stress via Down-Regulation of Glutathione Reductase and Cytochrome P450 Oxidoreductase in Liver Cells. Alcohol. Clin. Exp. Res. 2013, 38, 68–77. [Google Scholar] [CrossRef]
- Bian, E.; Xiong, Z.; Li, J. New advances of lncRNAs in liver fibrosis, with specific focus on lncRNA–miRNA interactions. J. Cell. Physiol. 2019, 234, 2194–2203. [Google Scholar] [CrossRef]
- Li, H.; Du, X.; Huang, H.; Chen, X.; Yang, Y.; Huang, C.; Meng, X.; Li, J. Noncoding RNAs in alcoholic liver disease. J. Cell. Physiol. 2019, 234, 14709–14720. [Google Scholar] [CrossRef]
- Li, M.; He, Y.; Zhou, Z.; Ramirez, T.; Gao, Y.; Gao, Y.; Ross, R.A.; Cao, H.; Cai, Y.; Xu, M.; et al. MicroRNA-223 ameliorates alcoholic liver injury by inhibiting the IL-6–p47phox–oxidative stress pathway in neutrophils. Gut 2016, 66, 705–715. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z. Progress and prospects of long noncoding RNAs in lipid homeostasis. Mol. Metab. 2016, 5, 164–170. [Google Scholar] [CrossRef]
- Torok, N.J. Update on Alcoholic Hepatitis. Biomolecules 2015, 5, 2978–2986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.; Feng, D.; Li, M.; Gao, Y.; Ramirez, T.; Cao, H.; Kim, S.-J.; Yang, Y.; Cai, Y.; Ju, C.; et al. Hepatic mitochondrial DNA/Toll-like receptor 9/MicroRNA-223 forms a negative feedback loop to limit neutrophil overactivation and acetaminophen hepatotoxicity in mice. Hepatology 2017, 66, 220–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lainiola, M.; Linden, A.-M. Alcohol intake in two different mouse drinking models after recovery from the lipopolysaccharide-induced sickness reaction. Alcohol 2017, 65, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bala, S.; Csak, T.; Saha, B.; Zatsiorsky, J.; Kodys, K.; Catalano, D.; Satishchandran, A.; Szabo, G. The pro-inflammatory effects of miR-155 promote liver fibrosis and alcohol-induced steatohepatitis. J. Hepatol. 2016, 64, 1378–1387. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Calin, G.A. Crucial role of non-coding RNAs in disease. Cancer Lett. 2018, 420, 127–128. [Google Scholar] [CrossRef]
- Lamas-Paz, A.; Hao, F.; Nelson, L.J.; Vázquez, M.T.; Canals, S.; Del Moral, M.G.; Martínez-Naves, E.; Nevzorova, Y.A.; Cubero, F.J. Alcoholic liver disease: Utility of animal models. World J. Gastroenterol. 2018, 24, 5063–5075. [Google Scholar] [CrossRef] [PubMed]
- Li, S. Expression of Concern: The functional role of long non-coding RNAs and epigenetics. Biol. Proced. Online 2016, 18, 1. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Chu, S.; Li, J.; Li, J.; Zhang, Z.; Xia, C.; Heng, Y.; Zhang, M.; Hu, J.; Wei, G.; et al. Anti-inflammatory function of ginsenoside Rg1 on alcoholic hepatitis through glucocorticoid receptor related nuclear factor-kappa B pathway. J. Ethnopharmacol. 2015, 173, 231–240. [Google Scholar] [CrossRef]
- Williams, J.A.; Manley, S.; Ding, W.-X. New advances in molecular mechanisms and emerging therapeutic targets in alcoholic liver diseases. World J. Gastroenterol. 2014, 20, 12908–12933. [Google Scholar] [CrossRef]
- Klieser, E.; Mayr, C.; Kiesslich, T.; Wissniowski, T.; Di Fazio, P.; Neureiter, D.; Ocker, M. The Crosstalk of miRNA and Oxidative Stress in the Liver: From Physiology to Pathology and Clinical Implications. Int. J. Mol. Sci. 2019, 20, 5266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawaratani, H.; Moriya, K.; Namisaki, T.; Uejima, M.; Kitade, M.; Takeda, K.; Okura, Y.; Kaji, K.; Takaya, H.; Nishimura, N.; et al. Therapeutic strategies for alcoholic liver disease: Focusing on inflammation and fibrosis (Review). Int. J. Mol. Med. 2017, 40, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Hu, M.; Zhang, R.; Shen, Z.; Flatow, L.; You, M. MicroRNA-217 Promotes Ethanol-induced Fat Accumulation in Hepatocytes by Down-regulating SIRT1. J. Biol. Chem. 2012, 287, 9817–9826. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Liu, X.; Zhou, Q.; Huang, C.; Meng, X.; Xu, F.; Li, J. Silent information regulator 1 (SIRT1) ameliorates liver fibrosis via promoting activated stellate cell apoptosis and reversion. Toxicol. Appl. Pharmacol. 2015, 289, 163–176. [Google Scholar] [CrossRef]
- Kharbanda, K.K.; Todero, S.L.; King, A.L.; Osna, N.A.; McVicker, B.L.; Tuma, D.J.; Wisecarver, J.L.; Bailey, S.M. Betaine Treatment Attenuates Chronic Ethanol-Induced Hepatic Steatosis and Alterations to the Mitochondrial Respiratory Chain Proteome. Int. J. Hepatol. 2011, 2012, 1–10. [Google Scholar] [CrossRef]
- Listenberger, L.; Townsend, E.; Rickertsen, C.; Hains, A.; Brown, E.; Inwards, E.G.; Stoeckman, A.K.; Matis, M.P.; Sampathkumar, R.S.; Osna, N.A.; et al. Decreasing Phosphatidylcholine on the Surface of the Lipid Droplet Correlates with Altered Protein Binding and Steatosis. Cells 2018, 7, 230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kharbanda, K.K.; Mailliard, M.E.; Baldwin, C.R.; Beckenhauer, H.C.; Sorrell, M.F.; Tuma, D.J. Betaine attenuates alcoholic steatosis by restoring phosphatidylcholine generation via the phosphatidylethanolamine methyltransferase pathway. J. Hepatol. 2007, 46, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Kharbanda, K.K. Methionine metabolic pathway in alcoholic liver injury. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 89–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arumugam, M.K.; Talawar, S.; Listenberger, L.; Donohue, J.T.M.; Osna, N.A.; Kharbanda, K.K. Role of Elevated Intracellular S-Adenosylhomocysteine in the Pathogenesis of Alcohol-Related Liver Disease. Cells 2020, 9, 1526. [Google Scholar] [CrossRef]
- Deng, X.; Von Keudell, G.; Suzuki, T.; Dohmae, N.; Nakakido, M.; Piao, L.; Yoshioka, Y.; Nakamura, Y.; Hamamoto, R. PRMT1 promotes mitosis of cancer cells through arginine methylation of INCENP. Oncotarget 2015, 6, 35173–35182. [Google Scholar] [CrossRef] [Green Version]
- Reintjes, A.; Fuchs, J.E.; Kremser, L.; Lindner, H.H.; Liedl, K.R.; Huber, L.A.; Valovka, T. Asymmetric arginine dimethylation of RelA provides a repressive mark to modulate TNFalpha/NF-kappaB response. Proc. Natl. Acad. Sci. USA 2016, 113, 4326–4331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, W.; Qin, W.; Qiao, F.; Xu, Z.; Yuan, Y.; Chen, H. Protein arginine methyltransferase 1 interacts with Gli1 and regulates its transcriptional activity. Tumor Biol. 2016, 37, 9071–9076. [Google Scholar] [CrossRef] [PubMed]
- Tikhanovich, I.; Zhao, J.; Olson, J.; Adams, A.; Taylor, R.; Bridges, B.; Marshall, L.; Roberts, B.; Weinman, S.A. Protein arginine methyltransferase 1 modulates innate immune responses through regulation of peroxisome proliferator-activated receptor γ-dependent macrophage differentiation. J. Biol. Chem. 2017, 292, 6882–6894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tikhanovich, I.; Zhao, J.; Bridges, B.; Kumer, S.; Roberts, B.; Weinman, S.A. Arginine methylation regulates c-Myc–dependent transcription by altering promoter recruitment of the acetyltransferase p300. J. Biol. Chem. 2017, 292, 13333–13344. [Google Scholar] [CrossRef] [Green Version]
- Jain, K.; Warmack, R.A.; Debler, E.W.; Hadjikyriacou, A.; Stavropoulos, P.; Clarke, S.G. Protein Arginine Methyltransferase Product Specificity Is Mediated by Distinct Active-site Architectures. J. Biol. Chem. 2016, 291, 18299–18308. [Google Scholar] [CrossRef] [Green Version]
- Chuang, C.-Y.; Chang, C.-P.; Lee, Y.-J.; Lin, W.-L.; Chang, W.-W.; Wu, J.-S.; Cheng, Y.-W.; Lee, H.; Li, C. PRMT1 expression is elevated in head and neck cancer and inhibition of protein arginine methylation by adenosine dialdehyde or PRMT1 knockdown downregulates proliferation and migration of oral cancer cells. Oncol. Rep. 2017, 38, 1115–1123. [Google Scholar] [CrossRef]
- Hsu, J.H.-R.; Hubbell-Engler, B.; Adelmant, G.; Huang, J.; Joyce, C.E.; Vazquez, F.; Weir, B.A.; Montgomery, P.; Tsherniak, A.; Giacomelli, A.O.; et al. PRMT1-Mediated Translation Regulation Is a Crucial Vulnerability of Cancer. Cancer Res. 2017, 77, 4613–4625. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Adams, A.; Roberts, B.; O’Neil, M.; Vittal, A.; Schmitt, T.; Kumer, S.; Cox, J.; Li, Z.; Weinman, S.A.; et al. Protein arginine methyl transferase 1- and Jumonji C domain-containing protein 6-dependent arginine methylation regulate hepatocyte nuclear factor 4 alpha expression and hepatocyte proliferation in mice. Hepatology 2018, 67, 1109–1126. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Adams, A.; Weinman, S.A.; Tikhanovich, I. Hepatocyte PRMT1 protects from alcohol induced liver injury by modulating oxidative stress responses. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Pár, A.; Pár, G. Alcoholic liver disease: The roles of genetic-epigenetic factors and the effect of abstinence. Orv. Hetil. 2019, 160, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Ni, X. ROS-mediated DNA methylation pattern alterations in carcinogenesis. Curr. Drug Targets 2015, 16, 13–19. [Google Scholar] [CrossRef] [PubMed]
- To, Y.; Ito, K.; Kizawa, Y.; Failla, M.; Ito, M.; Kusama, T.; Elliott, W.M.; Hogg, J.C.; Adcock, I.M.; Barnes, P.J. Targeting phosphoinositide-3-kinase-delta with theophylline reverses corticosteroid insensitivity in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2010, 182, 897–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, K.; Caramori, G.; Adcock, I.M. Therapeutic Potential of Phosphatidylinositol 3-Kinase Inhibitors in Inflammatory Respiratory Disease. J. Pharmacol. Exp. Ther. 2006, 321, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boccuto, L.; Abenavoli, L. Genetic and Epigenetic Profile of Patients with Alcoholic Liver Disease. Ann. Hepatol. 2017, 16, 490–500. [Google Scholar] [CrossRef]
- Meroni, M.; Longo, M.; Rametta, R.; Dongiovanni, P. Genetic and Epigenetic Modifiers of Alcoholic Liver Disease. Int. J. Mol. Sci. 2018, 19, 3857. [Google Scholar] [CrossRef] [Green Version]
- Zahs, A.; Curtis, B.J.; Waldschmidt, T.J.; Brown, L.A.S.; Gauthier, T.W.; Choudhry, M.A.; Kovacs, E.J.; Bird, M.D. Alcohol and epigenetic changes: Summary of the 2011 Alcohol and Immunology Research Interest Group (AIRIG) meeting. Alcohol 2012, 46, 783–787. [Google Scholar] [CrossRef] [Green Version]
- Tan, H.K.; Yates, E.; Lilly, K.; Dhanda, A.D. Oxidative stress in alcohol-related liver disease. World J. Hepatol. 2020, 12, 332–349. [Google Scholar] [CrossRef]
- Tkachenko, P.; Maevskaya, M.; Pavlov, A.; Komkova, I.; Pavlov, C.; Ivashkin, V. Prednisolone plus S-adenosil-l-methionine in severe alcoholic hepatitis. Hepatol. Int. 2016, 10, 983–987. [Google Scholar] [CrossRef]
- Medici, V.; Virata, M.C.; Peerson, J.M.; Stabler, S.P.; French, S.W.; Gregory, J.F., 3rd; Albanese, A.; Bowlus, C.L.; Devaraj, S.; Panacek, E.A.; et al. S-adenosyl-L-methionine treatment for alcoholic liver disease: A double-blinded, randomized, placebo-controlled trial. Alcohol. Clin. Exp. Res. 2011, 35, 1960–1965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, X.; Yang, Y.-D.; Chen, K.; Lv, Z.-Y.; Zheng, L.; Liu, Y.-P.; Chen, S.-H.; Yu, C.-H.; Jiang, X.-Y.; Zhang, C.-Y.; et al. HDMCP uncouples yeast mitochondrial respiration and alleviates steatosis in L02 and hepG2 cells by decreasing ATP and H2O2 levels: A novel mechanism for NAFLD. J. Hepatol. 2009, 50, 1019–1028. [Google Scholar] [CrossRef]
- Collins, P.; Jones, C.; Choudhury, S.; Damelin, L.; Hodgson, H. Increased expression of uncoupling protein 2 in HepG2 cells attenuates oxidative damage and apoptosis. Liver Int. 2005, 25, 880–887. [Google Scholar] [CrossRef]
- Phillips, M.; Curtis, H.; Portmann, B.; Donaldson, N.; Bomford, A.; O’Grady, J. Antioxidants versus corticosteroids in the treatment of severe alcoholic hepatitis—A randomised clinical trial. J. Hepatol. 2006, 44, 784–790. [Google Scholar] [CrossRef]
- Stewart, S.; Prince, M.; Bassendine, M.; Hudson, M.; James, O.; Jones, D.; Record, C.; Day, C.P. A randomized trial of antioxidant therapy alone or with corticosteroids in acute alcoholic hepatitis. J. Hepatol. 2007, 47, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Khac, E.; Thevenot, T.; Piquet, M.-A.; Benferhat, S.; Goria, O.; Chatelain, D.; Tramier, B.; Dewaele, F.; Ghrib, S.; Rudler, M.; et al. Glucocorticoids plusN-Acetylcysteine in Severe Alcoholic Hepatitis. N. Engl. J. Med. 2011, 365, 1781–1789. [Google Scholar] [CrossRef] [Green Version]
- Wenzel, G.; Kuklinski, B.; Rühlmann, C.; Ehrhardt, D. Alcohol-induced toxic hepatitis—A “free radical” associated disease. Lowering fatality by adjuvant antioxidant therapy. Z. fur die Gesamte Inn. Med. und Ihre Grenzgeb. 1993, 48, 490–496. [Google Scholar]
- McClain, C.; Vatsalya, V.; Cave, M. Role of Zinc in the Development/Progression of Alcoholic Liver Disease. Curr. Treat. Options Gastroenterol. 2017, 15, 285–295. [Google Scholar] [CrossRef]
- Correa, F.; Mallard, C.; Nilsson, M.; Sandberg, M. Activated microglia decrease histone acetylation and Nrf2-inducible anti-oxidant defence in astrocytes: Restoring effects of inhibitors of HDACs, p38 MAPK and GSK3β. Neurobiol. Dis. 2011, 44, 142–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Protein/Gene/Molecule | Role in ALD—Induced Oxidative Stress |
|---|---|
| miR-212 and iNOS | alcohol-induced gut leakiness |
| SREBP-1c and PPARα | promotion of liver steatosis |
| reactive aldehydes (e.g., 4-HNE) | promotion of liver steatosis |
| PAMPs and DAMPs | progression of inflammation |
| SIRT family | progression of oxidation and inflammation |
| miR-214 | suppression of cytochrome P450 |
| miR-223 | involved in neutrophils infiltration and ROS generation |
| miR-155 and miR-181b-3p | LPS-mediated inflammation |
| miR-291b | involved in TLR4/NF-κB pathway |
| miR-34a and miR-217 | inhibits the expression of SIRT1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michalak, A.; Lach, T.; Cichoż-Lach, H. Oxidative Stress—A Key Player in the Course of Alcohol-Related Liver Disease. J. Clin. Med. 2021, 10, 3011. https://doi.org/10.3390/jcm10143011
Michalak A, Lach T, Cichoż-Lach H. Oxidative Stress—A Key Player in the Course of Alcohol-Related Liver Disease. Journal of Clinical Medicine. 2021; 10(14):3011. https://doi.org/10.3390/jcm10143011
Chicago/Turabian StyleMichalak, Agata, Tomasz Lach, and Halina Cichoż-Lach. 2021. "Oxidative Stress—A Key Player in the Course of Alcohol-Related Liver Disease" Journal of Clinical Medicine 10, no. 14: 3011. https://doi.org/10.3390/jcm10143011
APA StyleMichalak, A., Lach, T., & Cichoż-Lach, H. (2021). Oxidative Stress—A Key Player in the Course of Alcohol-Related Liver Disease. Journal of Clinical Medicine, 10(14), 3011. https://doi.org/10.3390/jcm10143011






