Increased Mortality in Elderly Patients Admitted with Hyponatremia: A Prospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
- Patients admitted to the Internal Medicine Department;
- Age 65 years or more;
2.2. Demographics, Biochemical Analyses and Follow-Up
2.3. Evaluation of Hyponatremia
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hao, J.; Li, Y.; Zhang, X.; Pang, C.; Wang, Y.; Nigwekar, S.U.; Qiu, L.; Chen, L. The Prevalence and Mortality of Hyponatremia Is Seriously Underestimated in Chinese General Medical Patients: An Observational Retrospective Study. BMC Nephrol. 2017, 18, 328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, M.Y.; Sudhir, U.; Anil Kumar, T.; Saravanan, S.; Mahesh, E.; Punith, K. Hospital-Based Descriptive Study of Symptomatic Hyponatremia in Elderly Patients. J. Assoc. Physicians India 2010, 58, 667–669. [Google Scholar]
- Fenske, W.; Maier, S.K.G.; Blechschmidt, A.; Allolio, B.; Störk, S. Utility and Limitations of the Traditional Diagnostic Approach to Hyponatremia: A Diagnostic Study. Am. J. Med. 2010, 123, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Woodward, M.; Gonski, P.; Grossmann, M.; Obeid, J.; Scholes, R.; Topliss, D.J. Diagnosis and Management of Hyponatraemia in the Older Patient. Intern. Med. J. 2018, 48 (Suppl. 1), 5–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correia, L.; Ferreira, R.; Correia, I.; Lebre, A.; Carda, J.; Monteiro, R.; Simão, A.; Carvalho, A.; Costa, N. Severe Hyponatremia in Older Patients at Admission in an Internal Medicine Department. Arch. Gerontol. Geriatr. 2014, 59, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Liamis, G.; Filippatos, T.D.; Elisaf, M.S. Thiazide-Associated Hyponatremia in the Elderly: What the Clinician Needs to Know. J. Geriatr. Cardiol. 2016, 13, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Mannesse, C.K.; Vondeling, A.M.; van Marum, R.J.; van Solinge, W.W.; Egberts, T.C.G.; Jansen, P.A.F. Prevalence of Hyponatremia on Geriatric Wards Compared to Other Settings over Four Decades: A Systematic Review. Ageing Res. Rev. 2013, 12, 165–173. [Google Scholar] [CrossRef]
- Upadhyay, A.; Jaber, B.L.; Madias, N.E. Epidemiology of Hyponatremia. Semin. Nephrol. 2009, 29, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Terzian, C.; Frye, E.B.; Piotrowski, Z.H. Admission Hyponatremia in the Elderly: Factors Influencing Prognosis. J. Gen. Intern. Med. 1994, 9, 89–91. [Google Scholar] [CrossRef]
- Choudhury, M.; Aparanji, K.; Norkus, E.P.; Dharmarajan, T.S. Hyponatremia in Hospitalized Nursing Home Residents and Outcome: Minimize Hospitalization and Keep the Stay Short! J. Am. Med. Dir. Assoc. 2012, 13, e8–e9. [Google Scholar] [CrossRef]
- Ahamed, S.; Anpalahan, M.; Savvas, S.; Gibson, S.; Torres, J.; Janus, E. Hyponatraemia in Older Medical Patients: Implications for Falls and Adverse Outcomes of Hospitalisation. Intern. Med. J. 2014, 44, 991–997. [Google Scholar] [CrossRef]
- Ganguli, A.; Mascarenhas, R.C.; Jamshed, N.; Tefera, E.; Veis, J.H. Hyponatremia: Incidence, Risk Factors, and Consequences in the Elderly in a Home-Based Primary Care Program. Clin. Nephrol. 2015, 84, 75–85. [Google Scholar] [CrossRef]
- Spasovski, G.; Vanholder, R.; Allolio, B.; Annane, D.; Ball, S.; Bichet, D.; Decaux, G.; Fenske, W.; Hoorn, E.J.; Ichai, C.; et al. Clinical Practice Guideline on Diagnosis and Treatment of Hyponatraemia. Eur. J. Endocrinol. 2014, 170, G1–G47. [Google Scholar] [CrossRef] [Green Version]
- Hillier, T.A.; Abbott, R.D.; Barrett, E.J. Hyponatremia: Evaluating the Correction Factor for Hyperglycemia. Am. J. Med. 1999, 106, 399–403. [Google Scholar] [CrossRef]
- Collin, C.; Wade, D.T.; Davies, S.; Horne, V. The Barthel ADL Index: A Reliability Study. Int. Disabil. Stud. 1988, 10, 61–63. [Google Scholar] [CrossRef]
- Sujino, Y.; Nakano, S.; Tanno, J.; Shiraishi, Y.; Goda, A.; Mizuno, A.; Nagatomo, Y.; Kohno, T.; Muramatsu, T.; Nishimura, S.; et al. Clinical Implications of the Blood Urea Nitrogen/Creatinine Ratio in Heart Failure and Their Association with Haemoconcentration. ESC Heart Fail. 2019, 6, 1274–1282. [Google Scholar] [CrossRef]
- Cuesta, M.; Garrahy, A.; Thompson, C.J. SIAD: Practical Recommendations for Diagnosis and Management. J. Endocrinol. Investig. 2016, 39, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Akirov, A.; Diker-Cohen, T.; Steinmetz, T.; Amitai, O.; Shimon, I. Sodium Levels on Admission Are Associated with Mortality Risk in Hospitalized Patients. Eur. J. Intern. Med. 2017, 46, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Zilberberg, M.D.; Exuzides, A.; Spalding, J.; Foreman, A.; Jones, A.G.; Colby, C.; Shorr, A.F. Epidemiology, Clinical and Economic Outcomes of Admission Hyponatremia among Hospitalized Patients. Curr. Med. Res. Opin. 2008, 24, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Gill, G.; Huda, B.; Boyd, A.; Skagen, K.; Wile, D.; Watson, I.; van Heyningen, C. Characteristics and Mortality of Severe Hyponatraemia-a Hospital-Based Study. Clin. Endocrinol. 2006, 65, 246–249. [Google Scholar] [CrossRef]
- Waikar, S.S.; Mount, D.B.; Curhan, G.C. Mortality after Hospitalization with Mild, Moderate, and Severe Hyponatremia. Am. J. Med. 2009, 122, 857–865. [Google Scholar] [CrossRef] [Green Version]
- Zieschang, T.; Wolf, M.; Vellappallil, T.; Uhlmann, L.; Oster, P.; Kopf, D. The Association of Hyponatremia, Risk of Confusional State, and Mortality. Dtsch. Arztebl. Int. 2016, 113, 855–862. [Google Scholar] [CrossRef] [Green Version]
- Ryg, J.; Engberg, H.; Mariadas, P.; Pedersen, S.G.H.; Jorgensen, M.G.; Vinding, K.L.; Andersen-Ranberg, K. Barthel Index at Hospital Admission Is Associated with Mortality in Geriatric Patients: A Danish Nationwide Population-Based Cohort Study. Clin. Epidemiol. 2018, 10, 1789–1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, X.; Luo, Q.; Li, B.; Lin, Z.; Yu, X.; Huang, F. Serum Uric Acid and Mortality in Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Metabolism 2016, 65, 1326–1341. [Google Scholar] [CrossRef]
- Shao, Y.; Shao, H.; Sawhney, M.S.; Shi, L. Serum Uric Acid as a Risk Factor of All-Cause Mortality and Cardiovascular Events among Type 2 Diabetes Population: Meta-Analysis of Correlational Evidence. J. Diabetes Complicat. 2019, 33, 107409. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zhang, M.; Abeysekera, I.R.; Wang, X. High Serum Uric Acid Levels May Increase Mortality and Major Adverse Cardiovascular Events in Patients with Acute Myocardial Infarction. Saudi Med. J. 2017, 38, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Konta, T.; Ichikawa, K.; Kawasaki, R.; Fujimoto, S.; Iseki, K.; Moriyama, T.; Yamagata, K.; Tsuruya, K.; Narita, I.; Kondo, M.; et al. Association between Serum Uric Acid Levels and Mortality: A Nationwide Community-Based Cohort Study. Sci. Rep. 2020, 10, 6066. [Google Scholar] [CrossRef] [Green Version]
- Tasdemir, V.; Oguz, A.K.; Sayın, I.; Ergun, I. Hyponatremia in the Outpatient Setting: Clinical Characteristics, Risk Factors, and Outcome. Int. Urol. Nephrol. 2015, 47, 1977–1983. [Google Scholar] [CrossRef]
- Eckart, A.; Hausfater, P.; Amin, D.; Amin, A.; Haubitz, S.; Bernard, M.; Baumgartner, A.; Struja, T.; Kutz, A.; Christ-Crain, M.; et al. Hyponatremia and Activation of Vasopressin Secretion Are Both Independently Associated with 30-Day Mortality: Results of a Multicenter, Observational Study. J. Intern. Med. 2018, 284, 270–281. [Google Scholar] [CrossRef]
- Wald, R.; Jaber, B.L.; Price, L.L.; Upadhyay, A.; Madias, N.E. Impact of Hospital-Associated Hyponatremia on Selected Outcomes. Arch. Intern. Med. 2010, 170, 294–302. [Google Scholar] [CrossRef]
- Chawla, A.; Sterns, R.H.; Nigwekar, S.U.; Cappuccio, J.D. Mortality and Serum Sodium: Do Patients Die from or with Hyponatremia? Clin. J. Am. Soc. Nephrol. 2011, 6, 960–965. [Google Scholar] [CrossRef] [Green Version]
- Holland-Bill, L.; Christiansen, C.F.; Heide-Jørgensen, U.; Ulrichsen, S.P.; Ring, T.; Jørgensen, J.O.L.; Sørensen, H.T. Hyponatremia and Mortality Risk: A Danish Cohort Study of 279 508 Acutely Hospitalized Patients. Eur. J. Endocrinol. 2015, 173, 71–81. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.H.; Kim, H.W.; Lee, S.Y.; Sun, I.O.; Hwang, H.S.; Choi, S.R.; Chung, B.H.; Park, H.S.; Park, C.W.; Yang, C.W.; et al. Is the Sodium Level per Se Related to Mortality in Hospitalized Patients with Severe Hyponatremia? Clin. Nephrol. 2012, 77, 182–187. [Google Scholar] [CrossRef]
- Jiang, Y.; Cai, W.; Masquelin, M.E.; Gordon, K. An Unexpected Case of Lisinopril-Associated Severe Hyponatremia. Cureus 2020, 12, e9039. [Google Scholar] [CrossRef]
- Grattagliano, I.; Mastronuzzi, T.; D’Ambrosio, G. Hyponatremia Associated with Long-Term Medication Use in the Elderly: An Analysis in General Practice. J. Prim. Health Care 2018, 10, 167–173. [Google Scholar] [CrossRef]
- Soiza, R.L.; Cumming, K.; Clarke, J.M.; Wood, K.M.; Myint, P.K. Hyponatremia: Special Considerations in Older Patients. J. Clin. Med. 2014, 3, 944. [Google Scholar] [CrossRef] [Green Version]
- Renneboog, B.; Musch, W.; Vandemergel, X.; Manto, M.U.; Decaux, G. Mild Chronic Hyponatremia Is Associated with Falls, Unsteadiness, and Attention Deficits. Am. J. Med. 2006, 119, 71.e1–71.e8. [Google Scholar] [CrossRef] [PubMed]
- Al-Lamki, Z.; Farooqui, M.A.; Ahmed, S. Incidence and Outcome of Severe Hyponatremia in Children and Young Adults: A Single Institution Experience. Sultan Qaboos Univ. Med. J. 2006, 6, 13–16. [Google Scholar] [PubMed]
- Musch, W.; Thimpont, J.; Vandervelde, D.; Verhaeverbeke, I.; Berghmans, T.; Decaux, G. Combined Fractional Excretion of Sodium and Urea Better Predicts Response to Saline in Hyponatremia than Do Usual Clinical and Biochemical Parameters. Am. J. Med. 1995, 99, 348–355. [Google Scholar] [CrossRef]
- Hoyle, G.E.; Chua, M.; Soiza, R.L. Volaemic Assessment of the Elderly Hyponatraemic Patient: Reliability of Clinical Assessment and Validation of Bioelectrical Impedance Analysis. QJM 2011, 104, 35–39. [Google Scholar] [CrossRef] [Green Version]
- Cumming, K.; Hoyle, G.E.; Hutchison, J.D.; Soiza, R.L. Bioelectrical Impedance Analysis Is More Accurate than Clinical Examination in Determining the Volaemic Status of Elderly Patients with Fragility Fracture and Hyponatraemia. J. Nutr. Health Aging 2014, 18, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Clayton, J.A.; Le Jeune, I.R.; Hall, I.P. Severe Hyponatraemia in Medical In-Patients: Aetiology, Assessment and Outcome. QJM 2006, 99, 505–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filippatos, T.D.; Liamis, G.; Christopoulou, F.; Elisaf, M.S. Ten Common Pitfalls in the Evaluation of Patients with Hyponatremia. Eur. J. Intern. Med. 2016, 29, 22–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levey, A.S.; Eckardt, K.-U.; Tsukamoto, Y.; Levin, A.; Coresh, J.; Rossert, J.; De Zeeuw, D.; Hostetter, T.H.; Lameire, N.; Eknoyan, G. Definition and Classification of Chronic Kidney Disease: A Position Statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005, 67, 2089–2100. [Google Scholar] [CrossRef] [Green Version]
- Levey, A.S.; de Jong, P.E.; Coresh, J.; El Nahas, M.; Astor, B.C.; Matsushita, K.; Gansevoort, R.T.; Kasiske, B.L.; Eckardt, K.-U. The Definition, Classification, and Prognosis of Chronic Kidney Disease: A KDIGO Controversies Conference Report. Kidney Int. 2011, 80, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Nadal, J.; Channavajjhala, S.K.; Jia, W.; Clayton, J.; Hall, I.P.; Glover, M. Clinical and Molecular Features of Thiazide-Induced Hyponatremia. Curr. Hypertens. Rep. 2018, 20, 31. [Google Scholar] [CrossRef] [Green Version]
- Barber, J.; McKeever, T.M.; McDowell, S.E.; Clayton, J.A.; Ferner, R.E.; Gordon, R.D.; Stowasser, M.; O’Shaughnessy, K.M.; Hall, I.P.; Glover, M. A Systematic Review and Meta-Analysis of Thiazide-Induced Hyponatraemia: Time to Reconsider Electrolyte Monitoring Regimens after Thiazide Initiation? Br. J. Clin. Pharmacol. 2015, 79, 566–577. [Google Scholar] [CrossRef] [Green Version]
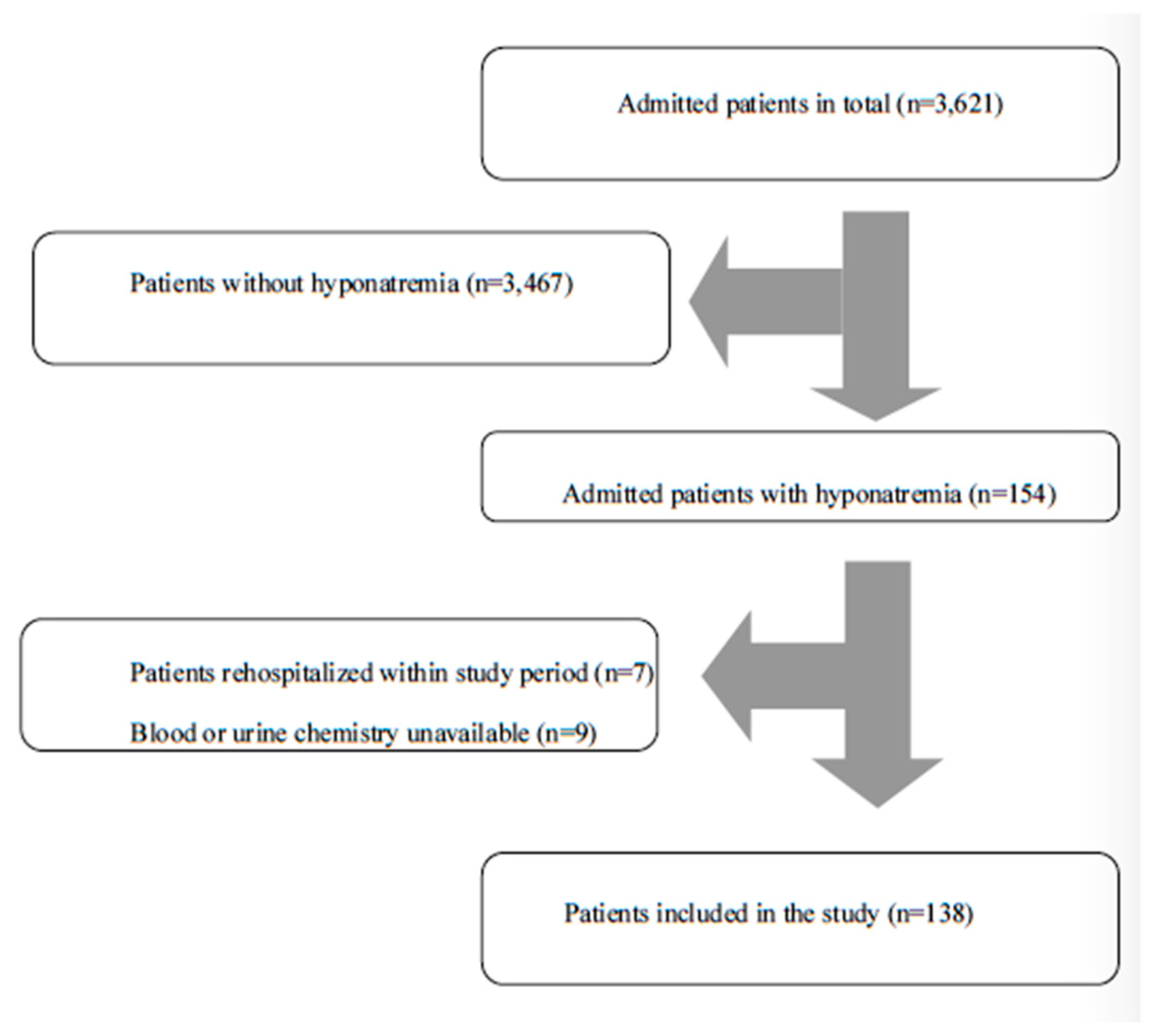
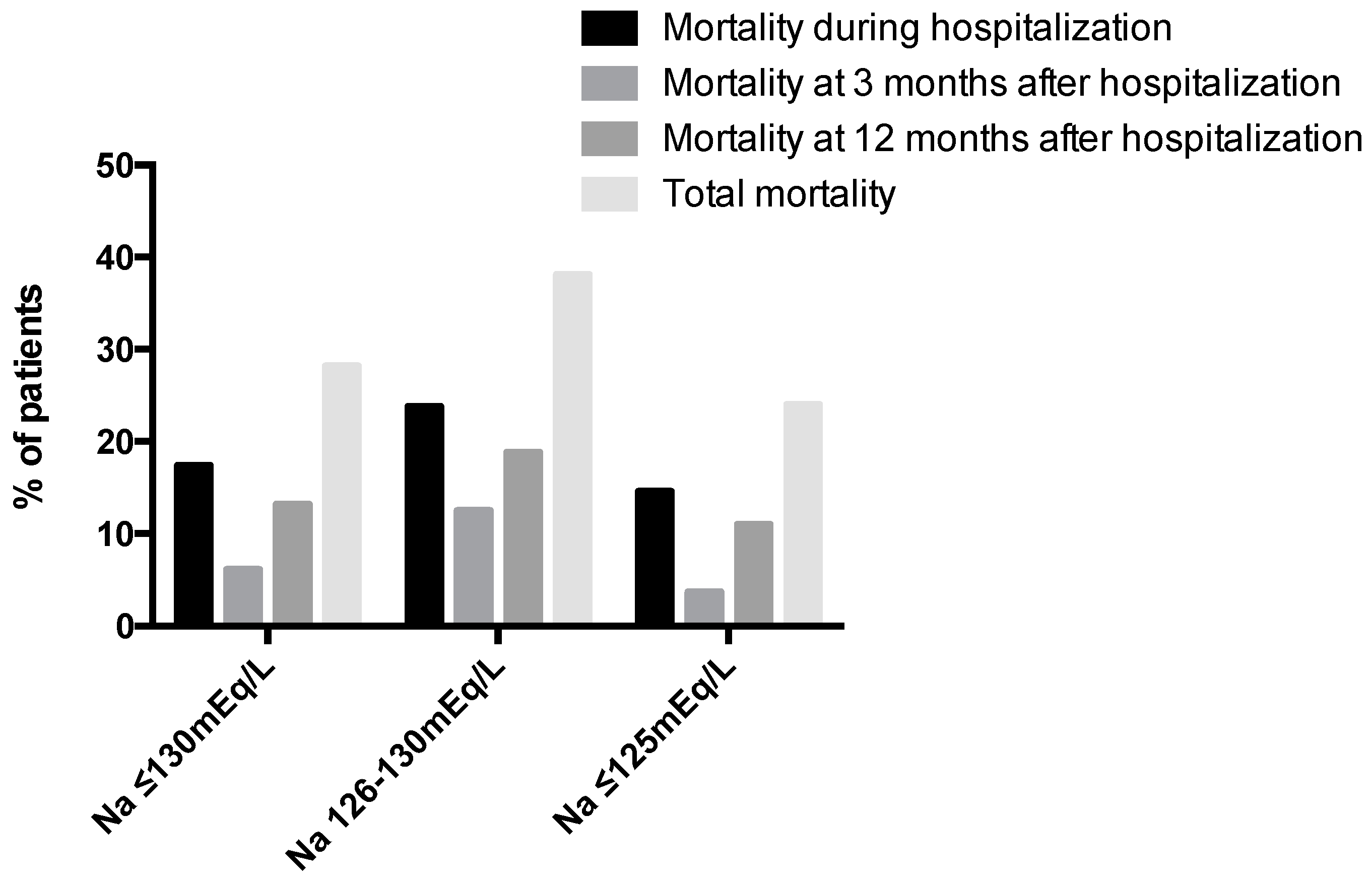
| Patient Characteristics | Na+ ≤ 130 mEq/L (n = 138) |
|---|---|
| Mean age, years (+/− SD) | 80.6 (7.5) |
| Male (n, %) | 50 (36.2) |
| Medical history | |
| Hypertension (n, %) | 121 (87.7) |
| Chronic kidney disease (n, %) | 88 (63.8) |
| Estimated GFR (EPI-CKD), mL/min/1.73 m2 (median, IQR) | 50 (33.8–69) |
| Heart failure (n, %) | 64 (46.4) |
| Diabetes mellitus (n, %) | 50 (36.2) |
| Ischemic heart disease (n, %) | 31 (22.5) |
| Dementia (n, %) | 27 (19.6) |
| Chronic lung disease (n, %) | 22 (15.9) |
| Cerebrovascular disease (n, %) | 19 (13.8) |
| Median Barthel Index (IQR) | 17 (8–20) |
| Hyponatremia in the past (n, %) | 52 (37.7) |
| Chronic medications | |
| ACE inhibitors and angiotensin receptor blockers (n, %) | 92 (66.7) |
| Thiazide diuretics (n, %) | 51 (37) |
| Furosemide (n, %) | 48 (34.8) |
| Calcium channel blockers (n, %) | 48 (34.8) |
| Selective serotonin receptor inhibitors (n, %) | 34 (24.6) |
| Acetylsalicylic acid (n, %) | 29 (21) |
| Aldosterone receptor antagonists (n, %) | 23 (16.7) |
| Antipsychotics (n, %) | 16 (11.6) |
| Symptomatic (n, %) | 80 (59.4) |
| Hypovolemic Hyponatremia (n = 73, 52.9%) | Euvolemic Hyponatremia (n = 37, 26.8%) | Hypervolemic Hyponatremia (n = 28, 20.3%) |
|---|---|---|
| Overt fluid losses (n = 73, 52.9%) | Thiazide use (n = 18, 13%) | Decompensated heart failure (n = 24, 17.4%) |
| Infection (n = 37, 26.8%) | SIAD (n = 17, 12.3%) | Kidney failure (n = 3, 2.2%) |
| Thiazide use (n = 30, 21.7%) | Excessive water intake (n = 1, 0.7%) | Liver cirrhosis (n = 1, 0.7%) |
| At least two causes (n = 34, 24,6%) | Corticoid insufficiency (n = 1, 0.7%) | At least two causes (additional causes: thiazide use n = 3; decompensated heart failure n = 1) (n = 3, 2.2%) |
| At least two causes (additional causes: urinary retention n = 3; hypothyroidism n = 3; kidney failure n = 2) (n = 7, 5.1%) |
| Patient Characteristics | Euvolemic, SIAD (n = 17) | Euvolemic, No SIAD (n = 20) | p |
|---|---|---|---|
| Mean age (+/− SD) | 77.7 (7.9) | 77.8 (7.8) | 0.9519 |
| Male (n, %) | 7 (41.2) | 7 (35) | 0.7447 |
| Median Barthel Index (IQR) | 16.5 (0–20) | 19 (15.8–20) | 0.1015 |
| Hyponatremia in the past (n, %) | 8 (57.1) | 9 (45) | 1 |
| Symptomatic (n, %) | 9 (52.9) | 17 (85) | 0.0689 |
| Mean corrected serum sodium mEq/L (+/− SD) | 121.6 (6.5) | 117.5 (6.5) | 0.0618 |
| Serum uric acid md/dL (+/− SD) | 3.1 (1.7) | 3.6 (2) | 0.4468 |
| Mean urine sodium mEq/L (+/− SD) | 71.4 (25.9) | 63.2 (40.1) | 0.4752 |
| Mean fractional excretion of sodium % (+/− SD) | 1.5 (1.7) | 1.3 (0.9) | 0.6314 |
| Mean plasma osmolarity mOsm/kg (+/− SD) | 254.4 (14.1) | 249.3 (12) | 0.2423 |
| Mean fractional excretion of uric acid % (+/− SD) | 24.5 (23.8) | 19.5 (18.3) | 0.4881 |
| Median length of hospitalization in days (IQR) | 6 (4–8.5) | 5 (4–7) | 0.4973 |
| Overall mortality (n, %) | 3 (17.6) | 0 (0) | 0.0875 |
| Re-admission at 3 months among survivors (n, %) | 5 (29.4) | 1 (5) | 0.0752 |
| Re-admission at 12 months among survivors (n, %) | 10 (58.8) | 3 (15) | 0.0075 |
| Hyponatremia after the hospitalization among survivors (n, %) | 8 (57.1) | 3 (15) | 0.0689 |
| Mortality at 3 months among discharged patients (n, %) | 2 (14.3) | 0 (0) | 0.1622 |
| Mortality at 12 months among discharged patients (n, %) | 2 (14.3) | 0 (0) | 0.1622 |
| Univariate Analysis p | Multivariate Analysis p | OR (95% CI) | |
|---|---|---|---|
| Symptomatic hyponatremia | 0.0457 | 0.988 | 0.992 (0.335–2.936) |
| Thiazide use | 0.0012 | 0.041 | 0.176 (0.033–0.934) |
| Barthel Index (per unit) | <0.0001 | 0.005 | 0.904 (0.843–0.971) |
| Uric acid (per mg/dL) | 0.0032 | 0.001 | 1.278 (1.099–1.485) |
| Proteins (per g/dL) | 0.0046 | 0.339 | 0.892 (0.706–1.127) |
| TSH (per mU/L) | 0.0073 | 0.617 | 0.998 (0.99–1.006) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ioannou, P.; Panagiotakis, S.; Tsagkaraki, E.; Tsioutis, C.; Fragkiadakis, K.; Gikas, A.; Filippatos, T.D. Increased Mortality in Elderly Patients Admitted with Hyponatremia: A Prospective Cohort Study. J. Clin. Med. 2021, 10, 3059. https://doi.org/10.3390/jcm10143059
Ioannou P, Panagiotakis S, Tsagkaraki E, Tsioutis C, Fragkiadakis K, Gikas A, Filippatos TD. Increased Mortality in Elderly Patients Admitted with Hyponatremia: A Prospective Cohort Study. Journal of Clinical Medicine. 2021; 10(14):3059. https://doi.org/10.3390/jcm10143059
Chicago/Turabian StyleIoannou, Petros, Symeon Panagiotakis, Emmanouela Tsagkaraki, Constantinos Tsioutis, Konstantinos Fragkiadakis, Achilleas Gikas, and Theodosios D. Filippatos. 2021. "Increased Mortality in Elderly Patients Admitted with Hyponatremia: A Prospective Cohort Study" Journal of Clinical Medicine 10, no. 14: 3059. https://doi.org/10.3390/jcm10143059
APA StyleIoannou, P., Panagiotakis, S., Tsagkaraki, E., Tsioutis, C., Fragkiadakis, K., Gikas, A., & Filippatos, T. D. (2021). Increased Mortality in Elderly Patients Admitted with Hyponatremia: A Prospective Cohort Study. Journal of Clinical Medicine, 10(14), 3059. https://doi.org/10.3390/jcm10143059








