Central and Branch Retinal Artery Occlusion—Do They Harbor the Same Risk of Further Ischemic Events?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Database
2.2. Study Sample
2.3. Statistical Analysis
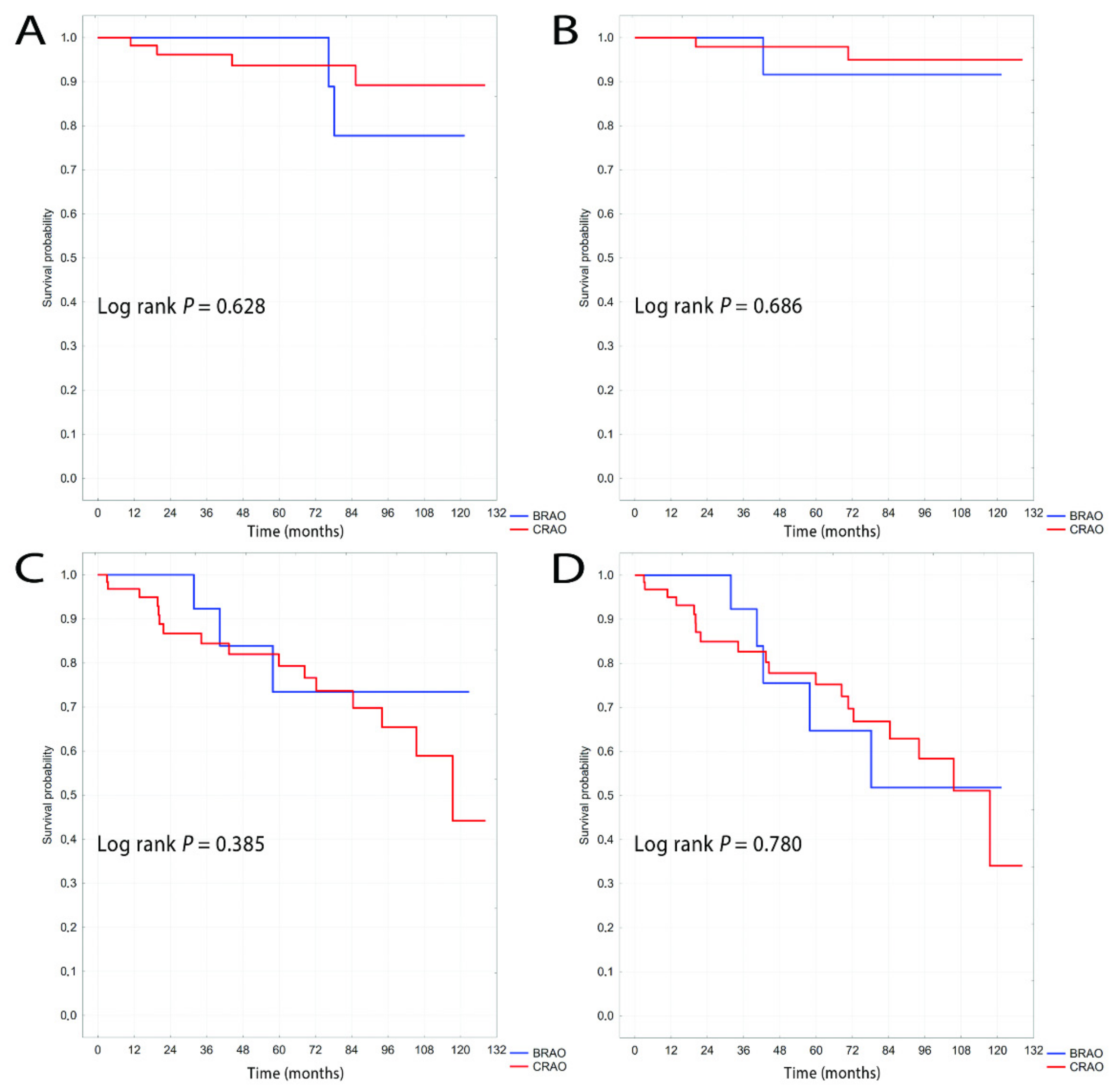
3. Results
4. Discussion
4.1. Ischemic Stroke
4.2. Myocardial Infarction
4.3. Mortality
4.4. Composite Endpoint
4.5. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dattilo, M.; Newman, N.J.; Biousse, V. Acute retinal arterial ischemia. Ann. Eye Sci. 2018, 3, 28. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.S.; Varma, D.; Lee, A. Arterial Occlusions to the Eye: From Retinal Emboli to Ocular Ischemic Syndrome. Asia-Pacific J. Ophthalmol. 2020, 9, 349–357. [Google Scholar] [CrossRef]
- Biousse, V.; Nahab, F.; Newman, N.J. Management of Acute Retinal Ischemia. Ophthalmology 2018, 125, 1597–1607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayreh, S.S.; Podhajsky, P.A.; Zimmerman, M.B. Retinal Artery Occlusion: Associated Systemic and Ophthalmic Abnormalities. Ophthalmology 2009, 116, 1928–1936. [Google Scholar] [CrossRef] [Green Version]
- Hayreh, S.S. Acute retinal arterial occlusive disorders. Prog. Retin. Eye Res. 2011, 30, 359–394. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.-S.; Jan, R.-L.; Weng, S.-F.; Wang, J.-J.; Chio, C.-C.; Wei, F.-T.; Chu, C.-C. Retinal Artery Occlusion and the 3-Year Risk of Stroke in Taiwan: A Nationwide Population-Based Study. Am. J. Ophthalmol. 2012, 154, 645–652.e1. [Google Scholar] [CrossRef]
- Sacco, R.L.; Kasner, S.E.; Broderick, J.P.; Caplan, L.R.; Connors, J.; Culebras, A.; Elkind, M.S.; George, M.G.; Hamdan, A.D.; Higashida, R.T.; et al. An Updated Definition of Stroke for the 21st Century. Stroke 2013, 44, 2064–2089. [Google Scholar] [CrossRef] [Green Version]
- Hayreh, S.S.; Zimmerman, M.B. Ocular Arterial Occlusive Disorders and Carotid Artery Disease. Ophthalmol. Retin. 2017, 1, 12–18. [Google Scholar] [CrossRef] [Green Version]
- Wilson, L.; Warlow, C.; Russell, R. Cardiovascular Disease in Patients with Retinal Arterial Occlusion. Lancet 1979, 313, 292–294. [Google Scholar] [CrossRef]
- Cho, K.H.; Kim, C.K.; Woo, S.J.; Park, K.H.; Park, S.J. Cerebral Small Vessel Disease in Branch Retinal Artery Occlusion. Investig. Opthalmolo. Vis. Sci. 2016, 57, 5818–5824. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.D.; Kim, J.Y.; Park, Y.J.; Park, S.J.; Baik, S.H.; Kang, J.; Jung, C.; Woo, S.J. Cerebral magnetic resonance imaging of coincidental infarction and small vessel disease in retinal artery occlusion. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Lauda, F.; Neugebauer, H.; Reiber, L.; Jüttler, E. Acute Silent Brain Infarction in Monocular Visual Loss of Ischemic Origin. Cerebrovasc. Dis. 2015, 40, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D.; Hetzel, A.; Geibel-Zehender, A.; Schulte-Mönting, J. Systemic diseases in non-inflammatory branch and central retinal artery occlusion--an overview of 416 patients. Eur. J. Med. Res. 2007, 12, 595–603. [Google Scholar]
- O’Donnell, M.J.; Xavier, D.; Liu, L.; Zhang, H.; Chin, S.L.; Rao-Melacini, P.; Rangarajan, S.; Islam, S.; Pais, P.; McQueen, M.J.; et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): A case-control study. Lancet 2010, 376, 112–123. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.W.; Lee, S.C.; Kwon, O.W.; Kim, Y.D.; Byeon, S.H. Co-occurrence of Acute Retinal Artery Occlusion and Acute Ischemic Stroke: Diffusion-Weighted Magnetic Resonance Imaging Study. Am. J. Ophthalmol. 2014, 157, 1231–1238. [Google Scholar] [CrossRef]
- Lavin, P.; Patrylo, M.; Hollar, M.; Espaillat, K.B.; Kirshner, H.; Schrag, M. Stroke Risk and Risk Factors in Patients with Central Retinal Artery Occlusion. Am. J. Ophthalmol. 2018, 196, 96–100. [Google Scholar] [CrossRef]
- Callizo, J.; Feltgen, N.; Pantenburg, S.; Wolf, A.; Neubauer, A.S.; Jurklies, B.; Wachter, R.; Schmoor, C.; Schumacher, M.; Junker, B.; et al. Cardiovascular Risk Factors in Central Retinal Artery Occlusion. Ophthalmology 2015, 122, 1881–1888. [Google Scholar] [CrossRef]
- Rim, T.H.; Han, J.; Choi, Y.S.; Hwang, S.-S.; Lee, C.; Lee, S.C.; Kim, S.S. Retinal Artery Occlusion and the Risk of Stroke Development. Stroke 2016, 47, 376–382. [Google Scholar] [CrossRef]
- Avery, M.B.; Magal, I.; Kherani, A.; Mitha, A.P. Risk of Stroke in Patients with Ocular Arterial Occlusive Disorders: A Retrospective Canadian Study. J. Am. Hear. Assoc. 2019, 8, e010509. [Google Scholar] [CrossRef] [Green Version]
- Hong, J.-H.; Sohn, S.-I.; Kwak, J.; Yoo, J.; Ahn, S.J.; Woo, S.J.; Jung, C.; Yum, K.S.; Bae, H.-J.; Chang, J.Y.; et al. Retinal artery occlusion and associated recurrent vascular risk with underlying etiologies. PLoS ONE 2017, 12, e0177663. [Google Scholar] [CrossRef] [Green Version]
- Helenius, J.; Arsava, E.M.; Goldstein, J.N.; Cestari, D.M.; Buonanno, F.S.; Rosen, B.R.; Ay, H. Concurrent acute brain infarcts in patients with monocular visual loss. Ann. Neurol. 2012, 72, 286–293. [Google Scholar] [CrossRef] [Green Version]
- Hankey, G.; Slattery, J.M.; Warlow, C.P. Prognosis and prognostic factors of retinal infarction: A prospective cohort study. BMJ 1991, 302, 499–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.-S.; Chu, C.-C.; Weng, S.-F.; Chang, C.; Wang, J.-J.; Jan, R.-L. The risk of acute coronary syndrome after retinal artery occlusion: A population-based cohort study. Br. J. Ophthalmol. 2014, 99, 227–231. [Google Scholar] [CrossRef] [Green Version]
- Deijle, I.A.; Van Schaik, S.M.; Van Wegen, E.E.; Weinstein, H.C.; Kwakkel, G.; Berg-Vos, R.M.V.D. Lifestyle Interventions to Prevent Cardiovascular Events After Stroke and Transient Ischemic Attack. Stroke 2017, 48, 174–179. [Google Scholar] [CrossRef]
- French, D.D.; Margo, C.E.; Greenberg, P.B. Ischemic Stroke Risk in Medicare Beneficiaries with Central Retinal Artery Occlusion: A Retrospective Cohort Study. Ophthalmol. Ther. 2018, 7, 125–131. [Google Scholar] [CrossRef]
- Mir, T.A.; Arham, A.Z.; Fang, W.; Alqahtani, F.; Alkhouli, M.; Gallo, J.; Hinkle, D.M. Acute Vascular Ischemic Events in Patients With Central Retinal Artery Occlusion in the United States: A Nationwide Study 2003-2014. Am. J. Ophthalmol. 2019, 200, 179–186. [Google Scholar] [CrossRef]
- Chodnicki, K.D.; Pulido, J.S.; Hodge, D.O.; Klaas, J.P.; Chen, J.J. Stroke Risk Before and After Central Retinal Artery Occlusion in a US Cohort. Mayo Clin. Proc. 2019, 94, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Choi, N.-K.; Yang, B.R.; Park, K.H.; Lee, J.; Jung, S.-Y.; Woo, S.J. Risk and Risk Periods for Stroke and Acute Myocardial Infarction in Patients with Central Retinal Artery Occlusion. Ophthalmology 2015, 122, 2336–2343. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, I.S.; Elsamna, S.T.; Zarbin, M.A.; Bhagat, N. Assessing the risk of stroke development following retinal artery occlusion. J. Stroke Cerebrovasc. Dis. 2020, 29. [Google Scholar] [CrossRef] [PubMed]
- De Potter, P.; Zografos, L. Survival prognosis of patients with retinal artery occlusion and associated carotid artery disease. Graefe’s Arch. Clin. Exp. Ophthalmol. 1993, 231, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Fallico, M.; Lotery, A.J.; Longo, A.; Avitabile, T.; Bonfiglio, V.; Russo, A.; Murabito, P.; Palmucci, S.; Pulvirenti, A.; Reibaldi, M. Risk of acute stroke in patients with retinal artery occlusion: A systematic review and meta-analysis. Eye 2020, 34, 683–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Zhu, W.; Wang, C. Relationship between retinal vascular occlusions and incident cerebrovascular diseases. Medicine 2016, 95, e4075. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Cugati, S.; Knudtson, M.D.; Rochtchina, E.; Klein, R.; Klein, B.E.; Wong, T.Y.; Mitchell, P. Retinal Arteriolar Emboli and Long-Term Mortality. Stroke 2006, 37, 1833–1836. [Google Scholar] [CrossRef] [Green Version]
- Klein, R.; Klein, B.E.K.; Moss, S.E.; Meuer, S.M. Retinal Emboli and Cardiovascular Disease. Arch. Ophthalmol. 2003, 121, 1446–1451. [Google Scholar] [CrossRef] [Green Version]
- Leisser, C.; Findl, O. Rate of strokes 1 year after retinal artery occlusion with analysis of risk groups. Eur. J. Ophthalmol. 2019, 30, 360–362. [Google Scholar] [CrossRef]
- Schorr, E.M.; Rossi, K.; Stein, L.K.; Park, B.L.; Tuhrim, S.; Dhamoon, M.S. Characteristics and Outcomes of Retinal Artery Occlusion. Stroke 2020, 51, 800–807. [Google Scholar] [CrossRef]
- Hayreh, S.S. Do Patients With Retinal Artery Occlusion Need Urgent Neurologic Evaluation? Am. J. Ophthalmol. 2018, 196, 53–56. [Google Scholar] [CrossRef]
- Laczynski, D.J.; Gallop, J.; Lyden, S.P.; Bena, J.; Yuan, A.; Smolock, C.J.; Caputo, F.J. Retinal artery occlusion does not portend an increased risk of stroke. J. Vasc. Surg. 2020, 72, 198–203. [Google Scholar] [CrossRef]
- Wolma, J.; Nederkoorn, P.J.; Goossens, A.; Vergouwen, M.D.I.; Van Schaik, I.N.; Vermeulen, M. Ethnicity a risk factor? The relation between ethnicity and large- and small-vessel disease in White people, Black people, and Asians within a hospital-based population. Eur. J. Neurol. 2009, 16, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Nichols, M.; Townsend, N.; Luengo-Fernandez, R.; Leal, J.; Gray, A.; Scarborough, P. European Cardiovascular Disease Statistics. European Heart Network, Brussels, European Society of Cardiology, Sophia Antipolis. 2012. Available online: https://www.escardio.org/static-file/Escardio/Press-media/press-releases/2013/EU-cardiovascular-disease-statistics-2012.pdf (accessed on 19 February 2021).
- Schilling, S.; Tzourio, C.; Dufouil, C.; Zhu, Y.; Berr, C.; Alpérovitch, A.; Crivello, F.; Mazoyer, B.; Debette, S. Plasma lipids and cerebral small vessel disease. Neurology 2014, 83, 1844–1852. [Google Scholar] [CrossRef] [PubMed]
- . Ahuja, R.M.; Chaturvedi, S.; Eliott, D.; Joshi, N.; Puklin, J.E.; Abrams, G.W. Mechanisms of retinal arterial occlusive disease in African American and Caucasian patients. Stroke 1999, 30, 1506–1509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hankey, G.J. Long-Term Outcome after Ischaemic Stroke/Transient Ischaemic Attack. Cerebrovasc. Dis. 2003, 16, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, M.; Béjot, Y.; Rothwell, P.M.; Touzé, E. Long-Term Risk of Myocardial Infarction Compared to Recurrent Stroke After Transient Ischemic Attack and Ischemic Stroke: Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2018, 7, e007267. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Total RAO population n = 131 | CRAO n = 94 (71.7%) | BRAO n = 37 (28.3%) | p-Value | |
|---|---|---|---|---|
| Sex (female), n (%) | 46 (35.1) | 35 (37.2) | 11 (29.7) | 0.417 |
| Age, years (SD) | 70 (11.2) | 70.9 (10.1) | 67.7 (13.7) | 0.449 |
| Age <65 years, n (%) | 38 (29.5) | 27 (29.4) | 11 (29.7) | 0.909 |
| Age <50 years, n (%) | 6 (4.6) | 2 (2.1) | 4 (10.8) | 0.032 |
| Age 50–59 years, n (%) | 13 (9.9) | 7 (7.5) | 6 (16.2) | 0.131 |
| Age 60–69 years, n (%) | 42 (32.1) | 35 (37.2) | 7 (18.9) | 0.043 |
| Age 70–79 years, n (%) | 45 (34.4) | 31 (33) | 14 (37.8) | 0.043 |
| Age ≥80 years, n (%) | 25 (19.1) | 19 (20.2) | 6 (16.2) | 0.600 |
| BMI 18.5–24.9, n (%) | 37 (28.2) | 29 (30.9) | 8 (21.6) | 0.291 |
| BMI 25–29.9, n (%) | 51 (38.9) | 39 (41.5) | 12 (32.4) | 0.338 |
| BMI 30–34.9, n (%) | 34 (26) | 22 (23.4) | 12 (32.4) | 0.289 |
| BMI 35–39.9, n (%) | 7 (5.3) | (3 (3.2) | 4 (10.8) | 0.081 |
| BMI ≥40, n (%) | 2 (1.5) | 1 (1.1) | 1 (2.7) | 0.491 |
| Systolic pressure, mmHg (SD) | 139 (23.7) | 138 (22.9) | 141.6 (25.7) | 0.380 |
| Diastolic pressure, mmHg (SD) | 81.9 (10.4) | 81.6 (11.2) | 82.6 (8) | 0.582 |
| Hypertension, n (%) | 111 (84.7) | 79 (84) | 32 (86.5) | 0.726 |
| Hypercholesterolemia, n (%) | 95 (72.5) | 68 (72.3) | 27 (73) | 0.942 |
| Coronary artery disease, n (%) | 52 (39.7) | 38 (40.4) | 14 (37.8) | 0.785 |
| Myocardial infarction before RAO, n (%) | 33 (25.2) | 24 (25.5) | 9 (24.3) | 0.886 |
| Diabetes mellitus, n (%) | 27 (20.6) | 22 (23.4) | 5 (13.5) | 0.208 |
| Ischemic stroke before RAO, n (%) | 17 (13) | 11 (11.7) | 6 (16.2) | 0.489 |
| Hemorrhagic stroke before RAO, n (%) | 3 (2.3) | 2 (2.1) | 1 (2.7) | 0.843 |
| Smoking habits, n (%) | 32 (24.4) | 26 (27.7) | 6 (16.2) | 0.170 |
| Atrial fibrillation, n (%) | 19 (14.5) | 13 (13.8) | 6 (16.2) | 0.856 |
| Permanent atrial fibrillation, n (%) | 9 (6.9) | 7 (7.5) | 2 (5.4) | 0.667 |
| Paroxysmal atrial fibrillation, n (%) | 10 (7.6) | 6 (6.4) | 4 (10.8) | 0.390 |
| Oral anticoagulant, n (%) | 17 (13) | 12 (12.8) | 5 (13.5) | 0.909 |
| Acetylsalicylic acid, n (%) | 30 (22.9) | 21 (22.3) | 9 (24.3) | 0.808 |
| Heart failure, n (%) | 27 (20.6) | 19 (20.2) | 8 (21.6) | 0.856 |
| Chronic kidney disease (stage) | 2.5 (0.7) | 2.5 (0.7) | 2.4 (0.6) | 0.321 |
| Chronic kidney disease, stage 4–5, n (%) | 4 (3.7) | 4 (5.3) | 0 (0) | 0.176 |
| Chronic kidney disease, stage ≤3, n (%) | 53(49.1) | 39 (52) | 14 (42.4) | 0.359 |
| RAO of the left eye, n (%) | 62 (47.3) | 45 (47.8) | 17 (46) | 0.842 |
| RAO of the right eye, n (%) | 69 (52.7) | 49 (52.1) | 20 (54.1) | 0.836 |
| Ischemic stroke after RAO, n (%) | 13 (9.9) | 10 (10.6) | 3 (8.1) | 0.662 |
| Myocardial infarction after RAO, n (%) | 3 (2.3) | 2 (2.1) | 1 (2.7) | 0.843 |
| Death after RAO, n (%) | 30 (22.9) | 24 (25.5) | 6 (16.2) | 0.253 |
| Composite endpoint after RAO, n (%) | 37 (28.2) | 29 (30.9) | 8 (21.6) | 0.338 |
| Median time to stroke, months (IQR) | 44.4 (57.2) | 32 (52.3) | 76.4 (56.8) | 0.352 |
| Median time to myocardial infarction, months (IQR) | 42.5 (50.5) | 45.4 (50.5) | -¹ | - |
| Median time to death, months (IQR) | 35.9 (43.3) | 35.9 (53.7) | 36.3 (11.6) | 0.876 |
| Median time to composite endpoint, months (IQR) | 40.4 (33.8) | 37.5 (37.2) | 41.5 (18.3) | 0.912 |
| Intima media measurement, mm (SD) 2 | 1.2 (0.4) | 1.2 (0.4) | 1.2 (0.3) | 0.844 |
| Atherosclerotic plaque in the carotid arteries, n (%) 3 | 94 (86.2) | 73 (91.3) | 21 (72.4) | 0.012 |
| Carotid stenosis >70%, n (%) 4 | 19 (17.1) | 14 (17.5) | 5 (16.1) | 0.863 |
| Ipsilateral stenosis to RAO, n (%) 4 | 15 (13.5) | 12 (15) | 3 (9.7) | 0.462 |
| Ipsilateral occlusion of the carotid artery, n (%) 4 | 4 (3.6) | 2 (2.5) | 2 (6.5) | 0.316 |
| Diameter of the ascending aorta, mm (SD) 5 | 34.1 (4.3) | 34.3 (4.3) | 33.6 (4.5) | 0.477 |
| Ivs., mm (SD) 5 | 11.2 (1.7) | 11.2 (1.8) | 11.2 (1.6) | 0.698 |
| LA, mm (SD) 5 | 39.7 (7) | 39.4 (7.1) | 40.4 (7) | 0.499 |
| LVEF, % (SD) 5 | 54.9 (7.7) | 55.2 (7.6) | 54.1 (8.1) | 0.731 |
| Calcifications on the mitral or aortic valves, n (%) 5 | 51 (43.6) | 41 (47.7) | 10 (32.3) | 0.138 |
| Mild aortic stenosis, n (%) 5 | 5 (4.2) | 5 (5.8) | 0 (0) | 0.172 |
| Moderate aortic stenosis, n (%) 5 | 4 (3.4) | 4 (4.7) | 0 (0) | 0.222 |
| Severe aortic stenosis, n (%) 5 | 1 (0.9) | 1 (1.2) | 0 (0) | 0.547 |
| PFO, n (%) 6 | 5 (4.3) | 2 (2.3) | 3 (9.7) | 0.083 |
| Total cholesterol, mg/dL (SD) | 196.5 (48.4) | 194.2 (45.7) | 201.8 (54.4) | 0.445 |
| LDL-cholesterol, mg/dL (SD) | 120.5 (42.2) | 119.4 (41.2) | 122.9 (44.9) | 0.360 |
| HDL-cholesterol, mg/dL (SD) | 46.3 (10.3) | 47.4 (10.9) | 43.6 (8.5) | 0.104 |
| Triglycerides, mg/dL (SD) | 153.6 (79.4) | 139 (45.7) | 186.9 (82.7) | <0.001 |
| Creatinine, mg/dL (SD) | 1.2 (0.35) | 1.2 (0.37) | 1.1 (0.3) | 0.614 |
| MDRD, mL/min (SD) | 61.9 (18.2) | 60.6 (18.9) | 64.8 (16.4) | 0.360 |
| Fasting glucose, mg/dL (SD) | 111.2 (33.8) | 112 (36.2) | 109.4 (27.8) | 0.914 |
| RAO | CRAO | BRAO | p-Value CRAO vs. BRAO | ||
|---|---|---|---|---|---|
| Stroke | PY | 635.9 | 464.6 | 173.3 | |
| IR | 20.7 | 21.7 | 17.9 | 0.767 | |
| Myocardial infarction | PY | 662.6 | 487.8 | 174.8 | |
| IR | 4.5 | 4.1 | 5.7 | 0.785 | |
| All-cause mortality | PY | 570.1 | 418.1 | 152 | |
| IR | 52.6 | 57.4 | 39.5 | 0.409 | |
| Composite endpoint | PY | 549.3 | 406.4 | 142.9 | |
| IR | 67.4 | 71.4 | 56.4 | 0.54 |
| CRAO | BRAO | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Age | 1.04 | 1–1.08 | 0.061 | 1.11 | 0.99–1.24 | 0.064 |
| Sex (male) | 1.14 | 0.52–2.49 | 0.756 | 0.7 | 0.14–3.52 | 0.661 |
| Age <65 years | 0.41 | 0.15–1.07 | 0.069 | - | - | - |
| Age ≥65 years | 2.46 | 0.93–6.51 | 0.069 | - | - | - |
| Age 60–69 years | 0.61 | 0.26–1.43 | 0.252 | - | - | - |
| Age 70–79 years | 1.11 | 0.52–2.39 | 0.786 | 3.66 | 0.73–18.24 | 0.114 |
| Age ≥80 years | 2.43 | 1.05–5.63 | 0.039 | 1.59 | 0.32–7.94 | 0.572 |
| BMI 18.5–24.9 | 2.03 | 0.94–4.37 | 0.072 | 2.48 | 0.48–12.88 | 0.281 |
| BMI 25–29.9 | 0.48 | 0.21–1.10 | 0.083 | 0.64 | 0.15–2.72 | 0.549 |
| BMI 30–34.9 | 0.95 | 0.40–2.28 | 0.917 | 1.08 | 0.21–5.44 | 0.930 |
| BMI 35–39.9 | 3.75 | 0.48–29.23 | 0.207 | 1.37 | 0.17–11.26 | 0.768 |
| Systolic pressure | 0.99 | 0.98–1.01 | 0.710 | 0.99 | 0.97–1.02 | 0.717 |
| Diastolic pressure | 4.02 | 0.98–1.05 | 0.295 | 0.99 | 0.91–1.08 | 0.850 |
| Hypertension | 1.63 | 0.56–4.72 | 0.369 | - | - | - |
| Hypercholesterolemia | 0.80 | 0.37–1.74 | 0.573 | 0.29 | 0.07–1.24 | 0.094 |
| Coronary artery disease | 1.43 | 0.68–3.00 | 0.348 | 1.84 | 0.43–7.77 | 0.408 |
| Myocardial infarction | 1.41 | 0.59–3.36 | 0.433 | 0.71 | 0.14–3.56 | 0.680 |
| Smoking habits | 0.99 | 0.44–2.27 | 0.997 | 1.09 | 0.22–5.41 | 0.918 |
| Diabetes mellitus | 1.32 | 0.56–3.11 | 0.530 | 0.82 | 0.11–7.56 | 0.942 |
| Ischemic stroke | 0.89 | 0.27–2.96 | 0.846 | 0.55 | 0.07–4.47 | 0.574 |
| Atrial fibrillation | 1.71 | 0.65–4.53 | 0.278 | 0.67 | 0.08–5.44 | 0.707 |
| Acetylsalicylic acid | 1.47 | 0.59–3.64 | 0.410 | 1.34 | 0.27–6.69 | 0.722 |
| Heart failure | 1.56 | 0.69–3.56 | 0.286 | 0.97 | 0.20–4.86 | 0.975 |
| CKD stage | 0.88 | 0.48–1.59 | 0.662 | 2.19 | 0.57–8.38 | 0.251 |
| Intima media thickness | 1.37 | 0.56–3.34 | 0.484 | 5.05 | 0.19–133.6 | 0.332 |
| Atherosclerotic plaque in the carotid arteries | 0.82 | 0.24–2.75 | 0.746 | - | - | - |
| Carotid stenosis >70% | 0.99 | 0.37–2.67 | 0.990 | 2.34 | 0.25–21.51 | 0.454 |
| Ipsilateral stenosis to RAO | 0.88 | 0.30–2.58 | 0.815 | 7.83 | 0.70–87.58 | 0.095 |
| Calcifications on the mitral or aortic valves | 1.64 | 0.70–3.81 | 0.254 | 2.27 | 0.51–10.21 | 0.284 |
| Mild aortic stenosis | 1.05 | 0.14–7.83 | 0.961 | - | - | - |
| Moderate aortic stenosis | 2.92 | 0.98–8.68 | 0.055 | - | - | - |
| Severe aortic stenosis | 5.96 | 0.77–46.26 | 0.088 | - | - | - |
| PFO | 0.68 | 0.09–5.19 | 0.710 | - | - | - |
| Total cholesterol | 0.99 | 0.98–0.998 | 0.023 | 0.99 | 0.98–1.01 | 0.319 |
| LDL-cholesterol | 0.99 | 0.98–1.00 | 0.183 | 0.99 | 0.97–1.01 | 0.239 |
| HDL-cholesterol | 0.94 | 0.90–0.99 | 0.020 | 0.96 | 0.88–1.04 | 0.319 |
| Triglycerides | 0.99 | 0.985–1.00 | 0.095 | 0.999 | 0.99–1.01 | 0.932 |
| Creatinine | 0.82 | 0.25–2.66 | 0.736 | 3.40 | 0.22–52.35 | 0.381 |
| MDRD | 0.98 | 0.96–1.01 | 0.208 | 0.94 | 0.88–1.01 | 0.009 |
| Fasting glucose | 1.00 | 0.99–1.01 | 0.925 | 0.96 | 0.91–1.01 | 0.123 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roskal-Wałek, J.; Wałek, P.; Biskup, M.; Odrobina, D.; Mackiewicz, J.; Głuszek, S.; Wożakowska-Kapłon, B. Central and Branch Retinal Artery Occlusion—Do They Harbor the Same Risk of Further Ischemic Events? J. Clin. Med. 2021, 10, 3093. https://doi.org/10.3390/jcm10143093
Roskal-Wałek J, Wałek P, Biskup M, Odrobina D, Mackiewicz J, Głuszek S, Wożakowska-Kapłon B. Central and Branch Retinal Artery Occlusion—Do They Harbor the Same Risk of Further Ischemic Events? Journal of Clinical Medicine. 2021; 10(14):3093. https://doi.org/10.3390/jcm10143093
Chicago/Turabian StyleRoskal-Wałek, Joanna, Paweł Wałek, Michał Biskup, Dominik Odrobina, Jerzy Mackiewicz, Stanisław Głuszek, and Beata Wożakowska-Kapłon. 2021. "Central and Branch Retinal Artery Occlusion—Do They Harbor the Same Risk of Further Ischemic Events?" Journal of Clinical Medicine 10, no. 14: 3093. https://doi.org/10.3390/jcm10143093






