Exercise and the Risk of Dementia in Patients with Newly Diagnosed Atrial Fibrillation: A Nationwide Population-Based Study
Abstract
:1. Introduction
2. Materials and Methods
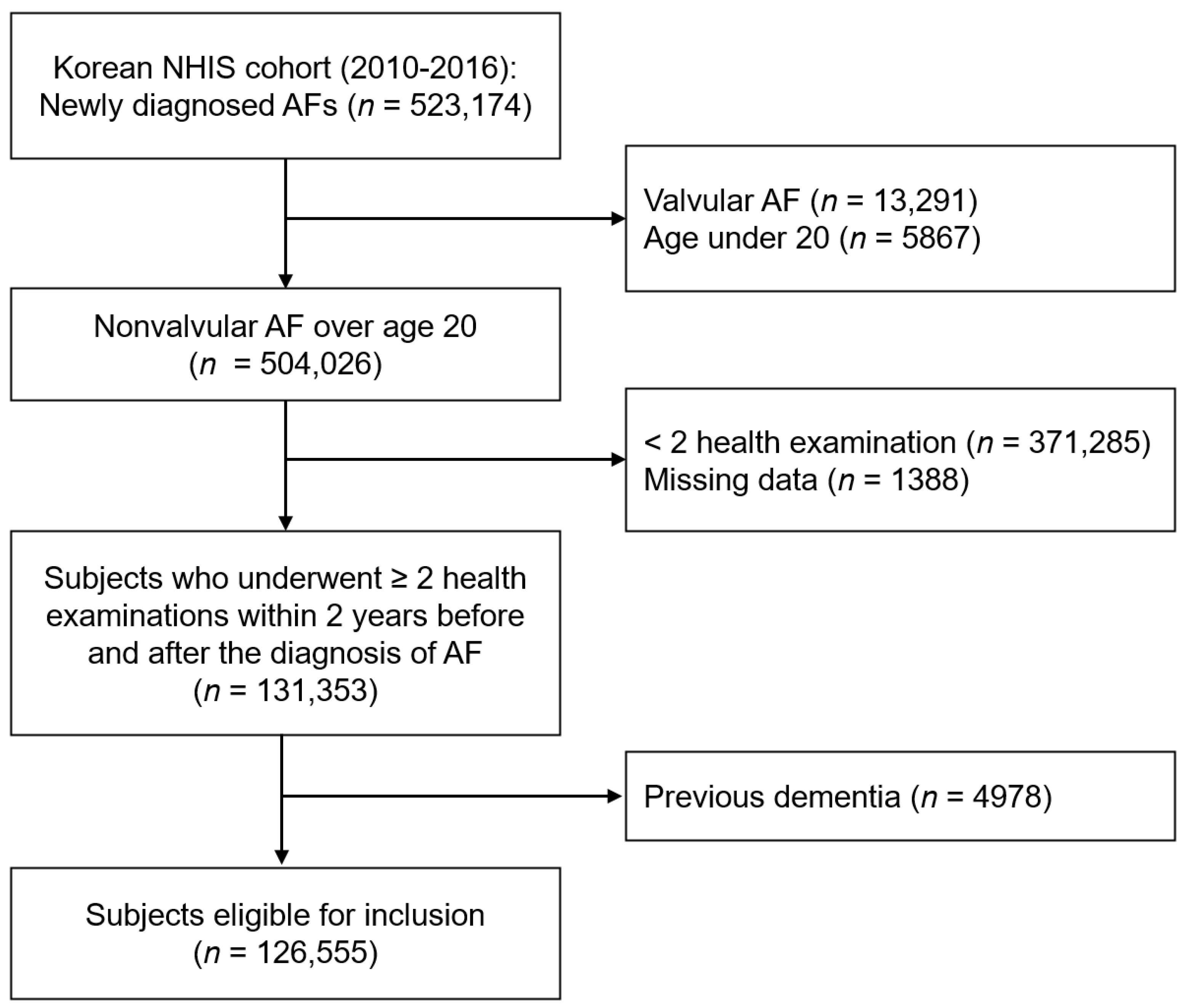
2.1. Data Source and Study Population
2.2. Evaluation of Physical Activity
2.3. Covariates
2.4. Study Outcomes and Follow-Up
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Changes in PA before and after New AF Diagnosis and the Risk of Incident Dementia
3.3. Sensitivity Analyses: One-Year and Three-Year Landmark Analysis
3.4. Subgroup Analyses
4. Discussion
4.1. Strengths
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chugh, S.S.; Havmoeller, R.; Narayanan, K.; Singh, D.; Rienstra, M.; Benjamin, E.J.; Gillum, R.F.; Kim, Y.; McAnulty, J.H., Jr.; Zheng, Z.; et al. Worldwide epidemiology of atrial fibrillation: A Global Burden of Disease 2010 Study. Circulation 2014, 129, 837–847. [Google Scholar] [CrossRef] [Green Version]
- Heeringa, J.; van der Kuip, D.A.M.; Hofman, A.; Kors, J.; van Herpen, G.; Stricker, B.H.C.; Stijnen, T.; Lip, G.Y.H.; Witteman, J.C.M. Prevalence, incidence and lifetime risk of atrial fibrillation: The Rotterdam study. Eur. Heart J. 2006, 27, 949–953. [Google Scholar] [CrossRef] [Green Version]
- Kwok, C.; Loke, Y.; Hale, R.; Potter, J.; Myint, P. Atrial fibrillation and incidence of dementia: A systematic review and meta-analysis. Neurology 2011, 76, 914–922. [Google Scholar] [CrossRef]
- Jacobs, L.G.; Billett, H.H.; Freeman, K.; Dinglas, C.; Jumaquio, L. Anticoagulation for stroke prevention in elderly patients with atrial fibrillation, including those with falls and/or early-stage dementia: A single-center, retrospective, observational study. Am. J. Geriatr. Pharmacother. 2009, 7, 159–166. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Action Plan on the Public Health Response to Dementia 2017–2025; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Rovio, S.; Kåreholt, I.; Helkala, E.-L.; Viitanen, M.; Winblad, B.; Tuomilehto, J.L.; Soininen, H.; Nissinen, A.; Kivipelto, M. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 2005, 4, 705–711. [Google Scholar] [CrossRef]
- Larson, E.B.; Wang, L.; Bowen, J.D.; McCormick, W.C.; Teri, L.; Crane, P.; Kukull, W. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann. Intern. Med. 2006, 144, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Lautenschlager, N.T.; Cox, K.L.; Flicker, L.; Foster, J.K.; Van Bockxmeer, F.M.; Xiao, J.; Greenop, K.R.; Almeida, O.P. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: A randomized trial. JAMA 2008, 300, 1027–1037. [Google Scholar] [CrossRef] [Green Version]
- Liu-Ambrose, T.; Best, J.R.; Davis, J.C.; Eng, J.J.; Lee, P.E.; Jacova, C.; Boyd, L.A.; Brasher, P.M.; Munkacsy, M.; Cheung, W.; et al. Aerobic exercise and vascular cognitive impairment: A randomized controlled trial. Neurology 2016, 87, 2082–2090. [Google Scholar] [CrossRef] [Green Version]
- Lamb, S.E.; Sheehan, B.; Atherton, N.; Nichols, V.; Collins, H.; Mistry, D.; Dosanjh, S.; Slowther, A.M.; Khan, I.; Petrou, S.; et al. Dementia and Physical Activity (DAPA) trial of moderate to high intensity exercise training for people with dementia: Randomised controlled trial. BMJ 2018, 361, k1675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ott, A.; Breteler, M.M.; de Bruyne, M.C.; van Harskamp, F.; Grobbee, D.E.; Hofman, A. Atrial fibrillation and dementia in a population-based study: The Rotterdam Study. Stroke 1997, 28, 316–321. [Google Scholar] [CrossRef]
- Hegbom, F.; Stavem, K.; Sire, S.; Heldal, M.; Orning, O.M.; Gjesdal, K. Effects of short-term exercise training on symptoms and quality of life in patients with chronic atrial fibrillation. Int. J. Cardiol. 2007, 116, 86–92. [Google Scholar] [CrossRef]
- Osbak, P.S.; Mourier, M.; Kjaer, A.; Henriksen, J.H.; Kofoed, K.F.; Jensen, G.B. A randomized study of the effects of exercise training on patients with atrial fibrillation. Am. Heart J. 2011, 162, 1080–1087. [Google Scholar] [CrossRef]
- Pathak, R.K.; Elliott, A.; Middeldorp, M.E.; Meredith, M.; Mehta, A.B.; Mahajan, R.; Hendriks, J.M.L.; Twomey, D.; Kalman, J.M.; Abhayaratna, W.P.; et al. Impact of CARDIOrespiratory FITness on arrhythmia recurrence in obese individuals with atrial fibrillation: The CARDIO-FIT study. J. Am. Coll. Cardiol. 2015, 66, 985–996. [Google Scholar] [CrossRef]
- Faselis, C.; Kokkinos, P.; Tsimploulis, A.; Pittaras, A.; Myers, J.; Lavie, C.J.; Kyritsi, F.; Lovic, D.; Karasik, P.; Moore, H. Exercise capacity and atrial fibrillation risk in veterans: A cohort study. Mayo Clin. Proc. 2016, 91, 558–566. [Google Scholar] [CrossRef]
- Choi, E.-K. Cardiovascular Research Using the Korean National Health Information Database. Korean Circ. J. 2020, 50, 754–772. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.W.; Kim, S.H.; Kang, S.H.; Kim, H.J.; Yoon, C.H.; Youn, T.J.; Chae, I.H. Mortality reduction with physical activity in patients with and without cardiovascular disease. Eur. Heart J. 2019, 40, 3547–3555. [Google Scholar] [CrossRef]
- Son, J.S.; Choi, S.; Kim, K.; Kim, S.M.; Choi, D.; Lee, G.; Jeong, S.; Park, S.Y.; Kim, Y.; Yun, J.; et al. Association of blood pressure classification in Korean young adults according to the 2017 American College of Cardiology/American Heart Association guidelines with subsequent cardiovascular disease events. JAMA 2018, 320, 1783–1792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.; Choi, S.; Hwang, S.E.; Son, J.S.; Lee, J.K.; Oh, J.; Park, S.M. Changes in exercise frequency and cardiovascular outcomes in older adults. Eur. Heart J. 2020, 41, 1490–1499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.-T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [PubMed]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease. A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 74, e177–e232. [Google Scholar] [CrossRef]
- Sperling, R.A.; Aisen, P.S.; Beckett, L.A.; Bennett, D.A.; Craft, S.; Fagan, A.M.; Iwatsubo, T.; Jack, C.R., Jr.; Kaye, J.; Montine, T.J.; et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 280–292. [Google Scholar] [CrossRef] [Green Version]
- Rastas, S.; Verkkoniemi, A.; Polvikoski, T.; Juva, K.; Niinistö, L.; Mattila, K.; Länsimies, E.; Pirttilä, T.; Sulkava, R. Atrial fibrillation, stroke, and cognition: A longitudinal population-based study of people aged 85 and older. Stroke 2007, 38, 1454–1460. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, V.; May, H.T.; Bair, T.L.; Crandall, B.G.; Cutler, M.J.; Day, J.D.; Mallender, C.; Osborn, J.S.; Stevens, S.M.; Weiss, J.P.; et al. Long-term population-based cerebral ischemic event and cognitive outcomes of direct oral anticoagulants compared with warfarin among long-term anticoagulated patients for atrial fibrillation. Am. J. Cardiol. 2016, 118, 210–214. [Google Scholar] [CrossRef]
- Swiger, K.J.; Manalac, R.J.; Blumenthal, R.S.; Blaha, M.J.; Martin, S.S. Statins and cognition: A systematic review and meta-analysis of short-and long-term cognitive effects. Mayo Clin. Proc. 2013, 88, 1213–1221. [Google Scholar] [CrossRef]
- Chiuve, S.E.; Rexrode, K.M.; Spiegelman, D.; Logroscino, G.; Manson, J.E.; Rimm, E.B. Primary prevention of stroke by healthy lifestyle. Circulation 2008, 118, 947–954. [Google Scholar] [CrossRef] [Green Version]
- Gligoroska, J.P.; Manchevska, S. The effect of physical activity on cognition–physiological mechanisms. Mater. Socio-Med. 2012, 24, 198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.Y.; Agarwal, S.K.; Norby, F.L.; Gottesman, R.F.; Loehr, L.R.; Soliman, E.Z.; Mosley, T.H.; Folsom, A.R.; Coresh, J.; Alonso, A. Persistent but not paroxysmal atrial fibrillation is independently associated with lower cognitive function: ARIC Study. J. Am. Coll. Cardiol. 2016, 67, 1379–1380. [Google Scholar] [CrossRef]
- Pathak, R.K.; Middeldorp, M.E.; Meredith, M.; Mehta, A.B.; Mahajan, R.; Wong, C.X.; Twomey, D.; Elliott, A.D.; Kalman, J.M.; Abhayaratna, W.P.; et al. Long-term effect of goal-directed weight management in an atrial fibrillation cohort: A long-term follow-up study (LEGACY). J. Am. Coll. Cardiol. 2015, 65, 2159–2169. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.J., Jr.; Entman, M.; North, W.C.; Kong, Y.; Mcintosh, H. The changes in cardiac output with reversion of atrial fibrillation to sinus rhythm. Circulation 1965, 31, 670–678. [Google Scholar] [CrossRef] [Green Version]
- Jefferson, A.L.; Beiser, A.S.; Himali, J.J.; Seshadri, S.; O’Donnell, C.J.; Manning, W.J.; Wolf, P.A.; Au, R.; Benjamin, E.J. Low cardiac index is associated with incident dementia and Alzheimer disease: The Framingham Heart Study. Circulation 2015, 131, 1333–1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lieshout, J.V. Middle cerebral artery blood velocity during exercise in patients with atrial fibrillation. Clin. Physiol. 1999, 19, 284–289. [Google Scholar]
- Malmo, V.; Nes, B.M.; Amundsen, B.H.; Tjonna, A.; Stoylen, A.; Rossvoll, O.; Wisloff, U.; Loennechen, J. Aerobic interval training reduces the burden of atrial fibrillation in the short term: A randomized trial. Circulation 2016, 133, 466–473. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Total (n = 126,555) | Non-Exerciser (n = 42,884) | Exercise Starter (n = 22,150) | Exercise Quitter (n = 22,993) | Exercise Maintainer (n = 38,528) |
|---|---|---|---|---|---|
| Age, years | 62.7 ± 12.0 | 65.7 ± 11.5 | 65.2 ± 12.0 | 63.6 ± 11.6 | 59.1 ± 11.8 |
| <65—no. (%) | 64,503 (51.0%) | 17,188 (40.1%) | 11,689 (52.8%) | 10,939 (47.6%) | 24,687 (64.1%) |
| 65–74—no. (%) | 41,349 (32.7%) | 15,424 (36.0%) | 7181 (32.4%) | 8091 (35.2%) | 10,653 (27.7%) |
| ≥75—no. (%) | 20,703 (16.4%) | 10,272 (24.0%) | 3280 (14.8%) | 3963 (17.2%) | 3188 (8.3%) |
| Male sex (%) | 78,446 (62.0%) | 21,819 (50.9%) | 13,537 (61.1%) | 14,103 (61.3%) | 28,987 (75.2%) |
| Comorbidities | |||||
| Hypertension | 84,014 (66.4%) | 29,468 (68.7%) | 14,584 (65.8%) | 15,432 (67.1%) | 24,530 (63.7%) |
| Diabetes | 29,024 (22.9%) | 10,655 (24.9%) | 5012 (22.6%) | 5559 (24.2%) | 7798 (20.2%) |
| Dyslipidemia | 13,218 (10.4%) | 4492 (10.5%) | 2318 (10.5%) | 2462 (10.7%) | 3946 (10.2%) |
| Previous MI | 6458 (5.1%) | 2391 (5.6%) | 1126 (5.1%) | 1172 (5.1%) | 1769 (4.6%) |
| Previous stroke | 16,800 (13.3%) | 6350 (14.8%) | 2864 (12.9%) | 3267 (14.2%) | 4319 (11.2%) |
| Previous CHF | 31,306 (24.7%) | 11,896 (27.7%) | 5285 (23.9%) | 5904 (25.7%) | 8221 (21.3%) |
| PAD | 27,380 (21.6%) | 10,552 (24.6%) | 4829 (21.8%) | 5318 (23.1%) | 6681 (17.3%) |
| COPD | 24,605 (19.4%) | 9785 (22.8%) | 4327 (19.5%) | 4691 (20.4%) | 5802 (15.1%) |
| Cancer | 7253 (5.73%) | 2344 (5.5%) | 1363 (6.2%) | 1435 (6.2%) | 2111 (5.5%) |
| CKD | 18,868 (14.9%) | 7951 (18.5%) | 3046 (13.8%) | 3616 (15.7%) | 4255 (11.0%) |
| CHA2DS2-VASc score | 2.7 ± 1.7 | 3.2 ± 1.8 | 2.7 ± 1.7 | 2.9 ± 1.7 | 2.2 ± 1.5 |
| 0 | 8238 (6.5%) | 1700 (4.0%) | 1382 (6.2%) | 1272 (5.5%) | 3884 (10.1%) |
| 1 | 25,590 (20.2%) | 6242 (14.6%) | 4537 (20.5%) | 4247 (18.5%) | 10,564 (27.4%) |
| 2 | 28,568 (22.6%) | 8498 (19.8%) | 5277 (23.8%) | 5122 (22.3%) | 9671 (25.1%) |
| ≥3 | 64,159 (50.7%) | 26,444 (61.6%) | 10,954 (49.5%) | 12,352 (53.7%) | 14,409 (37.4%) |
| Medication use | |||||
| OACs | |||||
| Warfarin | 26,185 (20.7%) | 8941 (20.9%) | 4542 (20.5%) | 4997 (21.7%) | 7705 (20.0%) |
| NOACs | 12,307 (9.7%) | 4523 (10.6%) | 2063 (9.3%) | 2348 (10.2%) | 3373 (8.8%) |
| Antiplatelet agent | |||||
| Aspirin | 26,752 (21.1%) | 9572 (22.3%) | 4508 (20.4%) | 4955 (21.6%) | 7717 (20.0%) |
| Clopidogrel | 8865 (7.0%) | 3277 (7.6%) | 1525 (6.9%) | 1717 (7.5%) | 2346 (6.1%) |
| Statin | 23,538 (18.6%) | 8466 (19.7%) | 4130 (18.7%) | 4367 (19.0%) | 6575 (17.1%) |
| Anthropometric measurements | |||||
| Weight (kg) | 65.3 ± 11.7 | 62.7 ± 11.5 | 65.3 ± 11.6 | 65.0 ± 11.5 | 68.3 ± 11.5 |
| Height (cm) | 162.8 ± 9.4 | 160.0 ± 9.5 | 162.8 ± 9.2 | 162.5 ± 9.2 | 166.1 ± 8.5 |
| Waist circumference (cm) | 84.6 ± 9.4 | 84.3 ± 10.3 | 84.4 ± 9.1 | 84.7 ± 9.0 | 84.8 ± 8.6 |
| BMI (kg/m2) | 24.6 ± 3.3 | 24.4 ± 3.5 | 24.6 ± 3.3 | 24.5 ± 3.3 | 24.7 ± 3.1 |
| Systolic BP (mmHg) | 125.7 ± 15.4 | 126.5 ± 16.0 | 125.6 ± 15.4 | 125.9 ± 15.7 | 124.9 ± 14.7 |
| Diastolic BP (mmHg) | 77.1 ± 10.3 | 77.1 ± 10.5 | 77.0 ± 10.2 | 77.0 ± 10.4 | 77.1 ± 10.1 |
| Laboratory Findings | |||||
| Fasting glucose (mg/dL) | 105.0 ± 27.3 | 105.4 ± 28.8 | 104.7 ± 27.1 | 105.5 ± 27.5 | 104.2 ± 25.6 |
| Triglyceride (mg/dL) | 132.9 ± 91.2 | 134.1 ± 88.5 | 132.6 ± 93.4 | 132.9 ± 88.9 | 131.7 ± 94.3 |
| Total cholesterol (mg/dL) | 81.0 ± 40.7 | 180.6 ± 40.5 | 181.4 ± 43.7 | 180.3 ± 41.4 | 181.8 ± 38.7 |
| HDL-cholesterol (mg/dL) | 52.2 ± 15.0 | 51.9 ± 14.6 | 52.3 ± 15.3 | 51.8 ± 16.2 | 52.7 ± 14.6 |
| LDL-cholesterol (mg/dL) | 103.2 ± 41.1 | 102.5 ± 38.9 | 103.6 ± 45.1 | 103.1 ± 45.7 | 103.7 ± 38.2 |
| AST (mg/dL) | 27.9 ± 19.0 | 27.8 ± 22.2 | 27.9 ± 16.7 | 27.9 ± 17.8 | 27.9 ± 16.9 |
| ALT (mg/dL) | 25.9 ± 20.8 | 24.9 ± 19.5 | 26.1 ± 19.4 | 25.8 ± 19.3 | 27.1 ± 23.7 |
| GGT (mg/dL) | 44.8 ± 60.7 | 43.3 ± 62.0 | 44.2 ± 58.8 | 45.3 ± 62.4 | 46.5 ± 59.4 |
| GFR (mL/min/1.73 m2) | 80.3 ± 28.1 | 78.7 ± 27.4 | 81.2 ± 29.7 | 79.9 ± 28.4 | 81.8 ± 27.7 |
| Lifestyle factors | |||||
| Alcohol | |||||
| Non-drinker | 84,398 (66.7%) | 32,752 (76.4%) | 14,644 (66.1%) | 16,271 (70.8%) | 20,731 (53.8%) |
| Mild-moderate drinker | 34,842 (27.5%) | 7986 (18.6%) | 6272 (28.3%) | 5446 (23.7%) | 15,138 (39.3%) |
| Heavy drinker | 7315 (5.8%) | 2146 (5.0%) | 1234 (5.6%) | 1276 (5.6%) | 2659 (6.9%) |
| Smoking | |||||
| Never smoker | 75,061 (59.3%) | 29,084 (67.8%) | 13,092 (59.1%) | 14,215 (61.8%) | 18,670 (48.5%) |
| Ex-smoker | 34,365 (27.2%) | 8311 (19.4%) | 5986 (27.0%) | 5764 (25.1%) | 14,304 (37.1%) |
| Current smoker | 17,129 (13.5%) | 5489 (12.8%) | 3072 (13.9%) | 3014 (13.1%) | 5554 (14.4%) |
| Low income | 21,262 (16.8%) | 7876 (18.4%) | 3759 (17.0%) | 4018 (17.5%) | 5609 (14.6%) |
| Follow-up duration | 3.1 ± 1.9 | 3.1 ± 1.9 | 3.2 ± 1.9 | 3.2 ± 1.9 | 3.2 ± 1.9 |
| Physical Activity | Primary Outcome | 1-Year Landmark Analysis a | 3-Year Landmark Analysis b |
|---|---|---|---|
| Overall Dementia | |||
| Persistent non-exerciser | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| Exercise starter | 0.87 (0.81–0.94) | 0.83 (0.76–0.91) | 0.80 (0.70–0.91) |
| Exercise quitter | 0.98 (0.92–1.05) | 0.96 (0.89–1.04) | 1.02 (0.91–1.15) |
| Exercise maintainer | 0.66 (0.61–0.72) | 0.66 (0.60–0.73) | 0.65 (0.56–0.74) |
| p-trend <0.001 | p-trend <0.001 | p-trend <0.001 | |
| Alzheimer’s Dementia | |||
| Persistent non-exerciser | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| Exercise starter | 0.88 (0.80–0.95) | 0.84 (0.76–0.93) | 0.80 (0.69–0.94) |
| Exercise quitter | 0.98 (0.91–1.06) | 0.96 (0.88–1.05) | 1.03 (0.90–1.18) |
| Exercise maintainer | 0.65 (0.59–0.72) | 0.65 (0.58–0.72) | 0.66 (0.56–0.77) |
| p-trend <0.001 | p-trend <0.001 | p-trend <0.001 | |
| Vascular Dementia | |||
| Persistent non-exerciser | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| Exercise starter | 0.87 (0.72–1.05) | 0.76 (0.61–0.95) | 0.61 (0.43–0.88) |
| Exercise quitter | 0.97 (0.82–1.15) | 0.93 (0.77–1.13) | 0.89 (0.66–1.20) |
| Exercise maintainer | 0.71 (0.59–0.86) | 0.70 (0.57–0.87) | 0.56 (0.40–0.79) |
| p-trend = 0.004 | p-trend = 0.004 | p-trend = 0.002 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, J.; Lee, S.-R.; Choi, E.-K.; Han, K.-D.; Jung, J.-H.; Ahn, H.-J.; Yun, J.P.; Kwon, S.; Oh, S.; Lip, G.Y.H. Exercise and the Risk of Dementia in Patients with Newly Diagnosed Atrial Fibrillation: A Nationwide Population-Based Study. J. Clin. Med. 2021, 10, 3126. https://doi.org/10.3390/jcm10143126
Lim J, Lee S-R, Choi E-K, Han K-D, Jung J-H, Ahn H-J, Yun JP, Kwon S, Oh S, Lip GYH. Exercise and the Risk of Dementia in Patients with Newly Diagnosed Atrial Fibrillation: A Nationwide Population-Based Study. Journal of Clinical Medicine. 2021; 10(14):3126. https://doi.org/10.3390/jcm10143126
Chicago/Turabian StyleLim, Jaehyun, So-Ryoung Lee, Eue-Keun Choi, Kyung-Do Han, Jin-Hyung Jung, Hyo-Jeong Ahn, Jun Pil Yun, Soonil Kwon, Seil Oh, and Gregory Y. H. Lip. 2021. "Exercise and the Risk of Dementia in Patients with Newly Diagnosed Atrial Fibrillation: A Nationwide Population-Based Study" Journal of Clinical Medicine 10, no. 14: 3126. https://doi.org/10.3390/jcm10143126








