Prognostic Value of Computed Tomographic Coronary Angiography for Long-Term Major Adverse Cardiac Events after Liver Transplantation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Routine Preoperative Cardiac Evaluation
2.3. Analysis of the CTCA Findings
2.4. Post-Transplant Follow-Up
2.5. Data Acquisition and Outcome Measures
2.6. Statistical Analyses
3. Results
3.1. Transplant Recipient’s Characteristics
3.2. Preoperative Evaluation Findings and Intraoperative Variables
3.3. CTCA Findings
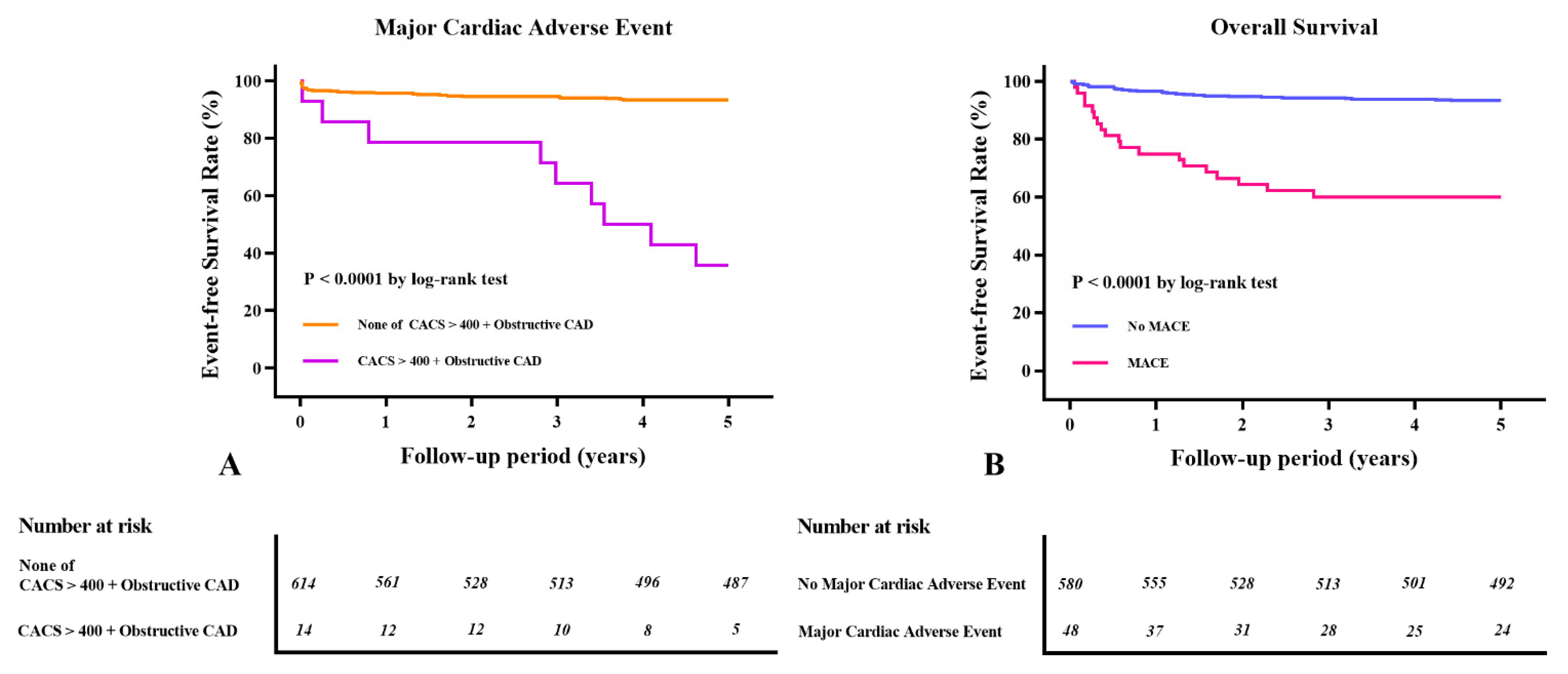
3.4. Long-Term Clinical Outcomes after LDLT According to the CTCA Findings and MACEs
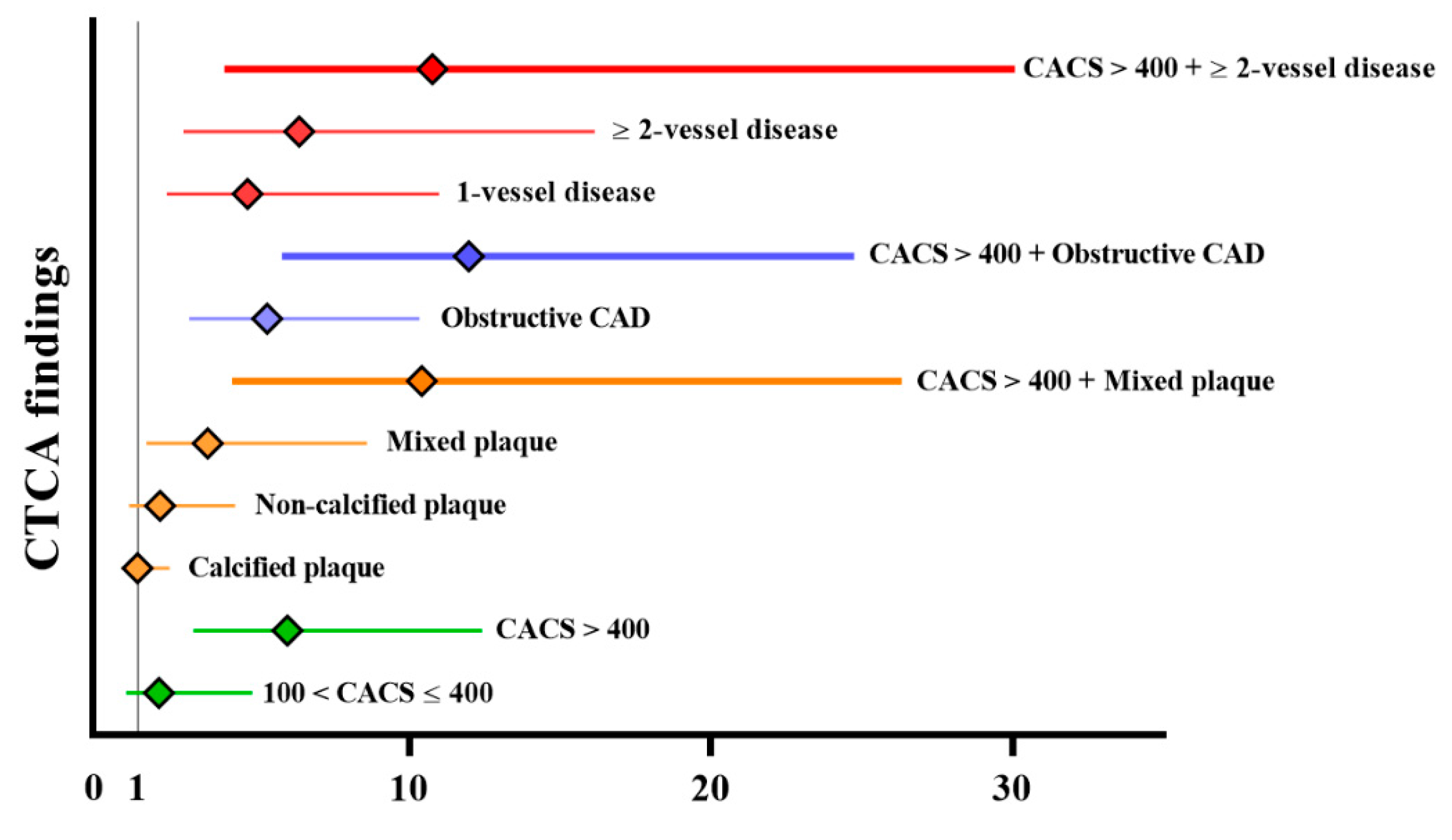
3.5. Prognostic Value of CTCA for Long-Term MACE
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- VanWagner, L.B.; Lapin, B.; Levitsky, J.; Wilkins, J.T.; Abecassis, M.M.; Skaro, A.I.; Lloyd-Jones, D.M. High early cardiovascular mortality after liver transplantation. Liver Transpl. 2014, 20, 1306–1316. [Google Scholar] [CrossRef]
- Kong, Y.G.; Kang, J.W.; Kim, Y.K.; Seo, H.; Lim, T.H.; Hwang, S.; Hwang, G.S.; Lee, S.G. Preoperative coronary calcium score is predictive of early postoperative cardiovascular complications in liver transplant recipients. Br. J. Anaesth. 2015, 114, 437–443. [Google Scholar] [CrossRef] [Green Version]
- Kong, Y.G.; Ha, T.Y.; Kang, J.W.; Hwang, S.; Lee, S.G.; Kim, Y.K. Incidence and predictors of increased coronary calcium scores in liver transplant recipients. Transplant Proc. 2015, 47, 1933–1938. [Google Scholar] [CrossRef]
- Fussner, L.A.; Heimbach, J.K.; Fan, C.; Dierkhising, R.; Coss, E.; Leise, M.D.; Watt, K.D. Cardiovascular disease after liver transplantation: When, what, and who is at risk. Liver. Transpl. 2015, 21, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Lentine, K.L.; Costa, S.P.; Weir, M.R.; Robb, J.F.; Fleisher, L.A.; Kasiske, B.L.; Carithers, R.L.; Ragosta, M.; Bolton, K.; Auerbach, A.D.; et al. Cardiac disease evaluation and management among kidney and liver transplantation candidates: A scientific statement from the American Heart Association and the American College of Cardiology Foundation. J. Am. Coll. Cardiol. 2012, 60, 434–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snipelisky, D.; Levy, M.; Shapiro, B. Utility of dobutamine stress echocardiography as part of the pre-liver transplant evaluation: An evaluation of its efficacy. Clin. Cardiol. 2014, 37, 468–472. [Google Scholar] [CrossRef]
- Mowatt, G.; Cook, J.A.; Hillis, G.S.; Walker, S.; Fraser, C.; Jia, X.; Waugh, N. 64-Slice computed tomography angiography in the diagnosis and assessment of coronary artery disease: Systematic review and meta-analysis. Heart 2008, 94, 1386–1393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.M.; Kong, Y.G.; Kang, J.W.; Kim, Y.K. Coronary computed tomography angiography in combination with coronary artery calcium scoring for the preoperative cardiac evaluation of liver transplant recipients. Biomed. Res. Int. 2017, 2017, 4081525. [Google Scholar] [CrossRef] [PubMed]
- McAvoy, N.C.; Kochar, N.; McKillop, G.; Newby, D.E.; Hayes, P.C. Prevalence of coronary artery calcification in patients undergoing assessment for orthotopic liver transplantation. Liver Transpl. 2008, 14, 1725–1731. [Google Scholar] [CrossRef]
- Kemmer, N.; Case, J.; Chandna, S.; Neff, G.W. The role of coronary calcium score in the risk assessment of liver transplant candidates. Transplant Proc. 2014, 46, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.J.; Kwon, H.M.; Jung, K.W.; Jeong, H.W.; Park, Y.S.; Jun, I.G.; Song, J.G.; Hwang, G.S. Risk stratification of myocardial injury after liver transplantation in patients with computed tomographic coronary angiography-diagnosed coronary artery disease. Am. J. Transplant. 2019, 19, 2053–2066. [Google Scholar] [CrossRef] [PubMed]
- Pohle, K.; Achenbach, S.; Macneill, B.; Ropers, D.; Ferencik, M.; Moselewski, F.; Hoffmann, U.; Brady, T.J.; Jang, I.K.; Daniel, W.G. Characterization of non-calcified coronary atherosclerotic plaque by multi-detector row CT: Comparison to IVUS. Atherosclerosis 2007, 190, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M., Jr.; Detrano, R. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef] [Green Version]
- Erbel, R.; Mohlenkamp, S.; Moebus, S.; Schmermund, A.; Lehmann, N.; Stang, A.; Dragano, N.; Gronemeyer, D.; Seibel, R.; Kalsch, H.; et al. Coronary risk stratification, discrimination, and reclassification improvement based on quantification of subclinical coronary atherosclerosis: The Heinz Nixdorf Recall study. J. Am. Coll. Cardiol. 2010, 56, 1397–1406. [Google Scholar] [CrossRef] [Green Version]
- Luca, L.; Westbrook, R.; Tsochatzis, E.A. Metabolic and cardiovascular complications in the liver transplant recipient. Ann. Gastroenterol. 2015, 28, 183–192. [Google Scholar]
- D’Avola, D.; Cuervas-Mons, V.; Martí, J.; Ortiz de Urbina, J.; Lladó, L.; Jimenez, C.; Otero, E.; Suarez, F.; Rodrigo, J.M.; Gómez, M.A.; et al. Cardiovascular morbidity and mortality after liver transplantation: The protective role of mycophenolate mofetil. Liver Transpl. 2017, 23, 498–509. [Google Scholar] [CrossRef]
- Vanwagner, L.B.; Bhave, M.; Te, H.S.; Feinglass, J.; Alvarez, L.; Rinella, M.E. Patients transplanted for nonalcoholic steatohepatitis are at increased risk for postoperative cardiovascular events. Hepatology 2012, 56, 1741–1750. [Google Scholar] [CrossRef]
- Austin, P.C.; Fine, J.P. Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat. Med. 2017, 36, 4391–4400. [Google Scholar] [CrossRef]
- Mandell, M.S.; Lindenfeld, J.; Tsou, M.Y.; Zimmerman, M. Cardiac evaluation of liver transplant candidates. World J. Gastroenterol. 2008, 14, 3445–3451. [Google Scholar] [CrossRef]
- Therapondos, G.; Flapan, A.D.; Plevris, J.N.; Hayes, P.C. Cardiac morbidity and mortality related to orthotopic liver transplantation. Liver Transpl. 2004, 10, 1441–1453. [Google Scholar] [CrossRef]
- An, J.; Shim, J.H.; Kim, S.O.; Lee, D.; Kim, K.M.; Lim, Y.S.; Lee, H.C.; Chung, Y.H.; Lee, Y.S. Prevalence and prediction of coronary artery disease in patients with liver cirrhosis: A registry-based matched case-control study. Circulation 2014, 130, 1353–1362. [Google Scholar] [CrossRef] [Green Version]
- Di Carli, M.F.; Blankstein, R. Low yield of routine preoperative coronary computed tomography angiography in patients evaluated for liver transplantation. Circulation 2014, 130, 1337–1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaw, L.J.; Raggi, P.; Schisterman, E.; Berman, D.S.; Callister, T.Q. Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. Radiology 2003, 228, 826–833. [Google Scholar] [CrossRef]
- Pletcher, M.J.; Tice, J.A.; Pignone, M.; Browner, W.S. Using the coronary artery calcium score to predict coronary heart disease events: A systematic review and meta-analysis. Arch. Intern. Med. 2004, 164, 1285–1292. [Google Scholar] [CrossRef]
- Rosário, M.A.; Lima, J.J.; Parga, J.R.; Avila, L.F.; Gowdak, L.H.; Lemos, P.A.; Rochitte, C.E. Coronary calcium score as predictor of stenosis and events in pretransplant renal chronic failure. Arq. Bras. Cardiol. 2010, 94, 236–243. [Google Scholar] [PubMed] [Green Version]
- Jeevarethinam, A.; Venuraju, S.; Dumo, A.; Ruano, S.; Atwal, S.; Mehta, V.S.; Rakhit, R.; Lahiri, A. Relationship between coronary artery calcification (CAC) and carotid atherosclerosis in asymptomatic diabetes: A prospective study. Heart 2014, 100, A87. [Google Scholar] [CrossRef]
- Michael, J.; Martin, B.; Sina, K.; Rajesh, T.-M.; Miguel, C.-A. Coronary artery calcium scoring: Is it time for a change in methodology? JACC Cardiovasc. Imaging 2017, 10, 923–937. [Google Scholar]
- Abdulla, J.; Abildstrom, S.Z.; Gotzsche, O.; Christensen, E.; Kober, L.; Torp-Pedersen, C. 64-multislice detector computed tomography coronary angiography as potential alternative to conventional coronary angiography: A systematic review and meta-analysis. Eur. Heart J. 2007, 28, 3042–3050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassagneau, P.; Jacquier, A.; Giorgi, R.; Amabile, N.; Gaubert, J.Y.; Cohen, F.; Muller, C.; Jolibert, M.; Louis, G.; Varoquaux, A.; et al. Prognostic value of preoperative coronary computed tomography angiography in patients treated by orthotopic liver transplantation. Eur. J. Gastroenterol. Hepatol. 2012, 24, 558–562. [Google Scholar] [CrossRef]
- Steinkohl, F.; Barbieri, F.; Senoner, T.; Strobl, S.; Finkenstedt, A.; Plank, F.; Langer, C.; Beyer, C.; Birkl, K.; Widmann, G.; et al. Coronary atherosclerosis profile in patients with end-stage liver disease prior to liver transplantation due to alcoholic fatty liver: A coronary CTA study. Eur. Radiol. 2021, 31, 494–503. [Google Scholar] [CrossRef]
- Hou, Z.H.; Lu, B.; Gao, Y.; Jiang, S.L.; Wang, Y.; Li, W.; Budoff, M.J. Prognostic value of coronary CT angiography and calcium score for major adverse cardiac events in outpatients. JACC Cardiovasc. Imaging 2012, 5, 990–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, S.W.; Kim, Y.J.; Shim, J.; Sung, J.M.; Han, M.E.; Kang, D.W.; Kim, J.Y.; Choi, B.W.; Chang, H.J. Coronary artery calcium scoring does not add prognostic value to standard 64-section CT angiography protocol in low-risk patients suspected of having coronary artery disease. Radiology 2011, 259, 92–99. [Google Scholar] [CrossRef] [Green Version]
- Jodocy, D.; Abbrederis, S.; Graziadei, I.W.; Vogel, W.; Pachinger, O.; Feuchtner, G.M.; Jaschke, W.; Friedrich, G. Coronary computer tomographic angiography for preoperative risk stratification in patients undergoing liver transplantation. Eur. J. Radiol. 2012, 81, 2260–2264. [Google Scholar] [CrossRef]
- Trevor, W.R.; Helena, K.; Tomohiro, T.; Paul, D.G.; Ian, D.M.; Mark, S.C.; Eberhard, L.R.; Markus, S.; Anand, G.; Gary, L.; et al. Living donor versus deceased donor liver transplantation: A surgeon-matched comparison of recipient morbidity and outcomes. Transpl. Int. 2013, 26, 780–787. [Google Scholar]
- Hogan, B.J.; Gonsalkorala, E.; Heneghan, M.A. Evaluation of coronary artery disease in potential liver transplant recipients. Liver Transpl. 2017, 23, 386–395. [Google Scholar] [CrossRef] [Green Version]
- Davidson, C.J.; Gheorghiade, M.; Flaherty, J.D.; Elliot, M.D.; Reddy, S.P.; Wang, N.C.; Sundaram, S.A.; Flamm, S.L.; Blei, A.T.; Abecassis, M.I.; et al. Predictive value of stress myocardial perfusion imaging in liver transplant candidates. Am. J. Cardiol. 2002, 89, 359–360. [Google Scholar] [CrossRef]
- Haywood, S.; Abecassis, M.; Levitsky, J. The renal benefit of mycophenolate mofetil after liver transplantation. Clin. Transplant. 2011, 25, E88–E95. [Google Scholar] [CrossRef]
- Wray, C.; Scovotti, J.C.; Tobis, J.; Niemann, C.U.; Planinsic, R.; Walia, A.; Findlay, J.; Wagener, G.; Cywinski, J.B.; Markovic, D.; et al. Liver transplantation outcome in patients with angiographically proven coronary artery disease: A multi-institutional study. Am. J. Transplant. 2013, 13, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Reyes, J.; Kashyap, R.; Dodson, S.F.; Demetris, A.J.; Ruppert, K.; Abu-Elmagd, K.; Marsh, W.; Madariaga, J.; Mazariegos, G.; et al. Long-term survival after liver transplantation in 4,000 consecutive patients at a single center. Ann. Surg. 2000, 232, 490–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- VanWagner, L.B.; Serper, M.; Kang, R.; Levitsky, J.; Hohmann, S.; Abecassis, M.; Skaro, A.; Lloyd-Jones, D.M. Factors associated with major adverse cardiovascular events after liver transplantation among a national sample. Am. J. Transplant. 2016, 16, 2684–2694. [Google Scholar] [CrossRef]
- Rabkin, J.M.; Corless, C.L.; Rosen, H.R.; Olyaei, A.J. Immunosuppression impact on long-term cardiovascular complications after liver transplantation. Am. J. Surg. 2002, 183, 595–599. [Google Scholar] [CrossRef]

| Variables | Total (n = 628) | Non-MACE (n = 580) | MACE (n = 48) | p Value |
|---|---|---|---|---|
| Age (years) | 53.0 (8.0) | 52.0 (9.0) | 54.0 (8.5) | 0.006 |
| Sex (male) | 502 (79.9) | 462 (79.7) | 40 (83.3) | 0.672 |
| Body mass index (kg/m2) | 23.5 (4.5) | 23.5 (4.5) | 22.6 (4.4) | 0.205 |
| AHA/ACCF risk factors for CAD | ||||
| Age > 60 years | 74 (11.8) | 64 (11.0) | 10 (20.8) | 0.073 |
| Diabetes mellitus | 144 (22.9) | 123 (21.2) | 21 (43.8) | 0.001 |
| Hypertension | 81 (12.9) | 69 (11.9) | 12 (25.0) | 0.017 |
| History of cardiovascular disease | 0 (0) | 0 (0) | 0 (0) | |
| Dyslipidemia | 307 (52.9) | 344 (54.8) | 37 (77.1) | 0.002 |
| Current smoker | 118 (18.8) | 109 (18.8) | 9 (18.8) | 0.999 |
| Left-ventricular hypertrophy | 63 (10.0) | 59 (10.2) | 4 (9.3) | 0.875 |
| Preoperative medication | ||||
| ACE inhibitor | 31 (4.9) | 27 (4.7) | 4 (8.3) | 0.433 |
| Beta-blocker | 160 (25.5) | 144 (24.8) | 16 (33.3) | 0.260 |
| Diuretics | 246 (39.2) | 222 (38.3) | 24 (50.0) | 0.148 |
| Statin | 11 (1.8) | 8 (1.4) | 3 (6.2) | 0.057 |
| Alcohol history | 171 (27.2) | 152 (26.2) | 19 (38.8) | 0.122 |
| Underlying liver diseases | 0.596 | |||
| Hepatitis B virus | 431 (68.6) | 399 (68.8) | 32 (66.7) | |
| Hepatitis C virus | 51 (8.1) | 45 (7.8) | 6 (12.5) | |
| Non-B non-C hepatitis | 19 (3.0) | 18 (3.1) | 1 (2.1) | |
| Alcoholic liver disease | 75 (11.9) | 68 (11.7) | 7 (14.6) | |
| Others | 52 (8.3) | 50 (8.6) | 2 (4.2) | |
| Child–Turcotte–Pugh score | 8.0 (4.0) | 8.0 (3.0) | 8.5 (3.0) | 0.045 |
| Model for end-stage liver disease score | 12.0 (8.0) | 12.0 (8.0) | 14.5 (10.5) | 0.025 |
| Variables | Total (n = 628) | Non-MACE (n = 580) | MACE (n = 48) | p Value |
|---|---|---|---|---|
| Hemoglobin (g/dL) | 10.9 (3.4) | 11.0 (3.4) | 9.9 (3.5) | 0.021 |
| Platelet (103/mm) | 58.0 (46.0) | 58.0 (46.5) | 61.0 (45) | 0.277 |
| Prothrombin time (INR) | 1.4 (0.4) | 1.4 (0.4) | 1.4 (0.4) | 0.510 |
| Albumin (g/dL) | 3.2 (0.9) | 3.2 (0.9) | 3.0 (0.9) | 0.186 |
| Creatinine (mg/dL) | 0.7 (0.3) | 0.7 (0.3) | 0.9 (0.8) | <0.001 |
| Total cholesterol (mg/dL) | 117.5 (59.5) | 120.0 (58.0) | 99.5 (69.5) | 0.023 |
| HDL cholesterol (mg/dL) | 39.0 (24.0) | 40.0 (25.0) | 32.0 (20.0) | 0.004 |
| LDL cholesterol (mg/dL) | 64.0 (42.0) | 65.0 (41.0) | 48.0 (53.5) | 0.044 |
| Triglyceride (mg/dL) | 61.0 (38.0) | 60.0 (39.0) | 65.0 (34.0) | 0.223 |
| Echocardiography | 0.315 | |||
| Valve diseases | 23 (3.7) | 21 (3.6) | 2 (4.2) | |
| Pulmonary hypertension | 9 (1.4) | 8 (1.4) | 1 (2.1) | |
| Other abnormalities | 6 (0.9) | 4 (0.9) | 2 (4.2) | |
| QTc (ms) | 446.0 (40.0) | 446.0 (39.0) | 449.5 (49.0) | 0.305 |
| Abnormal thallium SPECT | 6 (1.0) | 5 (0.9) | 1 (2.5) | 0.881 |
| Variables | Total (n = 628) | Non-MACE (n = 580) | MACE (n = 48) | p Value |
|---|---|---|---|---|
| Surgical time (min) | 787.0 (135.0) | 787.0 (135) | 786.0 (137.5) | 0.698 |
| RBC transfusion (unit) | 7.0 (11.0) | 7.0 (11.0) | 12.0 (10.5) | 0.002 |
| FFP transfusion (unit) | 10.0 (12.0) | 9.0 (11.0) | 12.0 (14.5) | 0.003 |
| Cryoprecipitate transfusion (unit) | 10.0 (10.0) | 10.0 (10.0) | 10.0 (17.5) | 0.728 |
| Apheresis platelet transfusion (unit) | 1.0 (2.0) | 1.0 (2.0) | 1.0 (2.0) | 0.243 |
| Graft volume (g) | 730.0 (180.0) | 730.0 (175) | 750.0 (202.5) | 0.411 |
| Graft-recipient weight ratio (%) | 1.1 (0.4) | 1.1 (0.4) | 1.1 (0.3) | 0.369 |
| Ischemic time (min) | 124.0 (37.0) | 123.5 (35.5) | 130.0 (47.0) | 0.460 |
| Variables | Total (n = 628) | Non-MACE (n = 580) | MACE (n = 48) | p Value |
|---|---|---|---|---|
| Coronary artery calcium score | <0.001 | |||
| None−mild (≤100) | 563 (89.6) | 528 (91.0) | 35 (72.9) | |
| Moderate (101−400) | 39 (6.2) | 35 (6.0) | 4 (8.3) | |
| Severe (>400) | 26 (4.1) | 17 (2.9) | 9 (18.8) | |
| Coronary artery plaque | 0.040 | |||
| Nonplaque | 403 (64.2) | 376 (64.8) | 27 (56.2) | |
| Calcified plaque | 150 (23.9) | 140 (24.1) | 10 (20.8) | |
| Noncalcified plaque | 23 (3.7) | 18 (3.1) | 5 (10.4) | |
| Mixed plaque | 52 (8.3) | 46 (7.9) | 6 (12.5) | |
| Coronary artery stenosis | <0.001 | |||
| Nonobstructive (<50%) | 592 (94.3) | 555 (95.7) | 37 (77.1) | |
| Obstructive (≥50%) | 36 (5.8) | 25 (4.3) | 11 (22.9) | |
| Coronary artery diseases | <0.001 | |||
| Noncoronary artery disease | 592 (94.3) | 555 (95.7) | 37 (77.1) | |
| 1-vessel disease | 21 (3.5) | 15 (2.6) | 6 (12.5) | |
| 2-vessel diseases | 14 (2.1) | 10 (1.7) | 4 (8.3) | |
| 3-vessel diseases | 1 (0.2) | 0 (0.0) | 1 (2.1) |
| Variables | Cox Proportional Hazards Model | Fine and Gray’s Subdistribution Hazards Model | ||||||
|---|---|---|---|---|---|---|---|---|
| Crude HR (95% CI) | p Value | Multivariable HR(95% CI) | p Value | Crude sHR (95% CI) | p Value | Multivariable sHR (95% CI) | p Value | |
| Age | 1.07 (1.02–1.12) | 0.002 | 1.05 (1.01–1.10) | 0.018 | 1.07 (1.03–1.11) | 0.001 | 1.05 (1.01–1.10) | 0.018 |
| Diabetes mellitus | 2.72 (1.54–4.81) | 0.001 | 2.22 (1.24–3.99) | 0.007 | 2.74 (1.55–4.84) | <0.001 | 2.43 (1.37–4.29) | 0.002 |
| Hypertension | 2.27 (1.18–4.36) | 0.014 | 2.29 (1.21–4.33) | 0.011 | ||||
| Dyslipidemia | 2.90 (1.48–5.69) | 0.002 | 2.27 (1.14–4.51) | 0.019 | 2.92 (1.52–5.70) | 0.003 | 2.45 (1.23–4.70) | 0.023 |
| Statin therapy | 4.03 (1.25–12.97) | 0.019 | 3.94 (1.28–12.08) | 0.017 | ||||
| Diuretics | 0.80 (0.60–1.06) | 0.112 | 1.58 (0.90–2.78) | 0.112 | ||||
| MELD score | 1.05 (1.01–1.08) | 0.006 | 1.05 (1.01–1.08) | 0.008 | ||||
| CTP score | 1.12 (0.99–1.26) | 0.071 | 1.11 (1.00–1.24) | 0.052 | ||||
| Hemoglobin | 0.86 (0.76–0.99) | 0.029 | 0.86 (0.74–1.00) | 0.054 | ||||
| Albumin | 0.70 (0.44–1.13) | 0.151 | 0.71 (0.43–1.18) | 0.189 | ||||
| Creatinine | 1.26 (1.14–1.39) | <0.001 | 1.20 (1.06–1.36) | 0.003 | 1.25 (1.12–1.41) | <0.001 | 1.19 (1.08–1.3) | <0.001 |
| Total cholesterol | 1.01 (0.99–1.01) | 0.034 | 0.99 (0.99–1.00) | 0.058 | ||||
| HDL cholesterol | 0.98 (0.97–0.99) | 0.016 | 0.98 (0.96–0.99) | 0.009 | ||||
| LDL cholesterol | 0.99 (0.98–1.00) | 0.067 | 0.99 (0.98–1.00) | 0.072 | ||||
| RBC transfusion | 1.02 (1.01–1.03) | 0.017 | 1.02 (1.00–1.03) | 0.005 | ||||
| FFP transfusion | 1.02 (1.01–1.03) | 0.031 | 1.01 (1.00–1.03) | 0.009 | ||||
| Abnormal echocardiography | 1.85 (0.73–4.67) | 0.194 | 1.90 (0.76–4.74) | 0.172 | ||||
| CACS > 400 + obstructive CAD | 11.97 (5.79–24.75) | <0.001 | 5.02 (2.25–11.21) | <0.001 | 11.70 (6.42~21.32) | <0.001 | 5.01 (2.37–10.58) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.-H.; Kim, Y.-K.; Ha, T.-Y.; Hwang, S.; Kim, W.; Koo, H.-J.; Yang, D.-H.; Kang, J.-W.; Lee, S.-G. Prognostic Value of Computed Tomographic Coronary Angiography for Long-Term Major Adverse Cardiac Events after Liver Transplantation. J. Clin. Med. 2021, 10, 3132. https://doi.org/10.3390/jcm10143132
Kim D-H, Kim Y-K, Ha T-Y, Hwang S, Kim W, Koo H-J, Yang D-H, Kang J-W, Lee S-G. Prognostic Value of Computed Tomographic Coronary Angiography for Long-Term Major Adverse Cardiac Events after Liver Transplantation. Journal of Clinical Medicine. 2021; 10(14):3132. https://doi.org/10.3390/jcm10143132
Chicago/Turabian StyleKim, Doo-Hwan, Young-Kug Kim, Tae-Yong Ha, Shin Hwang, Wooil Kim, Hyun-Jung Koo, Dong-Hyun Yang, Joon-Won Kang, and Sung-Gyu Lee. 2021. "Prognostic Value of Computed Tomographic Coronary Angiography for Long-Term Major Adverse Cardiac Events after Liver Transplantation" Journal of Clinical Medicine 10, no. 14: 3132. https://doi.org/10.3390/jcm10143132
APA StyleKim, D.-H., Kim, Y.-K., Ha, T.-Y., Hwang, S., Kim, W., Koo, H.-J., Yang, D.-H., Kang, J.-W., & Lee, S.-G. (2021). Prognostic Value of Computed Tomographic Coronary Angiography for Long-Term Major Adverse Cardiac Events after Liver Transplantation. Journal of Clinical Medicine, 10(14), 3132. https://doi.org/10.3390/jcm10143132







