Pharmacological Effects of Methotrexate and Infliximab in a Rats Model of Diet-Induced Dyslipidemia and Beta-3 Overexpression on Endothelial Cells
Abstract
1. Introduction
2. Materials and Method
2.1. Animals, Substances and Experimental Design
2.2. Biochemical Analysis
2.3. Determination of Inflammation Markers
2.4. Determination of Oxidative Stress Markers
2.5. Organ Index of the Liver, Kidney and Myocardium
2.6. Histopathological Analysis of Liver, Heart and Aorta
2.7. Statistical Analysis
3. Results
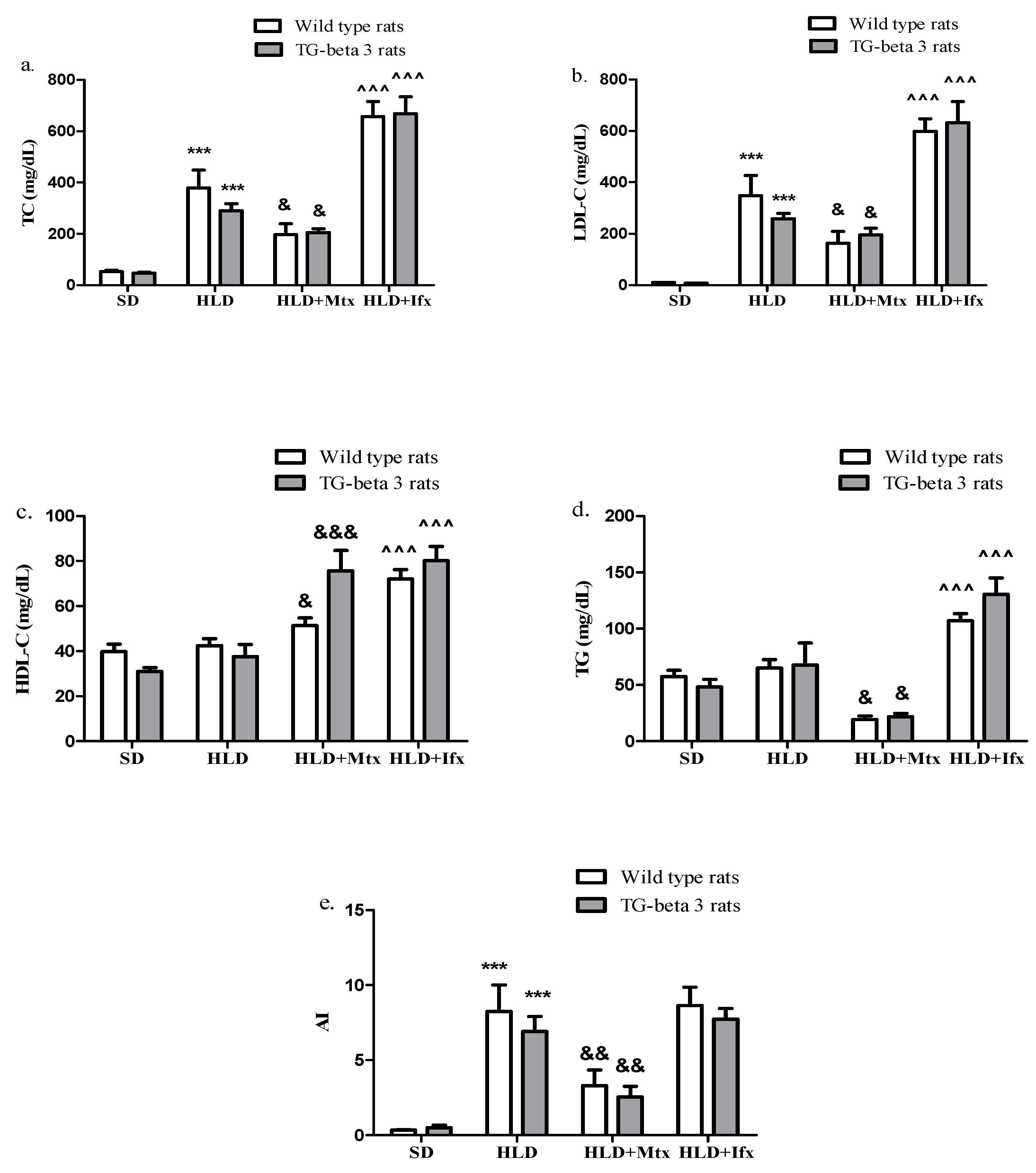
3.1. Effects on Plasma Lipid Levels
3.2. Inflammation Markers
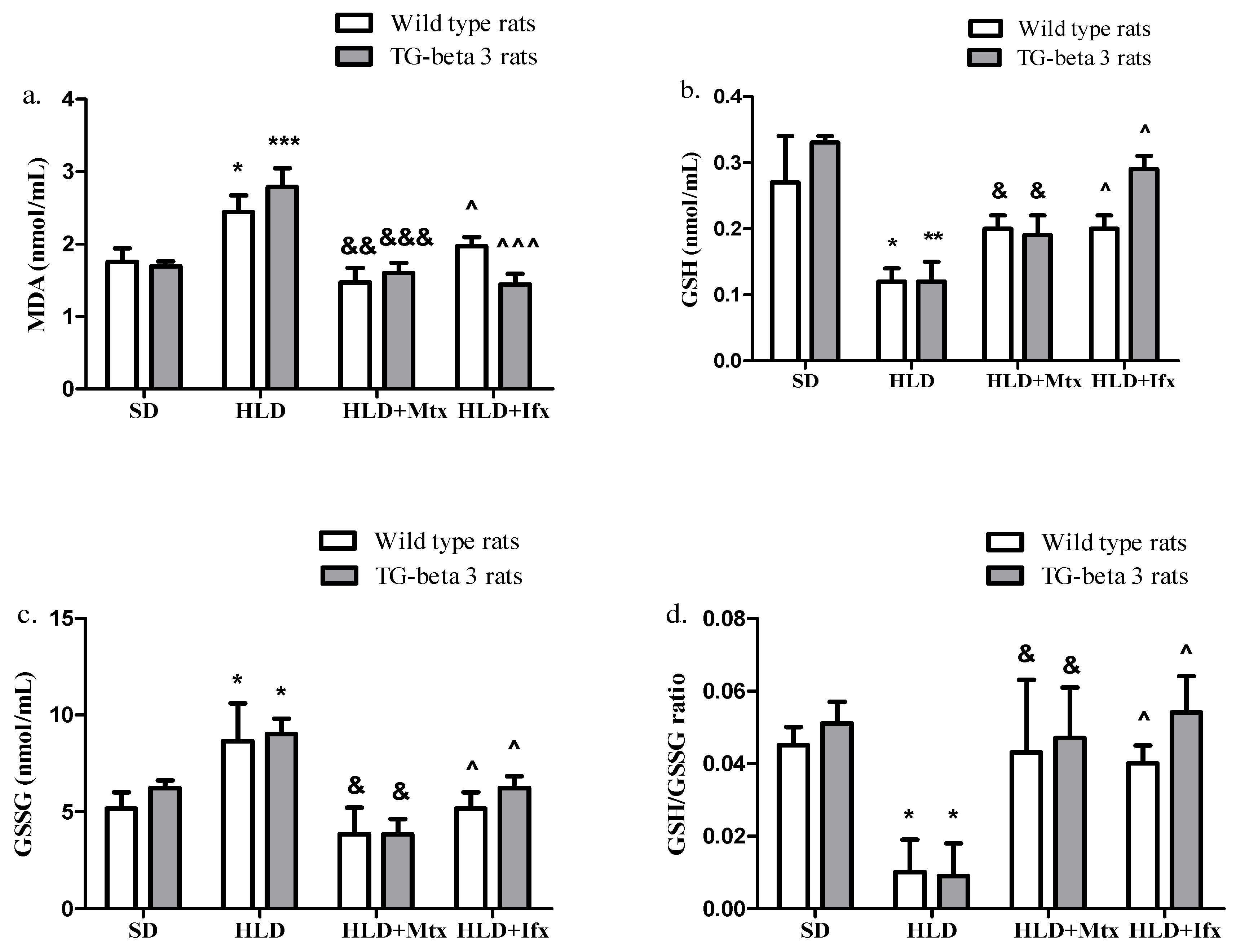
3.3. Oxidative Stress Markers
3.4. Effects on Liver Function Enzymes
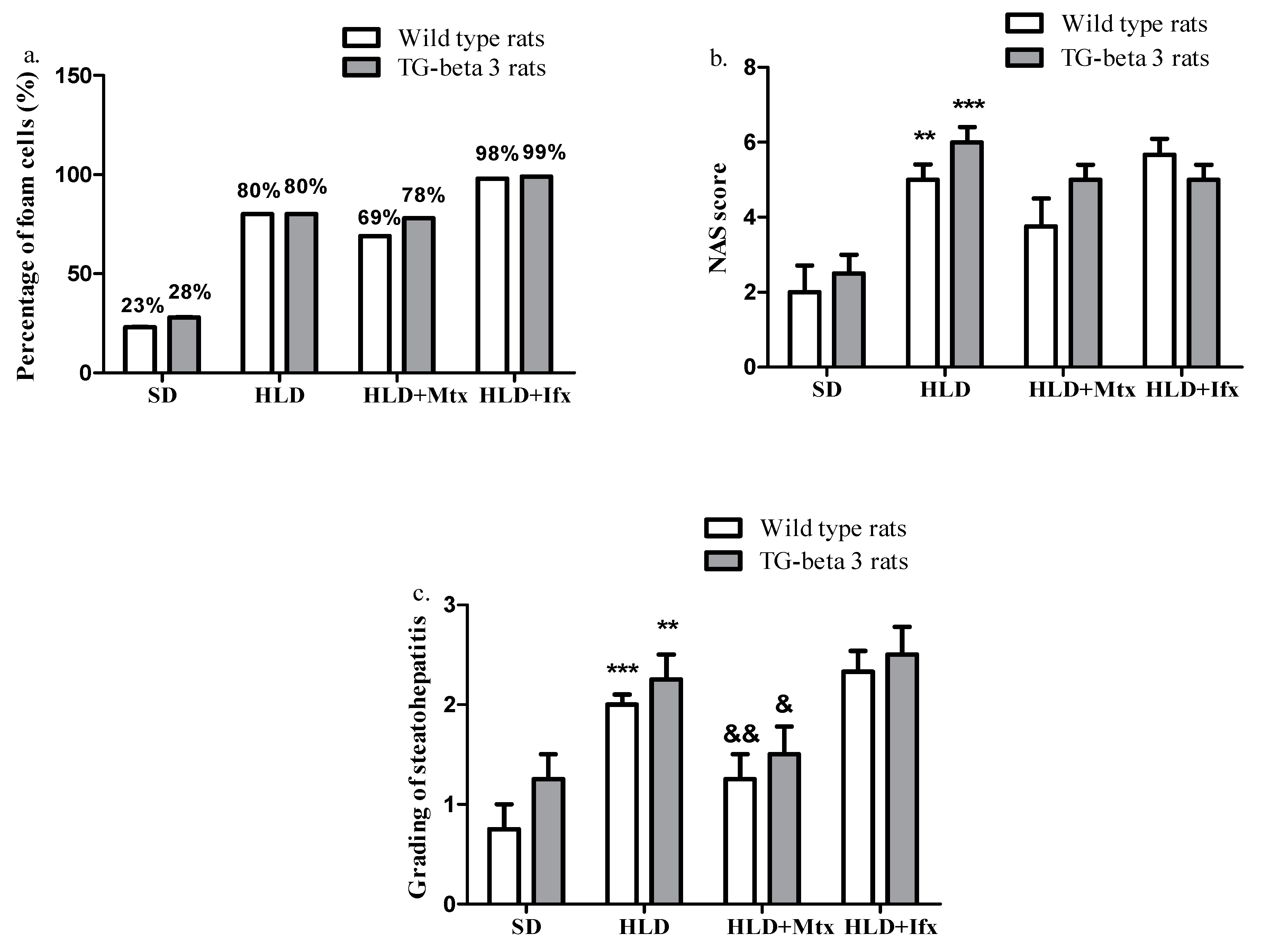
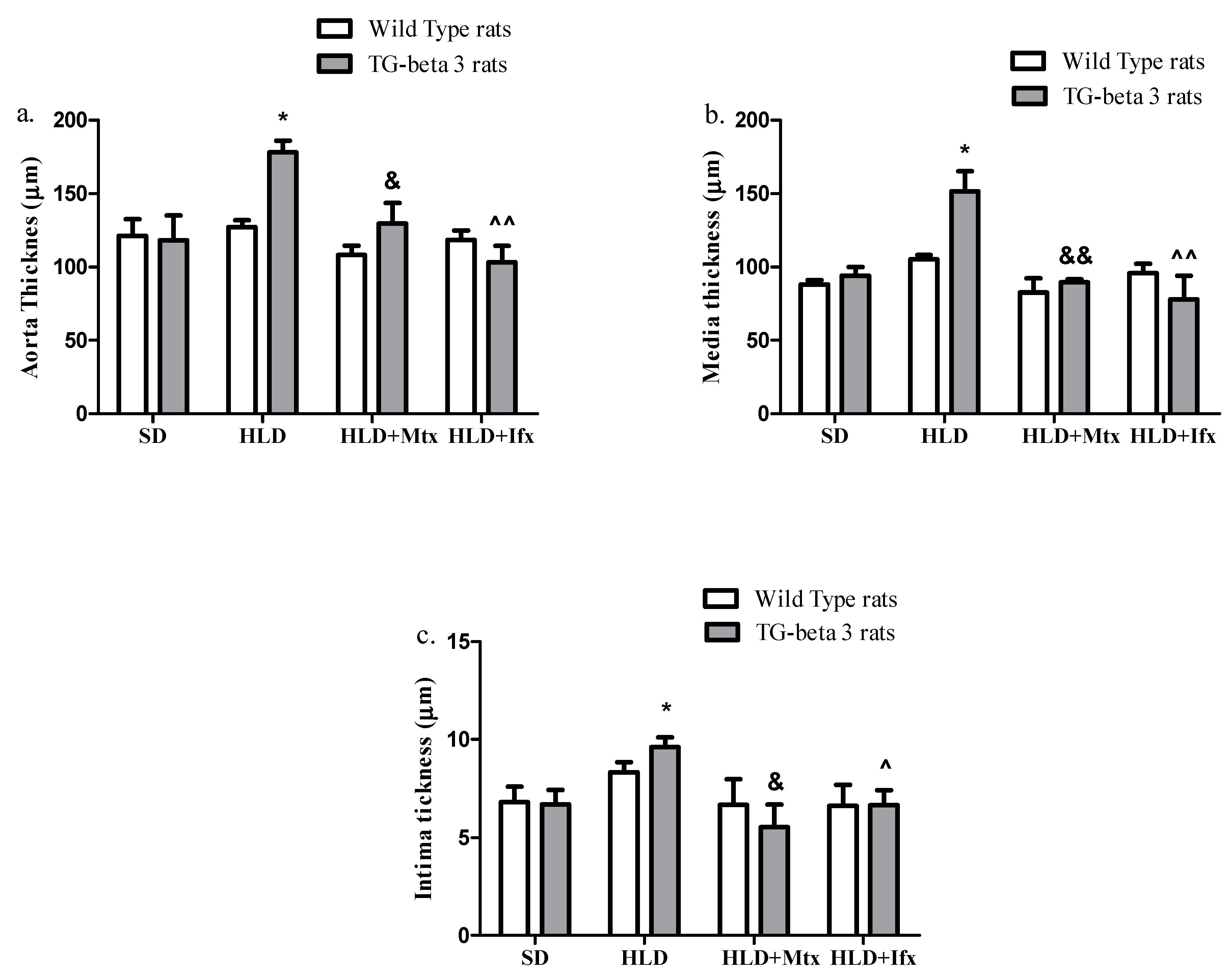
3.5. Histopathological Analysis
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart disease and stroke statistics—2019 update: A report from the American heart association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef] [PubMed]
- Sanjadi, M.; Sichanie, Z.R.; Totonchi, H.; Karami, J.; Rezaei, R.; Aslani, S. Atherosclerosis and autoimmunity: A growing relationship. Int. J. Rheum. Dis. 2018, 21, 908–921. [Google Scholar] [CrossRef]
- Soiza, R.L.; Donaldson, A.I.C.; Myint, P.K. Vaccine against arteriosclerosis: An update. Ther. Adv. Vaccines 2018, 9, 259–261. [Google Scholar]
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart disease and stroke statistics—2017 update a report from the American heart association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef]
- Wolf, D.; Ley, K. Immunity and Inflammation in Atherosclerosis. Circ. Res. 2019, 124, 315–327. [Google Scholar] [CrossRef]
- Taleb, S. L’inflammation dans l’athérosclérose. Arch. Cardiovasc. Dis. 2016, 109, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, W.; Li, X.; Zhou, H. Inflammation: A Novel Therapeutic Target/Direction in Atherosclerosis. Curr. Pharm. Des. 2017, 23, 1216–1227. [Google Scholar] [CrossRef] [PubMed]
- Jose, T.; Magnus, B.; Lina, B.; Marie-Luce, B.P.; Bertrand, C.; Mat, J.D.; Jesus, E.; Paul, C.E.; Sheila, E.F.; Daniel, F.K.; et al. Interplay between dyslipidemia and inflammation in atherosclerosis:Translating experimental targets into clinical practice. Eur. J. Prev. Cardiol. 2018, 25, 948–955. [Google Scholar]
- Zhu, Y.; Xian, X.; Wang, Z.; Bi, Y.; Chen, Q.; Han, X.; Tang, D.; Chen, R. Research Progress on the Relationship between Atherosclerosis and Inflammation. Biomolecules 2018, 8, 80. [Google Scholar] [CrossRef]
- Badimon, L.; Peña, E.; Arderiu, G.; Padró, T.; Slevin, M.; Vilahur, G.; Chiva-Blanch, G. C-reactive protein in atherothrombosis and angiogenesis. Front. Immunol. 2018, 9, 430. [Google Scholar] [CrossRef]
- Emerging Risk Factors Collaboration. C-Reactive Protein, Fibrinogen, and Cardiovascular Disease Prediction. N. Engl. J. Med. 2012, 367, 1310–1320. [Google Scholar] [CrossRef]
- Ridker, P.M.; Lüscher, T.F. Anti-inflammatory therapies for cardiovascular disease. Eur. Heart J. 2014, 35, 1782–1791. [Google Scholar] [CrossRef]
- Frostegård, J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. 2013, 11, 117. [Google Scholar] [CrossRef] [PubMed]
- Everett, B.M.; Pradhan, A.D.; Solomon, D.H.; Paynter, N.; MacFadyen, J.; Zaharris, E.; Gupta, M.; Clearfield, M.; Libby, P.; Hasan, A.A.; et al. Rationale and design of the Cardiovascular Inflammation Reduction Trial: A test of the inflammatory hypothesis of atherothrombosis. Am. Heart J. 2013, 166, 199–207.e15. [Google Scholar] [CrossRef] [PubMed]
- Golia, E.; Limongelli, G.; Natale, F.; Fimiani, F.; Maddaloni, V.; Pariggiano, I.; Bianchi, R.; Crisci, M.; D’Acierno, L.; Giordano, R.; et al. Inflammation and Cardiovascular Disease: From Pathogenesis to Therapeutic Target. Curr. Atheroscler. Rep. 2014, 16, 435. [Google Scholar] [CrossRef] [PubMed]
- Micha, R.; Imamura, F.; von Ballmoos, M.W.; Solomon, D.H.; Hernán, M.; Ridker, P.M.; Mozaffarian, D. Systematic Review and Meta-Analysis of Methotrexate Use and Risk of Cardiovascular Disease. Am. J. Cardiol. 2011, 108, 1362–1370. [Google Scholar] [CrossRef] [PubMed]
- Pincus, T.; Ferraccioli, G.; Sokka, T.; Larsen, A.; Rau, R.; Kushner, I.; Wolfe, F. Evidence from clinical trials and long-term observational studies that disease-modifying anti-rheumatic drugs slow radiographic progression in rheumatoid arthritis: Updating a 1983 review. Rheumatology 2002, 41, 1346–1356. [Google Scholar] [CrossRef][Green Version]
- Bernatsky, S.; Hudson, M.; Suissa, S. Anti-rheumatic drug use and risk of hospitalization for congestive heart failure in rheumatoid arthritis. Rheumatology 2005, 44, 677–680. [Google Scholar] [CrossRef][Green Version]
- Krishnan, E.; Lingala, V.B.; Singh, G. Declines in Mortality from Acute Myocardial Infarction in Successive Incidence and Birth Cohorts of Patients With Rheumatoid Arthritis. Circulation 2004, 110, 1774–1779. [Google Scholar] [CrossRef]
- Ridker, P.M. Targeting inflammatory pathways for the treatment of cardiovascular disease. Eur. Heart J. 2013, 35, 540–543. [Google Scholar] [CrossRef]
- Priyadharsini, R.P. Animal models to evaluate anti-atherosclerotic drugs. Fundam. Clin. Pharmacol. 2015, 29, 329–340. [Google Scholar] [CrossRef]
- Dhot, J.; Ferron, M.; Prat, V.; Persello, A.; Roul, D.; Stévant, D.; Guijarro, D.; Piriou, N.; Aillerie, V.; Erraud, A.; et al. Overexpression of endothelial β 3 -adrenergic receptor induces diastolic dysfunction in rats. ESC Heart Fail. 2020, 7, 4159–4171. [Google Scholar] [CrossRef]
- Institute of Laboratory Animal Resources (US); Committee on Care; Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th ed.; US Department of Health and Human Services, Public Health Service, National Institutes of Health: Bethesda, MD, USA, 2011.
- Gou, S.; Huang, H.-F.; Chen, X.-Y.; Liu, J.; He, M.; Ma, Y.-Y.; Zhao, X.-N.; Zhang, Y.; Ni, J.-M. Lipid-lowering, hepatoprotective, and atheroprotective effects of the mixture Hong-Qu and gypenosides in hyperlipidemia with NAFLD rats. J. Chin. Med. Assoc. 2016, 79, 111–121. [Google Scholar] [CrossRef]
- Gou, S.; Liu, B.-J.; Han, X.-F.; Wang, L.; Zhong, C.; Liang, S.; Liu, H.; Qiang, Y.; Zhang, Y.; Ni, J.-M. Anti-atherosclerotic effect of Fermentum Rubrum and Gynostemma pentaphyllum mixture in high-fat emulsion- and vitamin D3-induced atherosclerotic rats. J. Chin. Med. Assoc. 2018, 81, 398–408. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, H.; Lv, Y.; Wang, Z.; Xiao, W. Anti-atherosclerotic effect of Longxuetongluo Capsule in high cholesterol diet induced atherosclerosis model rats. Biomed. Pharmacother. 2018, 97, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Talbert, R.L. Pharmacotherapy a Pathophysiologic Approach, 10th ed.; McGraw-Hill Medical: New York, NY, USA, 2017; p. 271. [Google Scholar]
- Leong, X.F.; Ng, C.Y.; Jaarin, K. Animal Models in Cardiovascular Research: Hypertension and Atherosclerosis. BioMed Res. Int. 2015, 2015, 528757. [Google Scholar] [CrossRef]
- Ibrahim, A.Y.; Hendawy, S.; El-Sayed, A.; Omer, E.A. Evaluation of hypolipidemic Marrubium vulgare effect in Triton WR-1339-induced hyperlipidemia in mice. Asian Pac. J. Trop. Med. 2016, 9, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Park, S.-H.; Wang, F.; Zhou, C. Perinatal Bisphenol A Exposure Increases Atherosclerosis in Adult Male PXR-Humanized Mice. Endocrinology 2018, 159, 1595–1608. [Google Scholar] [CrossRef]
- Zălar, D.-M.; Pop, C.; Buzdugan, E.; Todea, D.; Mogoșan, C.I. The Atherosclerosis-Inflammation Relationship—A Pathophysiological approach. Farmacia 2019, 67, 6. [Google Scholar] [CrossRef]
- Ilhan, F. Atherosclerosis and the role of immune cells. World J. Clin. Cases 2015, 3, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Salwe, K.; Kumarappan, M. Evaluation of Antioxidant, Hypolipidemic, and Antiatherogenic Property of Lycopene and Astaxanthin in Atherosclerosis-induced Rats. Pharmacogn. Res. 2017, 9, 161–167. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E. Effect of High-Fat Diets on Oxidative Stress, Cellular Inflammatory Response and Cognitive Function. Nutrients 2019, 11, 2579. [Google Scholar] [CrossRef]
- Doostan, F.; Vafafar, R.; Zakeri-Milani, P.; Pouri, A.; Afshar, R.A.; Abbasi, M.M. Effects of Pomegranate (Punica granatum L.) Seed and Peel Methanolic Extracts on Oxidative Stress and Lipid Profile Changes Induced by Methotrexate in Rats. Adv. Pharm. Bull. 2017, 7, 269–274. [Google Scholar] [CrossRef]
- Gerards, A.H.; De Lathouder, S.; De Groot, E.R.; Dijkmans, B.A.C.; Aarden, L.A. Inhibition of cytokine production by methotrexate. Studies in healthy volunteers and patients with rheumatoid arthritis. Rheumatology 2003, 42, 1189–1196. [Google Scholar] [CrossRef]
- Wessels, J.A.M.; Huizinga, T.W.J.; Guchelaar, H.-J. Recent insights in the pharmacological actions of methotrexate in the treatment of rheumatoid arthritis. Rheumatology 2007, 47, 249–255. [Google Scholar] [CrossRef]
- Westlake, S.L.; Colebatch, A.N.; Baird, J.; Kiely, P.; Quinn, M.; Choy, E.; Ostor, A.J.K.; Edwards, C.J. The effect of methotrexate on cardiovascular disease in patients with rheumatoid arthritis: A systematic literature review. Rheumatology 2009, 49, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Rho, Y.H.; Oeser, A.; Chung, C.P.; Milne, G.L.; Stein, C.M. Drugs used in the treatment of rheumatoid arthritis: Relationship between current use and cardiovascular risk factors. Arch. Drug Inf. 2009, 2, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Naranjo, A.; The QUEST-RA Group; Sokka, T.; Descalzo, M.A.; Calvo-Alén, J.; Hørslev-Petersen, K.; Luukkainen, R.K.; Combe, B.; Burmester, G.R.; Devlin, J.; et al. Cardiovascular disease in patients with rheumatoid arthritis: Results from the QUEST-RA study. Arthritis Res. Ther. 2008, 10, R30. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Okada, Y.; Nawata, M.; Saito, K.; Tanaka, Y. Induction of hyperadiponectinemia following long-term treatment of patients with rheumatoid arthritis with infliximab (IFX), an anti-TNF-alpha antibody. Endocr. J. 2008, 55, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Skoog, T.; Dichtl, W.; Boquist, S.; Skoglund-Andersson, C.; Karpe, F.; Tang, R.; Bond, M.; De Faire, U.; Nilsson, J.; Eriksson, P.; et al. Plasma tumour necrosis factor-α and early carotid atherosclerosis in healthy middle-aged men. Eur. Heart J. 2002, 23, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Popa, C.; Hoogen, F.H.J.V.D.; Radstake, T.R.D.J.; Netea, M.G.; Eijsbouts, A.E.; Heijer, M.D.; Van Der Meer, J.W.M.; Van Riel, P.L.C.M.; Stalenhoef, A.F.H.; Barrera, P. Modulation of lipoprotein plasma concentrations during long-term anti-TNF therapy in patients with active rheumatoid arthritis. Ann. Rheum. Dis. 2007, 66, 1503–1507. [Google Scholar] [CrossRef]
- Popa, C.; Netea, M.G.; van Riel, P.L.C.M.; van der Meer, J.W.M.; Stalenhoef, A.F.H. The role of TNF-α in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk. J. Lipid Res. 2007, 48, 751–762. [Google Scholar] [CrossRef]
- Feingold, K.R.; Grunfeld, C. Tumor necrosis factor-alpha stimulates hepatic lipogenesis in the rat in vivo. J. Clin. Investig. 1987, 80, 184–190. [Google Scholar] [CrossRef]
- Foltz, I.N.; Karow, M.; Wasserman, S.M. Evolution and emergence of therapeutic monoclonal antibodies what cardiologists need to know. Circulation 2013, 127, 2222–2230. [Google Scholar] [CrossRef] [PubMed]
- Vis, M.; Nurmohamed, M.T.; Wolbink, G.; Voskuyl, A.E.; de Koning, M.; van de Stadt, R.; Twisk, J.W.; Dijkmans, B.A.; Lems, W.F. Short term effects of infliximab on the lipid profile in patients with rheumatoid arthritis. J. Rheumatol. 2005, 32, 252–255. [Google Scholar]
- Allanore, Y.; Kahan, A.; Sellam, J.; Ekindjian, O.G.; Borderie, D. Effects of repeated infliximab therapy on serum lipid profile in patients with refractory rheumatoid arthritis. Clin. Chim. Acta 2006, 365, 143–148. [Google Scholar] [CrossRef]
- Peters, M.J.L.; Vis, M.; Van Halm, V.; Wolbink, G.J.; Voskuyl, A.E.; Lems, W.; Dijkmans, B.; Twisk, J.; De Koning, M.H.M.T.; Van De Stadt, R.; et al. Changes in lipid profile during infliximab and corticosteroid treatment in rheumatoid arthritis. Ann. Rheum. Dis. 2007, 66, 958–961. [Google Scholar] [CrossRef]
- Hürlimann, D.; Forster, A.; Noll, G.; Enseleit, F.; Chenevard, R.; Distler, O.; Béchir, M.; Spieker, L.E.; Neidhart, M.; Michel, B.A.; et al. Anti—Tumor Necrosis Factor-α Treatment Improves Endothelial Function in Patients With Rheumatoid Arthritis. Circulation 2002, 106, 2184–2187. [Google Scholar] [CrossRef]
- Zhang, Q.; Qian, Z.-Y.; Zhou, P.-H.; Zhou, X.-L.; Zhang, D.-L.; He, N.; Zhang, J.; Liu, Y.-H.; Gu, Q. Effects of oral selenium and magnesium co-supplementation on lipid metabolism, antioxidative status, histopathological lesions, and related gene expression in rats fed a high-fat diet. Lipids Health Dis. 2018, 17, 165. [Google Scholar] [CrossRef]
- Singh, U.N.; Kumar, S.; Dhakal, S. Study of Oxidative Stress in Hypercholesterolemia. Int. J. Contemp. Med. Res. 2017, 4, 2454–7379. [Google Scholar]
- Piechota-Polanczyk, A.; Fichna, J. Review article: The role of oxidative stress in pathogenesis and treatment of inflammatory bowel diseases. Naunyn Schmiedeberg’s Arch. Pharmacol. 2014, 387, 605–620. [Google Scholar] [CrossRef]
- Kim, M.-H.; Lee, E.-J.; Cheon, J.-M.; Nam, K.-J.; Oh, T.-H.; Kim, K.-S. Antioxidant and hepatoprotective effects of fermented red ginseng against high fat diet-induced hyperlipidemia in rats. Lab. Anim. Res. 2016, 32, 217. [Google Scholar] [CrossRef]
- Zalewska, A.; Ziembicka, D.; Żendzian-Piotrowska, M.; Maciejczyk, M. The Impact of High-Fat Diet on Mitochondrial Function, Free Radical Production, and Nitrosative Stress in the Salivary Glands of Wistar Rats. Oxid. Med. Cell Longev. 2019, 2019, 2606120. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.-L.; Shi, Y.-H.; Hao, G.; Li, W.; Le, G.-W. Increasing Oxidative Stress with Progressive Hyperlipidemia in Human: Relation between Malondialdehyde and Atherogenic Index. J. Clin. Biochem. Nutr. 2008, 43, 154–158. [Google Scholar] [CrossRef]
- Kageyama, Y.; Takahashi, M.; Ichikawa, T.; Torikai, E.; Nagano, A. Reduction of oxidative stress marker levels by anti-TNF-α antibody, infliximab, in patients with rheumatoid arthritis. Clin. Exp. Rheumatol. 2008, 26, 73–80. [Google Scholar]
- Dogru, A.; Nazıroglu, M.; Cig, B. Modulator role of infliximab and methotrexate through the transient receptor potential melastatin 2 (TRPM2) channel in neutrophils of patients with rheumatoid arthritis: A pilot study. Arch. Med. Sci. 2019, 15, 1415–1424. [Google Scholar] [CrossRef]
- Zimmerman, M.C.; Clemens, D.L.; Duryee, M.; Sarmiento, C.; Chiou, A.; Hunter, C.D.; Tian, J.; Klassen, L.W.; O’Dell, J.R.; Thiele, G.M.; et al. Direct antioxidant properties of methotrexate: Inhibition of malondialdehyde-acetaldehyde-protein adduct formation and superoxide scavenging. Redox Biol. 2017, 13, 588–593. [Google Scholar] [CrossRef]
- Smith, C.J.; Ryckman, K.K.; Vanessa, M.B.; Barbara, H.; Carmen, R.I.; Gloria, S.; Tom, S.E.; Van Horn, L.V.; Wallace, R.B.; Robinson, J.G. The impact of birth weight on cardiovascular disease risk in the Women’s Health Initiative. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 239–245. [Google Scholar] [CrossRef]
- Kim, E.J.; Kim, B.-H.; Seo, H.S.; Lee, Y.J.; Kim, H.H.; Son, H.-H.; Choi, M.H. Cholesterol-Induced Non-Alcoholic Fatty Liver Disease and Atherosclerosis Aggravated by Systemic Inflammation. PLoS ONE 2014, 9, e97841. [Google Scholar] [CrossRef]
- Saiki, O.; Takao, R.; Naruse, Y.; Kuhara, M.; Imai, S.; Uda, H. Infliximab but not methotrexate induces extra-high levels of VLDL-triglyceride in patients with rheumatoid arthritis. J. Rheumatol. 2007, 34, 1997–2004. [Google Scholar]
- Di Micco, P.; Ferrazzi, P.; Librè, L.; Mendolicchio, L.; Quaglia, I.; De Marco, M.; Colombo, A.; Bacci, M.; Rota, L.L.; Lodigiani, C. Intima-media thickness evolution after treatment with infliximab in patients with rheumatoid arthritis. Int. J. Gen. Med. 2009, 2, 141–144. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zălar, D.-M.; Pop, C.; Buzdugan, E.; Kiss, B.; Ştefan, M.-G.; Ghibu, S.; Bâlteanu, V.-A.; Crişan, D.; Buruiană-Simic, A.; Grozav, A.; et al. Pharmacological Effects of Methotrexate and Infliximab in a Rats Model of Diet-Induced Dyslipidemia and Beta-3 Overexpression on Endothelial Cells. J. Clin. Med. 2021, 10, 3143. https://doi.org/10.3390/jcm10143143
Zălar D-M, Pop C, Buzdugan E, Kiss B, Ştefan M-G, Ghibu S, Bâlteanu V-A, Crişan D, Buruiană-Simic A, Grozav A, et al. Pharmacological Effects of Methotrexate and Infliximab in a Rats Model of Diet-Induced Dyslipidemia and Beta-3 Overexpression on Endothelial Cells. Journal of Clinical Medicine. 2021; 10(14):3143. https://doi.org/10.3390/jcm10143143
Chicago/Turabian StyleZălar, Denisa-Mădălina, Cristina Pop, Elena Buzdugan, Bela Kiss, Maria-Georgia Ştefan, Steliana Ghibu, Valentin-Adrian Bâlteanu, Doiniţa Crişan, Alexandra Buruiană-Simic, Adriana Grozav, and et al. 2021. "Pharmacological Effects of Methotrexate and Infliximab in a Rats Model of Diet-Induced Dyslipidemia and Beta-3 Overexpression on Endothelial Cells" Journal of Clinical Medicine 10, no. 14: 3143. https://doi.org/10.3390/jcm10143143
APA StyleZălar, D.-M., Pop, C., Buzdugan, E., Kiss, B., Ştefan, M.-G., Ghibu, S., Bâlteanu, V.-A., Crişan, D., Buruiană-Simic, A., Grozav, A., & Mogoșan, C. I. (2021). Pharmacological Effects of Methotrexate and Infliximab in a Rats Model of Diet-Induced Dyslipidemia and Beta-3 Overexpression on Endothelial Cells. Journal of Clinical Medicine, 10(14), 3143. https://doi.org/10.3390/jcm10143143





