Increased Plasma Levels of Myosin Heavy Chain 11 Is Associated with Atherosclerosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Clinical and Biochemical Analysis
2.3. Tissue Staining and Immunohistochemistry
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Patients with Atherosclerosis
3.2. Myosin-11 Plasma Levels Were Upregulated in Patients with Atherosclerosis
3.3. Circulating Myosin-11 Levels in Patients with CAD or PAD
3.4. Circulating Myosin-11 Levels and Clinical Parameters
3.5. The Effects of Renal Function on Circulating Myosin-11 Levels
3.6. Efficacy of Myosin-11 for Diagnosis
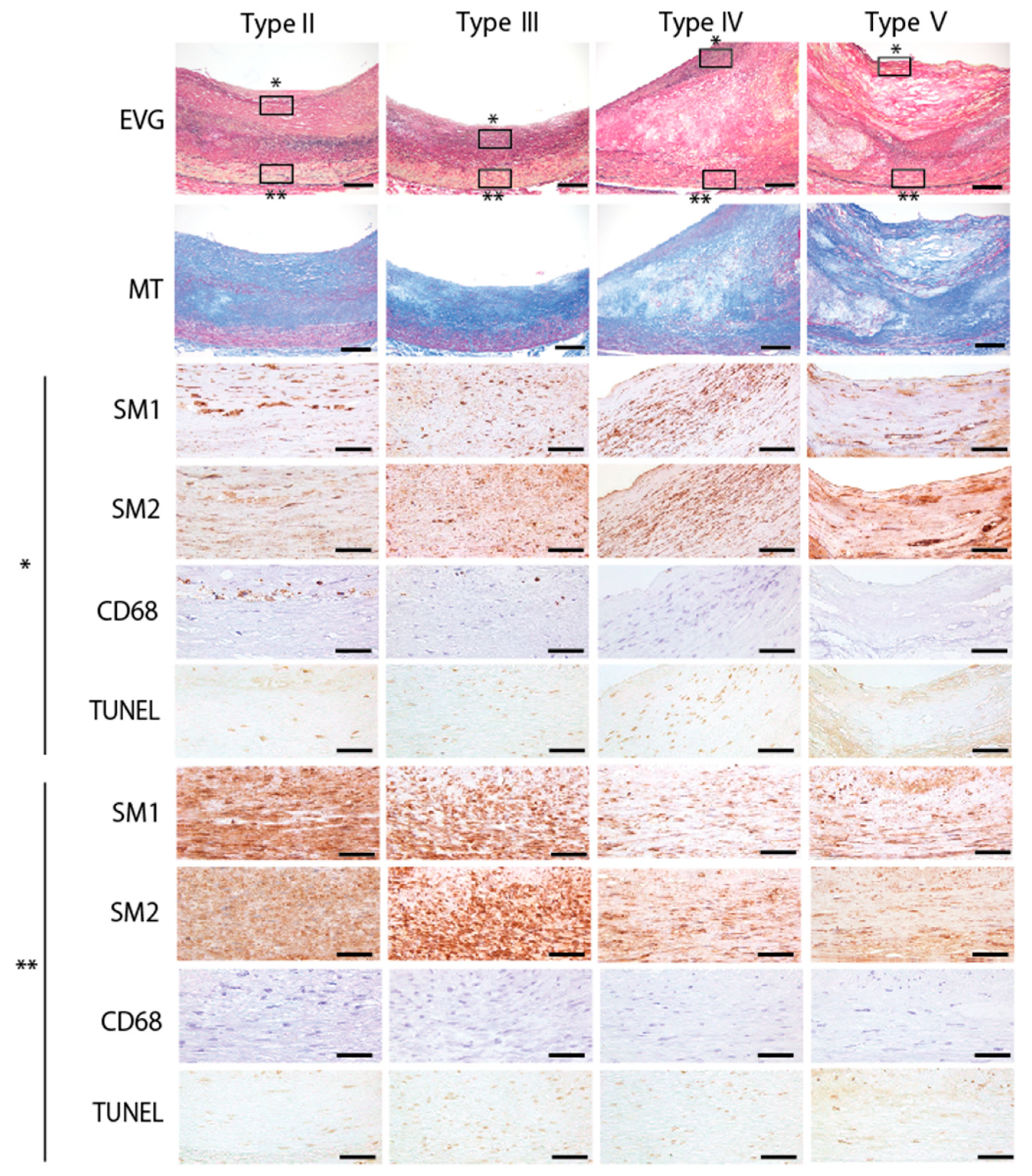
3.7. Expression of Myosin-11 Isoforms in the Coronary Arteries
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Basatemur, G.L.; Jørgensen, H.F.; Clarke, M.C.H.; Bennett, M.R.; Mallat, Z. Vascular smooth muscle cells in atherosclerosis. Nat. Rev. Cardiol. 2019, 16, 727–744. [Google Scholar] [CrossRef]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef]
- Gisterå, A.; Hansson, G.K. The immunology of atherosclerosis. Nat. Rev. Nephrol. 2017, 13, 368–380. [Google Scholar] [CrossRef]
- Davies, M.J.; Richardson, P.D.; Woolf, N.; Katz, D.R.; Mann, J. Risk of thrombosis in human atherosclerotic plaques: Role of extracellular lipid, macrophage, and smooth muscle cell content. Heart 1993, 69, 377–381. [Google Scholar] [CrossRef]
- Hoefer, I.E.; Steffens, S.; Ala-Korpela, M.; Back, M.; Badimon, L.; Bochaton-Piallat, M.L.; Boulanger, C.M.; Caligiuri, G.; Dimmeler, S.; Egido, J.; et al. Novel methodologies for biomarker discovery in atherosclerosis. Eur. Heart J. 2015, 36, 2635–2642. [Google Scholar] [CrossRef] [PubMed]
- da Silva, P.M.; Duarte, J.S.; von Hafe, P.; Gil, V.; de Oliveira, J.N.; de Sousa, G. Standardization of laboratory and lipid profile evaluation: A call for action with a special focus in 2016 ESC/EAS dyslipidaemia guidelines—Full report. Atheroscler. Suppl. 2018, 31, e1–e12. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.K. Biomarkers of plaque instability. Curr. Cardiol. Rep. 2014, 16, 547. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, V.; Jamthikar, A.D.; Gupta, D.; Shanu, N.; Puvvula, A.; Khanna, N.N.; Saba, L.; Omerzum, T.; Viskovic, K.; Mavrogeni, S.; et al. Low-cost preventive screening using carotid ultrasound in patients with diabetes. Front. Biosci. 2020, 25, 1132–1171. [Google Scholar]
- Eslava-Alcon, S.; Extremera-García, M.J.; González-Rovira, A.; Rosal-Vela, A.; Rojas-Torres, M.; Beltran-Camacho, L.; Sanchez-Gomar, I.; Jiménez-Palomares, M.; Alonso-Piñero, J.A.; Conejero, R.; et al. Molecular signatures of atherosclerotic plaques: An up-dated panel of protein related markers. J. Proteom. 2020, 221, 103757. [Google Scholar] [CrossRef]
- Schwartz, S.M.; Virmani, R.; Rosenfeld, M.E. The good smooth muscle cells in atherosclerosis. Curr. Atheroscler. Rep. 2000, 2, 422–429. [Google Scholar] [CrossRef]
- Shankman, L.S.; Gomez, D.; Cherepanova, O.A.; Salmon, M.; Alencar, G.F.; Haskins, R.M.; Swiatlowska, P.; Newman, A.A.; Greene, E.S.; Straub, A.C.; et al. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat. Med. 2015, 21, 628–637. [Google Scholar] [CrossRef]
- Feil, S.; Fehrenbacher, B.; Lukowski, R.; Essmann, F.; Schulze-Osthoff, K.; Schaller, M.; Feil, R. Transdifferentiation of vascular smooth muscle cells to macrophage-like cells during atherogenesis. Circ. Res. 2014, 115, 662–667. [Google Scholar] [CrossRef]
- Chappell, J.; Harman, J.L.; Narasimhan, V.M.; Yu, H.; Foote, K.; Simons, B.D.; Bennett, M.R.; Jorgensen, H.F. Extensive Proliferation of a Subset of Differentiated, yet Plastic, Medial Vascular Smooth Muscle Cells Contributes to Neointimal Formation in Mouse Injury and Atherosclerosis Models. Circ. Res. 2016, 119, 1313–1323. [Google Scholar] [CrossRef]
- Jacobsen, K.; Lund, M.B.; Shim, J.; Gunnersen, S.; Fuchtbauer, E.M.; Kjolby, M.; Carramolino, L.; Bentzon, J.F. Diverse cellular architecture of atherosclerotic plaque derives from clonal expansion of a few medial SMCs. JCI Insight 2017, 2. [Google Scholar] [CrossRef] [PubMed]
- Dobnikar, L.; Taylor, A.L.; Chappell, J.; Oldach, P.; Harman, J.L.; Oerton, E.; Dzierzak, E.; Bennett, M.R.; Spivakov, M.; Jorgensen, H.F. Disease-relevant transcriptional signatures identified in individual smooth muscle cells from healthy mouse vessels. Nat. Commun. 2018, 9, 4567. [Google Scholar] [CrossRef]
- Kockx, M.M.; De Meyer, G.R.; Muhring, J.; Jacob, W.; Bult, H.; Herman, A.G. Apoptosis and related proteins in different stages of human atherosclerotic plaques. Circulation 1998, 97, 2307–2315. [Google Scholar] [CrossRef] [PubMed]
- Okura, Y.; Brink, M.; Itabe, H.; Scheidegger, K.J.; Kalangos, A.; Delafontaine, P. Oxidized low-density lipoprotein is associated with apoptosis of vascular smooth muscle cells in human atherosclerotic plaques. Circulation 2000, 102, 2680–2686. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.C.; Figg, N.; Maguire, J.J.; Davenport, A.P.; Goddard, M.; Littlewood, T.D.; Bennett, M.R. Apoptosis of vascular smooth muscle cells induces features of plaque vulnerability in atherosclerosis. Nat. Med. 2006, 12, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.C.; Littlewood, T.D.; Figg, N.; Maguire, J.J.; Davenport, A.P.; Goddard, M.; Bennett, M.R. Chronic apoptosis of vascular smooth muscle cells accelerates atherosclerosis and promotes calcification and medial degeneration. Circ. Res. 2008, 102, 1529–1538. [Google Scholar] [CrossRef]
- Hartman, M.A.; Spudich, J.A. The myosin superfamily at a glance. J. Cell Sci. 2012, 125, 1627–1632. [Google Scholar] [CrossRef]
- Myronovkij, S.; Negrych, N.; Nehrych, T.; Redowicz, M.J.; Souchelnytskyi, S.; Stoika, R.; Kit, Y. Identification of a 48 kDa form of unconventional myosin 1c in blood serum of patients with autoimmune diseases. Biochem. Biophys. Rep. 2016, 5, 175–179. [Google Scholar] [CrossRef][Green Version]
- Seguin, J.R.; Saussine, M.; Ferriere, M.; Leger, J.J.; Leger, J.; Larue, C.; Calzolari, C.; Grolleau, R.; Chaptal, P.A. Myosin: A highly sensitive indicator of myocardial necrosis after cardiac operations. J. Thorac. Cardiovasc. Surg. 1989, 98, 397–401. [Google Scholar] [CrossRef]
- Astorri, E.; Fiorina, P.; Gavaruzzi, G.; Contini, G.A.; Fesani, F. Perioperative myocardial cell damage assessed by immunoradiometric assay of beta-myosin heavy chain serum levels in patients undergoing coronary bypass surgery. Int. J. Cardiol. 1996, 55, 157–162. [Google Scholar] [CrossRef]
- Onuoha, G.N.; Alpar, E.K.; Laprade, M.; Rama, D.; Pau, B. Levels of myosin heavy chain fragment in patients with tissue damage. Arch. Med Res. 2001, 32, 27–29. [Google Scholar] [CrossRef]
- Nagai, R.; Kuro-o, M.; Babij, P.; Periasamy, M. Identification of two types of smooth muscle myosin heavy chain isoforms by cDNA cloning and immunoblot analysis. J. Biol. Chem. 1989, 264, 9734–9737. [Google Scholar] [CrossRef]
- Yokoyama, U.; Arakawa, N.; Ishiwata, R.; Yasuda, S.; Minami, T.; Goda, M.; Uchida, K.; Suzuki, S.; Matsumoto, M.; Koizumi, N.; et al. Proteomic analysis of aortic smooth muscle cell secretions reveals an association of myosin heavy chain 11 with abdominal aortic aneurysm. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1012–H1018. [Google Scholar] [CrossRef]
- Koenig, W.; Khuseyinova, N. Biomarkers of atherosclerotic plaque instability and rupture. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 15–26. [Google Scholar] [CrossRef]
- Emerging Risk Factors, C.; Kaptoge, S.; Di Angelantonio, E.; Pennells, L.; Wood, A.M.; White, I.R.; Gao, P.; Walker, M.; Thompson, A.; Sarwar, N.; et al. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N. Engl. J. Med. 2012, 367, 1310–1320. [Google Scholar] [CrossRef] [PubMed]
- Leguillette, R.; Gil, F.R.; Zitouni, N.; Lajoie-Kadoch, S.; Sobieszek, A.; Lauzon, A.M. (+)Insert smooth muscle myosin heavy chain (SM-B) isoform expression in human tissues. Am. J. Physiol. Cell Physiol. 2005, 289, C1277–C1285. [Google Scholar] [CrossRef][Green Version]
- Stary, H.C. Natural history and histological classification of atherosclerotic lesions: An update. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1177–1178. [Google Scholar] [CrossRef]
- Aikawa, M.; Sivam, P.N.; Kuro-o, M.; Kimura, K.; Nakahara, K.; Takewaki, S.; Ueda, M.; Yamaguchi, H.; Yazaki, Y.; Periasamy, M.; et al. Human smooth muscle myosin heavy chain isoforms as molecular markers for vascular development and atherosclerosis. Circ. Res. 1993, 73, 1000–1012. [Google Scholar] [CrossRef] [PubMed]
- Lutgens, E.; de Muinck, E.D.; Kitslaar, P.J.; Tordoir, J.H.; Wellens, H.J.; Daemen, M.J. Biphasic pattern of cell turnover characterizes the progression from fatty streaks to ruptured human atherosclerotic plaques. Cardiovasc. Res. 1999, 41, 473–479. [Google Scholar] [CrossRef]
- Blankenberg, S.; Zeller, T.; Saarela, O.; Havulinna, A.S.; Kee, F.; Tunstall-Pedoe, H.; Kuulasmaa, K.; Yarnell, J.; Schnabel, R.B.; Wild, P.S.; et al. Contribution of 30 biomarkers to 10-year cardiovascular risk estimation in 2 population cohorts: The MONICA, risk, genetics, archiving, and monograph (MORGAM) biomarker project. Circulation 2010, 121, 2388–2397. [Google Scholar] [CrossRef]
- Churov, A.; Summerhill, V.; Grechko, A.; Orekhova, V.; Orekhov, A. MicroRNAs as Potential Biomarkers in Atherosclerosis. Int. J. Mol. Sci. 2019, 20, 5547. [Google Scholar] [CrossRef]
- Owens, G.K.; Kumar, M.S.; Wamhoff, B.R. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol. Rev. 2004, 84, 767–801. [Google Scholar] [CrossRef]
- Misra, A.; Feng, Z.; Chandran, R.R.; Kabir, I.; Rotllan, N.; Aryal, B.; Sheikh, A.Q.; Ding, L.; Qin, L.; Fernandez-Hernando, C.; et al. Integrin beta3 regulates clonality and fate of smooth muscle-derived atherosclerotic plaque cells. Nat. Commun. 2018, 9, 2073. [Google Scholar] [CrossRef]
- Bentzon, J.F.; Sondergaard, C.S.; Kassem, M.; Falk, E. Smooth muscle cells healing atherosclerotic plaque disruptions are of local, not blood, origin in apolipoprotein E knockout mice. Circulation 2007, 116, 2053–2061. [Google Scholar] [CrossRef]
- Bentzon, J.F.; Weile, C.; Sondergaard, C.S.; Hindkjaer, J.; Kassem, M.; Falk, E. Smooth muscle cells in atherosclerosis originate from the local vessel wall and not circulating progenitor cells in ApoE knockout mice. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2696–2702. [Google Scholar] [CrossRef]
- Yu, H.; Stoneman, V.; Clarke, M.; Figg, N.; Xin, H.B.; Kotlikoff, M.; Littlewood, T.; Bennett, M. Bone marrow-derived smooth muscle-like cells are infrequent in advanced primary atherosclerotic plaques but promote atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1291–1299. [Google Scholar] [CrossRef]
- van der Wal, A.C.; Becker, A.E.; van der Loos, C.M.; Das, P.K. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation 1994, 89, 36–44. [Google Scholar] [CrossRef]
- Krysko, D.V.; Vanden Berghe, T.; D’Herde, K.; Vandenabeele, P. Apoptosis and necrosis: Detection, discrimination and phagocytosis. Methods 2008, 44, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Holdenrieder, S.; Nagel, D.; Schalhorn, A.; Heinemann, V.; Wilkowski, R.; von Pawel, J.; Raith, H.; Feldmann, K.; Kremer, A.E.; Müller, S.; et al. Clinical relevance of circulating nucleosomes in cancer. Ann. N. Y. Acad. Sci. 2008, 1137, 180–189. [Google Scholar] [CrossRef]
- Golledge, J. Abdominal aortic aneurysm: Update on pathogenesis and medical treatments. Nat. Rev. Cardiol. 2019, 16, 225–242. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, K.; Morikage, N.; Nishino-Fujimoto, S.; Furutani, A.; Shirasawa, B.; Hamano, K. Current Status and Perspectives on Pharmacologic Therapy for Abdominal Aortic Aneurysm. Curr. Drug Targets 2018, 19, 1265–1275. [Google Scholar] [CrossRef]
- Suzuki, T.; Katoh, H.; Watanabe, M.; Kurabayashi, M.; Hiramori, K.; Hori, S.; Nobuyoshi, M.; Tanaka, H.; Kodama, K.; Sato, H.; et al. Novel biochemical diagnostic method for aortic dissection. Results of a prospective study using an immunoassay of smooth muscle myosin heavy chain. Circulation 1996, 93, 1244–1249. [Google Scholar] [CrossRef]
- Katoh, H.; Suzuki, T.; Hiroi, Y.; Ohtaki, E.; Suzuki, S.; Yazaki, Y.; Nagai, R. Diagnosis of aortic dissection by immunoassay for circulating smooth muscle myosin. Lancet 1995, 345, 191–192. [Google Scholar] [CrossRef]
- Rensen, S.S.; Doevendans, P.A.; van Eys, G.J. Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Neth. Heart J. 2007, 15, 100–108. [Google Scholar] [CrossRef]
- Bentzon, J.F.; Majesky, M.W. Lineage tracking of origin and fate of smooth muscle cells in atherosclerosis. Cardiovasc. Res. 2018, 114, 492–500. [Google Scholar] [CrossRef]
- Mikawa, T.; Gourdie, R.G. Pericardial mesoderm generates a population of coronary smooth muscle cells migrating into the heart along with ingrowth of the epicardial organ. Dev. Biol. 1996, 174, 221–232. [Google Scholar] [CrossRef]
- Wasteson, P.; Johansson, B.R.; Jukkola, T.; Breuer, S.; Akyurek, L.M.; Partanen, J.; Lindahl, P. Developmental origin of smooth muscle cells in the descending aorta in mice. Development 2008, 135, 1823–1832. [Google Scholar] [CrossRef] [PubMed]



| Variables | A | B | C | p-value | |||
|---|---|---|---|---|---|---|---|
| Controls (n = 34) | CAD or PAD (n = 35) | CAD + PAD (n = 10) | A vs. B vs. C | A vs. B | A vs. C | B vs. C | |
| Age, years | 71.2 ± 3.7 | 69.9 ± 7.4 | 72 ± 8.5 | 0.348 | |||
| Male gender, n (%) | 28 (82.4) | 30 (85.7) | 8 (80.0) | 0.923 | |||
| Body mass index | 23.3 ± 1.2 | 22.8 ± 3.2 | 21.7 ± 3.0 | 0.383 | |||
| Systolic BP, mmHg | 132 ± 12 | 131 ± 19 | 138 ± 22 | 0.477 | |||
| Diastolic BP, mmHg | 80 ± 8 | 66 ± 14 | 71 ± 13 | <0.001 * | <0.001 * | 0.047 * | 0.262 |
| HDL-cholesterol, mg/dL | 58 ± 16 | 52 ± 14 | 44 ± 12 | 0.042 * | 0.073 | 0.024 * | 0.262 |
| LDL-cholesterol, mg/dL | 118 ± 19 | 83 ± 23 | 96 ± 27 | <0.001 * | <0.001* | 0.018 * | 0.105 |
| Triglyceride, mg/dL | 101 ± 43 | 118 ± 67 | 125 ± 68 | 0.575 | |||
| HbA1c, % | 5.5 ± 0.4 | 6.3 ± 0.9 | 6.0 ± 0.7 | <0.001 * | <0.001 * | 0.047 * | 0.358 |
| Creatinine, mg/dL | 0.7 ± 0.2 | 3.8 ± 4.7 | 6.6 ± 5.8 | <0.001 * | <0.001 * | <0.001 * | 0.25 |
| eGFR, mL/min/1.73 m2 | 78 ± 17 | 45 ± 32 | 30 ± 33 | <0.001 * | <0.001* | <0.001 * | 0.198 |
| Hemodialysis, n (%) | 0 (0) | 11 (31.4) | 6 (60.0) | <0.001 * | <0.001 * | <0.001 * | 0.179 |
| Hypertension, n (%) | 15 (44.1) | 28 (80.0) | 9 (90.0) | 0.001 * | 0.002 * | 0.013 * | 0.661 |
| Dyslipidemia, n (%) | 14 (41.2) | 28 (80.0) | 6 (60.0) | 0.004 * | 0.001 * | 0.472 | 0.228 |
| Brinkman index | 356 ± 423 | 710 ± 511 | 948 ± 681 | 0.006 * | 0.008 * | 0.014 * | 0.286 |
| Diabetes mellitus, n (%) | 0 (0) | 19 (54.3) | 6 (60.0) | <0.001 * | <0.001 * | <0.001 * | >0.999 |
| Statins, n (%) | 1 (2.9) | 28 (80.0) | 3 (30.0) | <0.001 * | <0.001 * | 0.032 * | 0.005 * |
| ACE inhibitor or ARB, n (%) | 4 (11.8) | 20 (57.1) | 7 (70.0) | <0.001 * | <0.001 * | 0.001* | 0.716 |
| β-blocker, n (%) | 2 (5.9) | 21 (60.0) | 3 (30.0) | <0.001 * | <0.001 * | 0.069 | 0.151 |
| Acetylsalicylic acid, n (%) | 0 (0) | 30 (85.7) | 9 (90.0) | <0.001 * | <0.001 * | <0.001 * | >0.999 |
| Variables | A | B | p-Value |
|---|---|---|---|
| CAD (n = 24) | PAD (n = 11) | A vs. B | |
| Age, years | 70 ± 8.2 | 70 ± 5.3 | 0.587 |
| Male gender, n (%) | 21 (87.5) | 9 (81.8) | 0.656 |
| Body mass index | 22.4 ± 2.9 | 23.8 ± 3.6 | 0.238 |
| Systolic BP, mmHg | 127 ± 19 | 139 ± 17 | 0.085 |
| Diastolic BP, mmHg | 65 ± 12 | 67 ± 18 | 0.687 |
| HDL-cholesterol, mg/dL | 50 ± 12 | 56 ± 17 | 0.494 |
| LDL-cholesterol, mg/dL | 86 ± 22 | 78 ± 26 | 0.163 |
| Triglyceride, mg/dL | 113 ± 67 | 126 ± 69 | 0.472 |
| HbA1c, % | 6.3 ± 0.9 | 6.5 ± 0.8 | 0.18 |
| Creatinine, mg/dL | 2.7 ± 4.0 | 6.1 ± 5.7 | 0.252 |
| eGFR, mL/min/1.73 m2 | 49 ± 28 | 37 ± 39 | 0.43 |
| Hemodialysis, n (%) | 6 (25.0) | 5 (45.5) | 0.115 |
| Hypertension, n (%) | 19 (79.2) | 9 (81.8) | 0.856 |
| Dyslipidemia, n (%) | 20 (83.3) | 8 (72.7) | 0.466 |
| Brinkman index | 724 ± 561 | 679 ± 402 | 0.847 |
| Diabetes mellitus, n (%) | 10 (41.7) | 9 (81.8) | 0.027 * |
| Statins, n (%) | 20 (83.3) | 8 (72.7) | 0.466 |
| ACE inhibitor or ARB, n (%) | 16 (66.7) | 4 (36.4) | 0.093 |
| β-blocker, n (%) | 16 (66.7) | 5 (45.5) | 0.234 |
| Acetylsalicylic acid, n (%) | 24 (100) | 6 (54.5) | <0.001 * |
| Controls | CAD or PAD | CAD + PAD | |||||
|---|---|---|---|---|---|---|---|
| AOR | 95%CI | p-Value | (Reference) | AOR | 95%CI | p-Value | |
| Model 1 | |||||||
| Myosin-111 | 0.01 | 0.01–0.03 | 0.002 * | (1.00) | 4.10 | 1.43–11.69 | 0.008 * |
| Brinkman index 2 | 0.71 | 0.49–1.03 | 0.065 | (1.00) | 1.07 | 0.92–1.24 | 0.070 |
| Hypertension | 0.03 | 0.01–0.90 | 0.044 * | (1.00) | 8.76 | 0.24–323.81 | 0.598 |
| Dyslipidemia | 0.12 | 0.01–3.52 | 0.219 | (1.00) | 0.31 | 0.05–1.91 | 0.276 |
| Model 2 | |||||||
| hsCRP 1 | 0.02 | 0.01–0.37 | 0.008 * | (1.00) | 0.58 | 0.23–1.42 | 0.232 |
| Brinkman index 2 | 0.92 | 0.80–1.05 | 0.208 | (1.00) | 1.10 | 0.96–1.26 | 0.183 |
| Hypertension | 0.20 | 0.05–0.85 | 0.029 * | (1.00) | 2.12 | 0.21–21.37 | 0.523 |
| Dyslipidemia | 0.17 | 0.04–0.72 | 0.016 * | (1.00) | 0.29 | 0.05–1.49 | 0.136 |
| Age | Gender | Diagnosis | Hypertension | Dyslipidemia | Diabetes Mellitus | Smoking History | Atherosclerosis Lesions | |
|---|---|---|---|---|---|---|---|---|
| Patient 1 | 48 | M | HCC | − | − | − | − | Type II, III |
| Patient 2 | 66 | M | Liver cirrhosis | − | − | − | + | Type II, III |
| Patient 3 | 71 | M | Lung cancer | + | − | + | + | Type III, IV, V |
| Patient 4 | 72 | M | HCC | − | − | − | + | Type II, III |
| Patient 5 | 88 | F | CHF | + | − | − | + | Type III, IV, V |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, L.; Ishigami, T.; Tomiyama, H.; Kato, Y.; Kikuchi, H.; Tasaki, K.; Yamashita, J.; Inoue, S.; Taguri, M.; Nagao, T.; et al. Increased Plasma Levels of Myosin Heavy Chain 11 Is Associated with Atherosclerosis. J. Clin. Med. 2021, 10, 3155. https://doi.org/10.3390/jcm10143155
Takahashi L, Ishigami T, Tomiyama H, Kato Y, Kikuchi H, Tasaki K, Yamashita J, Inoue S, Taguri M, Nagao T, et al. Increased Plasma Levels of Myosin Heavy Chain 11 Is Associated with Atherosclerosis. Journal of Clinical Medicine. 2021; 10(14):3155. https://doi.org/10.3390/jcm10143155
Chicago/Turabian StyleTakahashi, Lisa, Tomoaki Ishigami, Hirofumi Tomiyama, Yuko Kato, Hiroyuki Kikuchi, Koichiro Tasaki, Jun Yamashita, Shigeru Inoue, Masataka Taguri, Toshitaka Nagao, and et al. 2021. "Increased Plasma Levels of Myosin Heavy Chain 11 Is Associated with Atherosclerosis" Journal of Clinical Medicine 10, no. 14: 3155. https://doi.org/10.3390/jcm10143155
APA StyleTakahashi, L., Ishigami, T., Tomiyama, H., Kato, Y., Kikuchi, H., Tasaki, K., Yamashita, J., Inoue, S., Taguri, M., Nagao, T., Chikamori, T., Ishikawa, Y., & Yokoyama, U. (2021). Increased Plasma Levels of Myosin Heavy Chain 11 Is Associated with Atherosclerosis. Journal of Clinical Medicine, 10(14), 3155. https://doi.org/10.3390/jcm10143155








