Premedication Does Not Influence the Incidence of Infliximab Infusion Reactions in Pediatric Patients with Inflammatory Bowel Disease—A Single Center Case–Control Study
Abstract
:1. Introduction
2. Methods
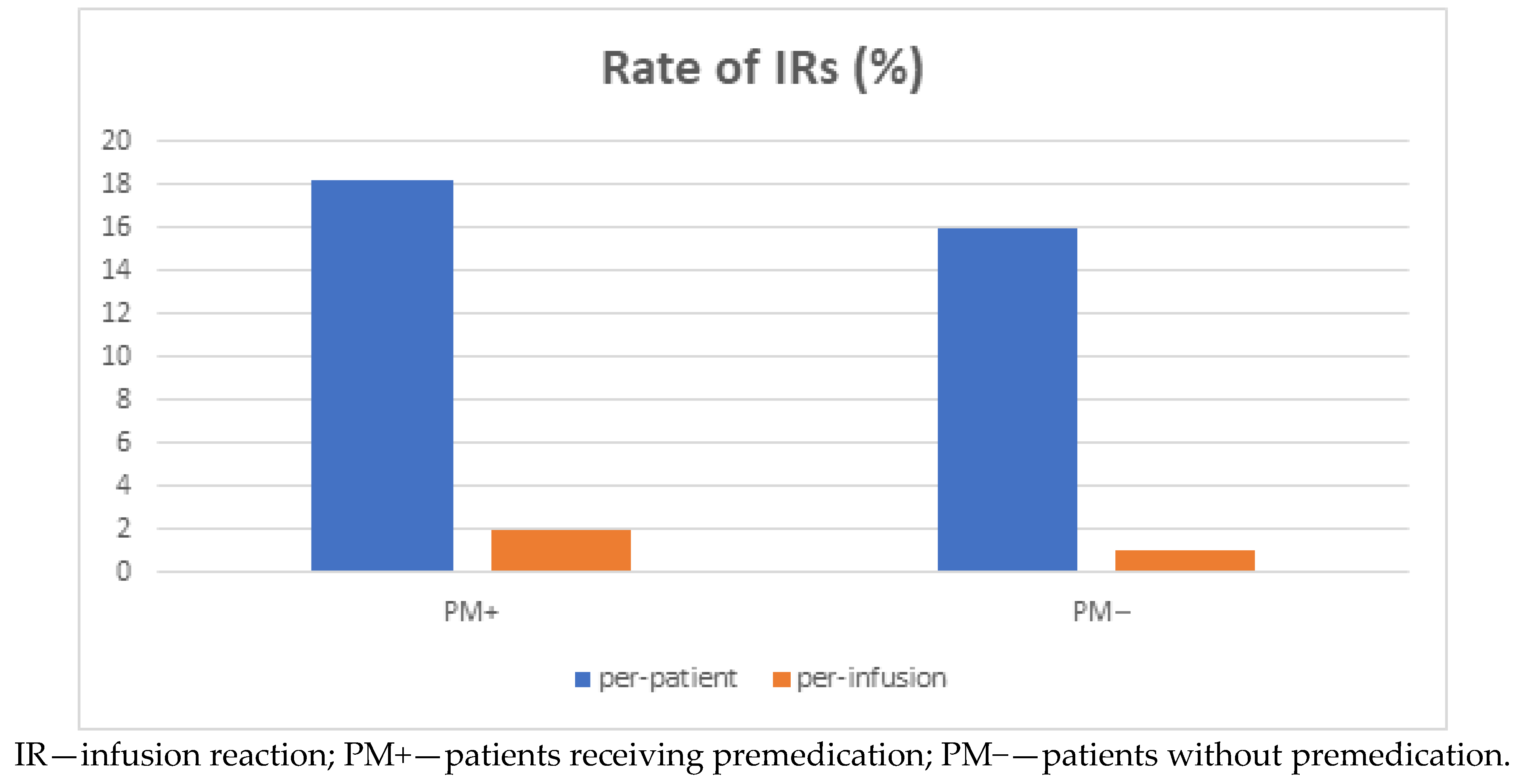
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, Y.; Lu, N.; Bai, A. Clinical use and mechanisms of infliximab treatment on inflammatory bowel disease: A recent update. Biomed. Res. Int. 2013, 2013, 581631. [Google Scholar] [CrossRef] [Green Version]
- Hyams, J.; Crandall, W.; Kugathasan, S.; Griffiths, A.; Olson, A.; Johanns, J. Induction and maintenance infliximab therapy for the treatment of moderate-to-severe Crohn’s disease in children. Gastroenterology 2007, 132, 863–873. [Google Scholar] [CrossRef]
- Hyams, J.S.; Lerer, T.; Griffiths, A.; Pfefferkorn, M.; Kugathasan, S.; Evans, J.; Markowitz, J. Long-term outcome of maintenance infliximab therapy in children with Crohn’s disease. Inflamm. Bowel Dis. 2009, 15, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, G.R.; Feagan, B.G.; Cohen, R.D.; Salzberg, B.A.; Safdi, M.; Popp, J.W.; Langholff, W.; Sandborn, W.J. Infliximab for Crohn’s Disease: More Than 13 Years of Real-world Experience. Inflamm. Bowel Dis. 2018, 24, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Corica, D.; Romano, C. Biological Therapy in Pediatric Inflammatory Bowel Disease: A Systematic Review. J. Clin. Gastroenterol. 2017, 51, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Armuzzi, A.; Fiorino, G.; Variola, A.; Manetti, N.; Fries, W.; Orlando, A.; Maconi, G.; Bossa, F.; Cappello, M.; Biancone, L.; et al. The PROSIT Cohort of Infliximab Biosimilar in IBD: A Prolonged Follow-up on the Effectiveness and Safety Across Italy. Inflamm. Bowel Dis. 2018, 25, 568–579. [Google Scholar] [CrossRef] [Green Version]
- Kim, N.H.; Lee, J.H.; Hong, S.N.; Yoon, H.; Kang, H.W.; Lee, S.H.; Park, D.I. Long-term Efficacy and Safety of CT-P13, a Biosimilar of Infliximab, in Patients with In-flammatory Bowel Disease: A Retrospective Multicenter Study. J. Gastroenterol. Hepatol. 2019, 34, 1523–1532. [Google Scholar] [CrossRef]
- Fumery, M.; Tilmant, M.; Yzet, C.; Brazier, F.; Loreau, J.; Turpin, J.; Le Mouel, J.P.; Goeb, V.; Nguyen-Khac, E.; Singh, S.; et al. Premedication as primary prophylaxis does not influence the risk of acute infliximab infusion reactions in immune-mediated inflammatory diseases: A systematic review and meta-analysis. Dig. Liver Dis. 2019, 51, 484–488. [Google Scholar] [CrossRef]
- Cheifetz, A.; Smedley, M.; Martin, S.; Reiter, M.; Leone, G.; Mayer, L.; Plevy, S. The incidence and man- agement of infusion reactions to infliximab: A large center experience. Am. J. Gastroenterol. 2003, 98, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Ducharme, J.; Pelletier, C.; Zacharias, R. The safety of inflixi- mab infusions in the community setting. Can. J. Gastroenterol. 2010, 24, 307–311. [Google Scholar] [CrossRef] [Green Version]
- Choquette, D.; Faraawi, R.; Chow, A.; Rodrigues, J.; Bensen, W.J.; Nantel, F. Incidence and management of infusion reactions to infliximab in a prospective real-world community registry. J. Rheumatol. 2015, 42, 1105–1111. [Google Scholar] [CrossRef]
- Lichtenstein, L.; Ron, Y.; Kivity, S.; Ben-Horin, S.; Israeli, E.; Fraser, G.M.; Dotan, I.; Chowers, Y.; Confino-Cohen, R.; Weiss, B. Infliximab-Related Infusion Reactions: Systematic Review. J. Crohn’s Colitis 2015, 9, 806–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, B.P.; Secord, E. Innate Immunity. Pediatr. Clin. N. Am. 2019, 66, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Picoraro, J.; Winberry, G.; Siegel, C.A.; El-Matary, W.; Moses, J.; Grossman, A.; Park, K.T. Premedication Use Be-fore Infliximab Administration: A Cross-sectional Analysis. Inflamm. Bowel Dis. 2017, 23, 174–180. [Google Scholar] [CrossRef]
- Checkley, L.A.; Kristofek, L.; Kile, S.; Bolgar, W. Incidence and Management of Infusion Reactions to Infliximab in an Alternate Care Setting. Dig. Dis. Sci. 2018, 64, 855–862. [Google Scholar] [CrossRef] [Green Version]
- Kostev, K.; Bauer, A.; Jacob, L. Lines of therapy with biological drugs in dermatology, gastroenterology, and rheumatology practices in Germany. Int. J. Clin. Pharmacol. Ther. 2019, 57, 182–187. [Google Scholar] [CrossRef]
- Szymanska, E.; Dadalski, M.; Oracz, G.; Kierkus, J. Safety profile of biologic therapy in Polish paediatric patients with Crohn’s disease. Gastroenterol. Rev. 2015, 3, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Van Wassenaer, E.A.; Meester, V.L.; Kindermann, A.; Koot, B.G.; Benninga, M.A.; de Meij, T.G. Premedication with Intravenous Steroids Does Not Influence the Incidence of Infusion Reactions Following Infliximab Infusions in Pediatric Inflammatory Bowel Disease Patients-A Case-Control Study. Eur. J. Clin. Pharmacol. 2019, 75, 1445–1450. [Google Scholar] [CrossRef] [Green Version]
- Jacobstein, D.A.; Markowitz, J.E.; Kirschner, B.S.; Ferry, G.; Cohen, S.A.; Gold, B.D.; Baldassano, R.N. Premedication and Infusion Reactions with Infliximab: Results from a Pediatric Inflammatory Bowel Disease Consortium. Inflamm. Bowel Dis. 2005, 11, 442–446. [Google Scholar] [CrossRef]
- Lichtenstein, G.R.; Feagan, B.G.; Cohen, R.D.; Salzberg, B.A.; Diamond, R.H.; Price, S.; Langholff, W.; Londhe, A.; Sandborn, W.J. Serious Infection and Mortality in Patients with Crohn’s Disease: More Than 5 Years of Follow-Up in the TREAT™ Registry. Am. J. Gastroenterol. 2012, 107, 1409–1422. [Google Scholar] [CrossRef] [Green Version]
- Sieczkowska-Golub, J.; Meglicka, M.; Plocek, A.; Banaszkiewicz, A.; Jarzebicka, D.; Toporowska-Kowalska, E.; Gawrońska, A.; Oracz, G.; Kierkus, J. Induction Therapy with Biosimilar Infliximab in Children with Crohn Disease. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 285–288. [Google Scholar] [CrossRef] [PubMed]
| (a) | ||||
|---|---|---|---|---|
| Remicade | Remsima | Flixabi | All Patients | |
| No of patients | 19 | 70 | 16 | 105 |
| PM+ | 19 | 36 | 0 | 55 |
| PM− | 0 | 34 | 16 | 50 |
| IR | 8 | 2 | 8 | 18 |
| -anaphylactic shock | 3 | 1 | 7 | 11 |
| -urtricaria | 2 | 1 | 0 | 3 |
| -erythema/rush | 3 | 1 | 1 | 5 |
| (b) | ||||
| Male | 44 (41.9%) | |||
| Age range yr. | 15 (6–18) | |||
| No of infusions | 1276 | |||
| CD | 91 (86.7%) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymanska, E.; Dadalski, M.; Sieczkowska-Golub, J.; Jarzebicka, D.; Meglicka, M.; Osiecki, M.; Wiernicka, A.; Lebensztejn, D.M.; Korczowski, B.; Kierkus, J. Premedication Does Not Influence the Incidence of Infliximab Infusion Reactions in Pediatric Patients with Inflammatory Bowel Disease—A Single Center Case–Control Study. J. Clin. Med. 2021, 10, 3177. https://doi.org/10.3390/jcm10143177
Szymanska E, Dadalski M, Sieczkowska-Golub J, Jarzebicka D, Meglicka M, Osiecki M, Wiernicka A, Lebensztejn DM, Korczowski B, Kierkus J. Premedication Does Not Influence the Incidence of Infliximab Infusion Reactions in Pediatric Patients with Inflammatory Bowel Disease—A Single Center Case–Control Study. Journal of Clinical Medicine. 2021; 10(14):3177. https://doi.org/10.3390/jcm10143177
Chicago/Turabian StyleSzymanska, Edyta, Maciej Dadalski, Joanna Sieczkowska-Golub, Dorota Jarzebicka, Monika Meglicka, Marcin Osiecki, Anna Wiernicka, Dariusz M. Lebensztejn, Bartosz Korczowski, and Jaroslaw Kierkus. 2021. "Premedication Does Not Influence the Incidence of Infliximab Infusion Reactions in Pediatric Patients with Inflammatory Bowel Disease—A Single Center Case–Control Study" Journal of Clinical Medicine 10, no. 14: 3177. https://doi.org/10.3390/jcm10143177
APA StyleSzymanska, E., Dadalski, M., Sieczkowska-Golub, J., Jarzebicka, D., Meglicka, M., Osiecki, M., Wiernicka, A., Lebensztejn, D. M., Korczowski, B., & Kierkus, J. (2021). Premedication Does Not Influence the Incidence of Infliximab Infusion Reactions in Pediatric Patients with Inflammatory Bowel Disease—A Single Center Case–Control Study. Journal of Clinical Medicine, 10(14), 3177. https://doi.org/10.3390/jcm10143177






