Seasonality of Hypoplastic Left Heart Syndrome and Single Ventricle Heart in Poland in the Context of Air Pollution
Abstract
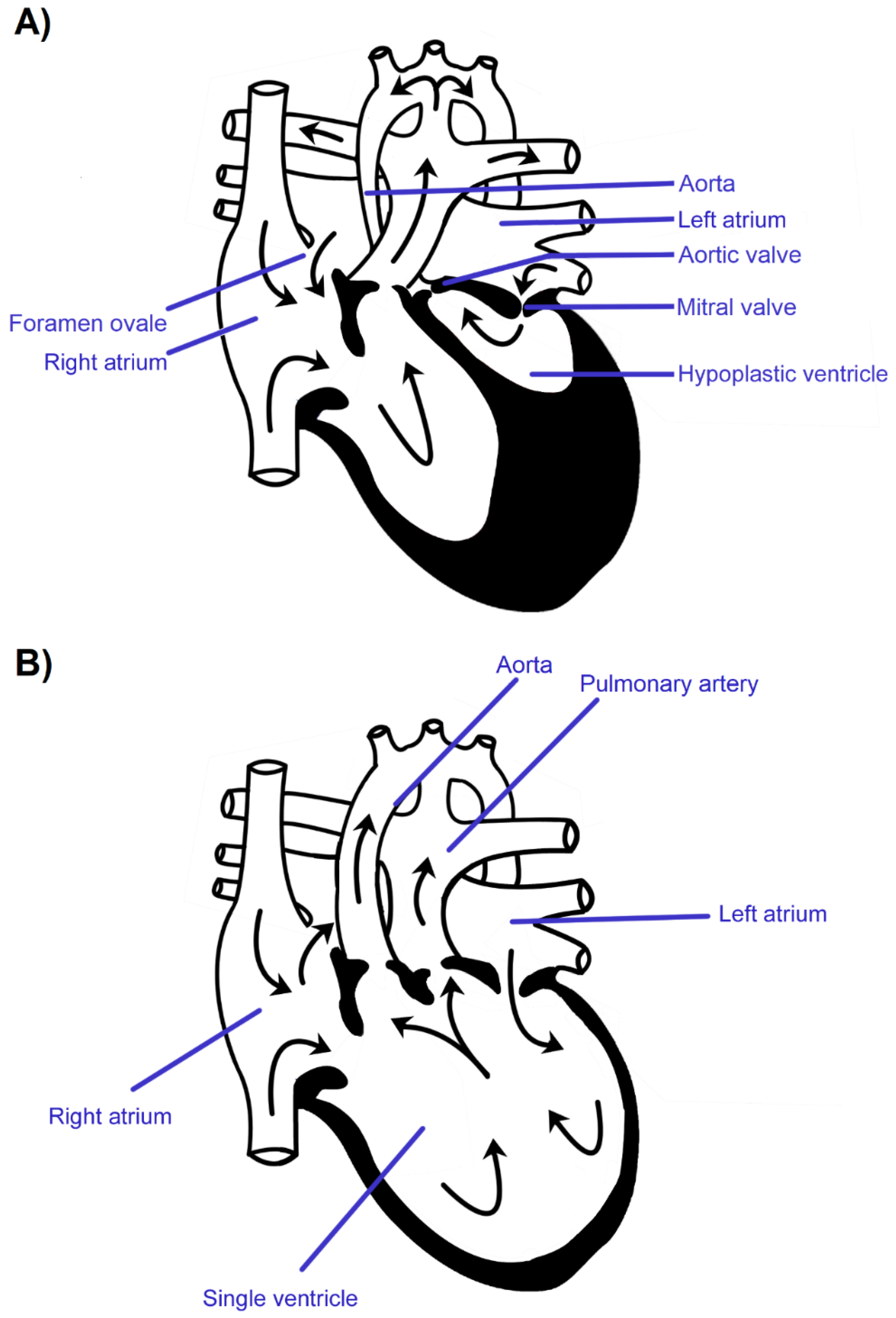
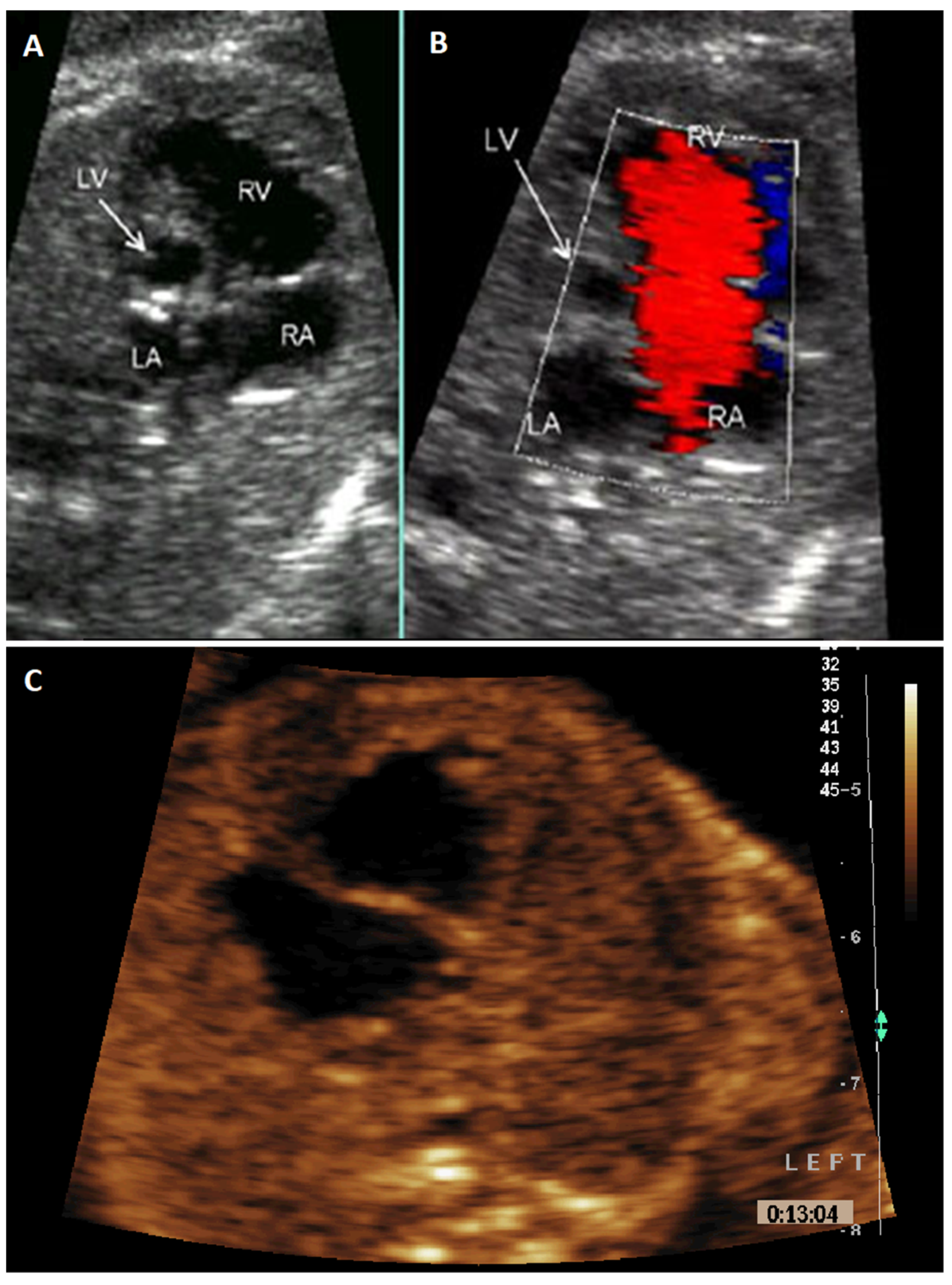
:1. Introduction
2. Materials and Methods
2.1. Study Group Selection
2.2. Measurement Tools
2.3. Statistical Analysis
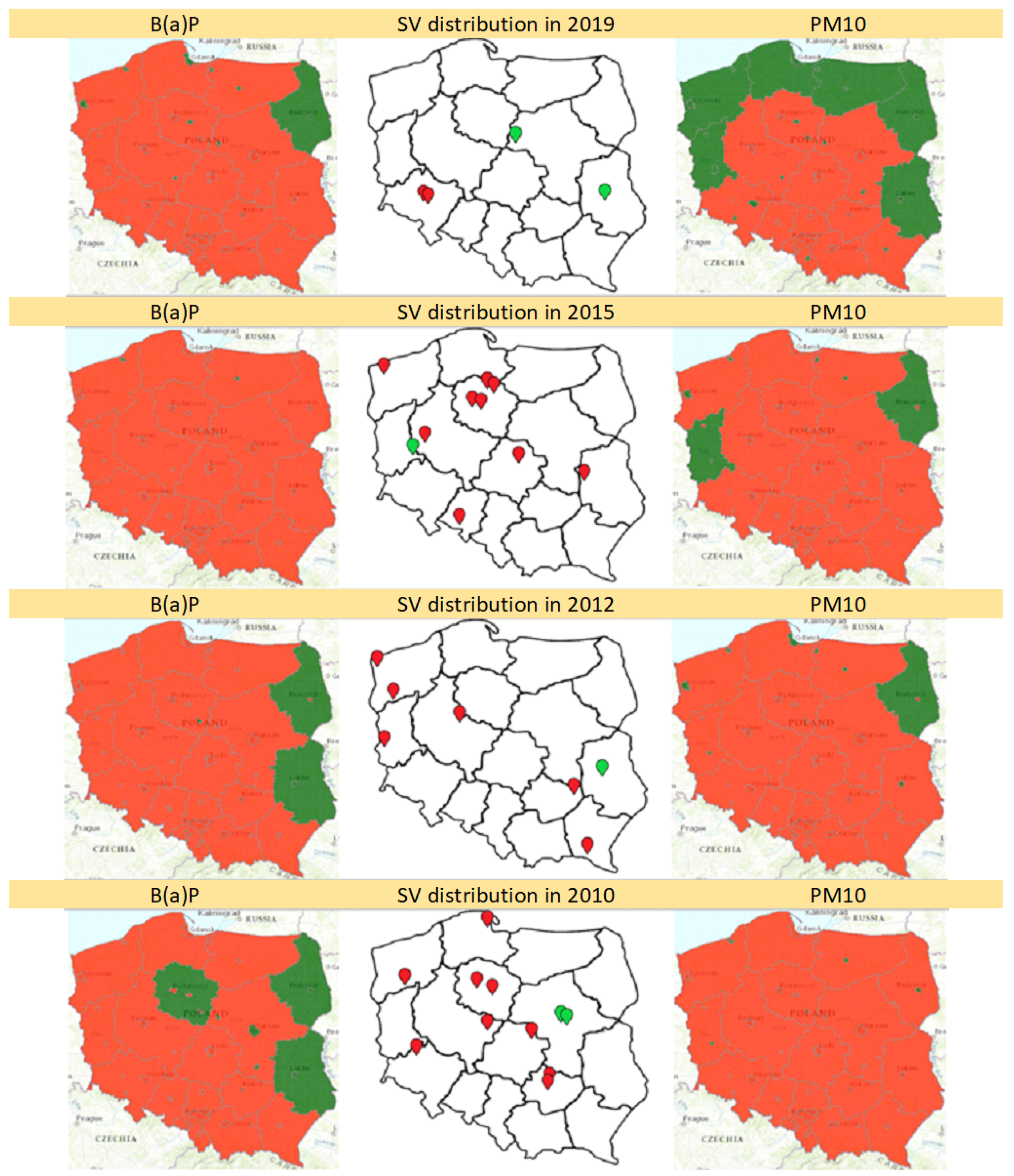
3. Results
3.1. Variability Over Time
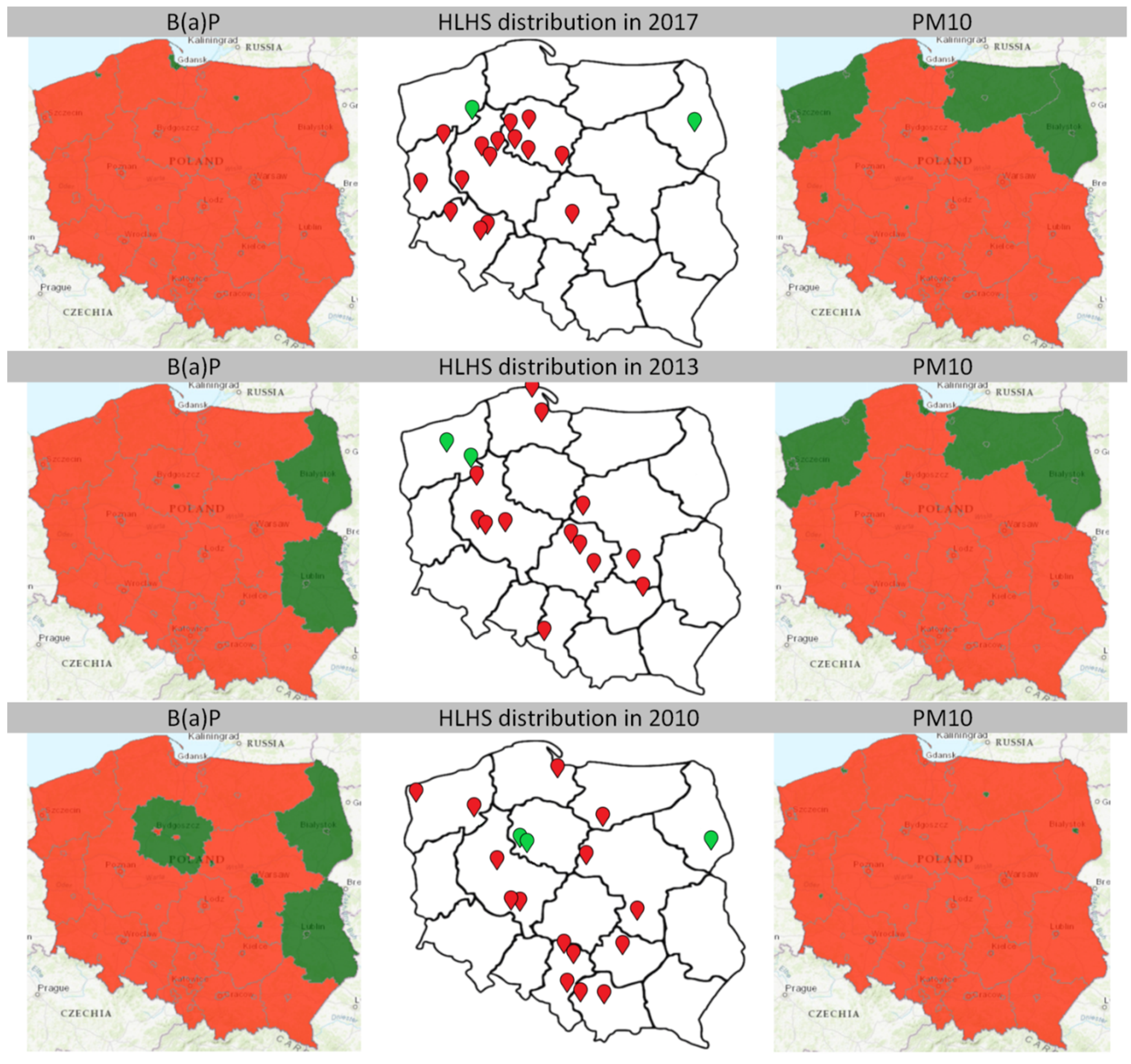
3.2. Territorial Distribution
4. Discussion
4.1. Variability Over Time
4.2. Potential Effect of Air Pollution on HLHS Formation
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paige, S.L.; Yang, W.; Priest, J.R.; Botto, L.D.; Shaw, G.M.; Thomas, R.; Ii, C. Risk factors associated with the development of double-inlet ventricle congenital heart disease. Birth Defects Res. 2019, 111, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Allen, H.D.; Driscoll, D.J.; Shaddy, R.E.; Feltes, T.F. Moss & Adams’ Heart Disease in Infants, Children, and Adolescents: Including the Fetus and Young Adult; Wolters Kluwer Health: Alphen aan den Rijn, The Netherlands, 2013; ISBN 9781451171273. [Google Scholar]
- Steven, J.M.; Marino, B.S.; Jobes, D.R. Chapter 40-Hypoplastic Left Heart Syndrome. In Critical Heart Disease in Infants and Children, 2nd ed.; Nichols, D.G., Ungerleider, R.M., Spevak, P.J., Greeley, W.J., Cameron, D.E., Lappe, D.G., Wetzel, R.C., Eds.; Mosby: Philadelphia, PA, USA, 2006; pp. 823–844. ISBN 978-0-323-01281-2. [Google Scholar]
- Malec, E.; Januszewska, K.; Pawłowska, M. Moje dziecko ma wadę serca. In Warszawa: Wyd. Fundacja Mam Serce. Fundacja Na rzecz Dzieci z Wadami Serca Cor Infantis; Fundacja Mam serce, Fundacja na rzecz Dzieci z Wadami Serca Cor Infantis: Lublin, Poland, 2014. [Google Scholar]
- Artman, M.; Teitel, D.F.; Mahoney, L. Neonatal Cardiology, 3rd ed.; McGraw Hill Education: New York, NY, USA, 2017. [Google Scholar]
- Strzelecka, I.; Moll, J.; Kornacka, K.; Zieliński, A.; Respondek-Liberska, M. Does evolving fetal heart defects pose a separate clinical program? Prenat. Cardiol. 2013, 3, 9–14. [Google Scholar]
- Strzelecka, I.; Michalska, E.; Zych-Krekora, K.; Respondek-Liberska, M. Follow-Up on 107 Fetuses with Normal Us + Echo After 37TH Week of Gestation. Prenat. Cardiol. 2017, 7, 26–30. [Google Scholar] [CrossRef] [Green Version]
- Rao, P. Single Ventricle—A Comprehensive Review. Children 2021, 8, 441. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, M.K.; Rychik, J. Outcomes in Hypoplastic Left Heart Syndrome. Pediatr. Clin. N. Am. 2020, 67, 945–962. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.M.; Beatty, J.T.; Nakano, S.J. Heart failure in single right ventricle congenital heart disease: Physiological and molecular considerations. Am. J. Physiol. Hear. Circ. Physiol. 2020, 318, H947–H965. [Google Scholar] [CrossRef]
- Rebizant, B.; Koleśnik, A.; Grzyb, A.; Chaberek, K.; Sękowska, A.; Witwicki, J.; Szymkiewicz-Dangel, J.; Dębska, M. Fetal Cardiac Interventions—Are They Safe for the Mothers? J. Clin. Med. 2021, 10, 851. [Google Scholar] [CrossRef]
- Bartel, H. Embriologia: Podręcznik Dla Studentów; Wydawnictwo Lekarskie PZWL: Warszawa, Poland, 2010. [Google Scholar]
- Feinstein, J.A.; Benson, D.W.; Dubin, A.M.; Cohen, M.S.; Maxey, D.M.; Mahle, W.T.; Pahl, E.; Villafae, J.; Bhatt, A.B.; Peng, L.F.; et al. Hypoplastic left heart syndrome: Current considerations and expectations. J. Am. Coll. Cardiol. 2012, 59. [Google Scholar] [CrossRef] [Green Version]
- Debska, M.; Kolesnik, A.; Rebizant, B.; Sekowska, A.; Grzyb, A.; Chaberek, K.; Witwicki, J.; Debski, R.; Dangel, J. Fetal Cardiac Interventions—Polish Experience from “Zero” to the Third World Largest Program. J. Clin. Med. 2020, 9, 2888. [Google Scholar] [CrossRef]
- Dolk, H.; Loane, M.; Garne, E. A European Surveillance of Congenital Anomalies (EUROCAT) Working Group Congenital Heart Defects in Europe. Circulation 2011, 123, 841–849. [Google Scholar] [CrossRef] [Green Version]
- Yabrodi, M.; Mastropietro, C.W. Hypoplastic left heart syndrome: From comfort care to long-term survival. Pediatr. Res. 2017, 81, 142–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Respondek-Liberska, M. (Ed.) Kardiologia Prenatalna Dla Położników I Kardiologów Dziecięcych; Czelej: Lublin, Poland, 2006. [Google Scholar]
- Alboliras, E.; Hijazi, Z.M.; Lopez, L.H.D. Visual Guide to Neonatal Cardiology; John Wiley Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Hogge, W.A.; Wilkins, I.; Hill, L.C.B. Sanders’ Structural Fetal Abnormalities, 3rd ed.; Mc Graw Hill Education: New York, NY, USA, 2017. [Google Scholar]
- Kobylińska, J.; Dworzański, W.; Cendrowska-Pinkosz, M.; Dworzańska, A.; Hermanowicz-Dryka, T.; Kiszka, J.; Starosławska, E.B.F. Morfologiczne i molekularne podstawy rozwoju serca. Adv. Hyg. Exp. Med. Postepy Hig. i Med. Dosw. 2013, 67, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Chief, I. For E.P. Air Quality Assessment—Annual Air Quality Assessments. Available online: http://powietrze.gios.gov.pl/pjp/maps/air/quality/type/R?lang=en (accessed on 8 April 2021).
- Sokołowski, Ł.; Fendler, W.; Tobota, Z.; Kordjalik, P.; Krekora, M.; Słodki, M.; Respondek-Liberska, M. Detection screening and seasonality evaluation of hypoplastic left heart syndrome in the polish national registry for fetal cardiac anomalies from the years 2004 to 2016. Prenat. Diagn. 2020, 40, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Siebert, J.R. Analysis of seasonal variation of birth defects in Atlanta. Birth Defects Res. Part A: Clin. Mol. Teratol. 2006, 76, 636–637. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, Y.; Tong, S.; Jiang, Z.; Xu, Z.; Li, X.; Wang, W. Analysis of the Seasonal Trend of Congenital Heart Defects. J. Pediatr. 2019, 207, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Eghtesady, P.; Brar, A.; Hall, M. Seasonality of hypoplastic left heart syndrome in the United States: A 10-year time-series analysis. J. Thorac. Cardiovasc. Surg. 2011, 141, 432–438. [Google Scholar] [CrossRef] [Green Version]
- Dai, L.; Zhu, J.; Liang, J.; Wang, Y.P.; Wang, H.; Mao, M. Birth defects surveillance in China. World. J. Pediatr. 2011, 7, 302–310. [Google Scholar] [CrossRef]
- Hinton, R.B.; Martin, L.J.; Tabangin, M.E.; Mazwi, M.L.; Cripe, L.H.; Benson, D.W. Hypoplastic Left Heart Syndrome Is Heritable. J. Am. Coll. Cardiol. 2007, 50, 1590–1595. [Google Scholar] [CrossRef] [Green Version]
- Yagi, H.; Liu, X.; Gabriel, G.C.; Wu, Y.; Peterson, K.; Murray, S.A.; Aronow, B.J.; Martin, L.J.; Benson, D.W.; Lo, C.W. The Genetic Landscape of Hypoplastic Left Heart Syndrome. Pediatr. Cardiol. 2018, 39, 1069–1081. [Google Scholar] [CrossRef]
- Ye, M.; Coldren, C.; Liang, X.; Mattina, T.; Goldmuntz, E.; Benson, D.W.; Ivy, D.; Perryman, M.B.; Garrett-Sinha, L.A.; Grossfeld, P. Deletion of ETS-1, a gene in the Jacobsen syndrome critical region, causes ventricular septal defects and abnormal ventricular morphology in mice. Hum. Mol. Genet. 2009, 19, 648–656. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Yagi, H.; Saeed, S.; Bais, A.S.; Gabriel, G.C.; Chen, Z.; Peterson, K.A.; Li, Y.; Schwartz, M.C.; Reynolds, W.T.; et al. The complex genetics of hypoplastic left heart syndrome. Nat Genet 2017, 49, 1152–1159. [Google Scholar] [CrossRef]
- Tomita-Mitchell, A.; Stamm, K.D.; Mahnke, D.K.; Kim, M.S.; Hidestrand, P.M.; Liang, H.L.; Goetsch, M.A.; Hidestrand, M.; Simpson, P.; Pelech, A.N.; et al. Impact of MYH6 variants in hypoplastic left heart syndrome. Physiol. Genomics 2016, 48, 912–921. [Google Scholar] [CrossRef] [Green Version]
- Stingone, J.A.; Luben, T.J.; Daniels, J.L.; Fuentes, M.; Richardson, D.B.; Aylsworth, A.S.; Herring, A.H.; Anderka, M.; Botto, L.; Correa, A.; et al. Maternal exposure to criteria air pollutants and congenital heart defects in offspring: Results from the National Birth Defects Prevention Study. Environ. Health Perspect. 2014, 122, 863–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eghtesady, P. Hypoplastic left heart syndrome: Rheumatic heart disease of the fetus? Med. Hypotheses 2006, 66, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Kuehl, K.S.; Loffredo, C.A. A cluster of hypoplastic left heart malformation in Baltimore, Maryland. Pediatr. Cardiol. 2006, 27, 25–31. [Google Scholar] [CrossRef]
- Jones, H.N.; Olbrych, S.K.; Smith, K.L.; Cnota, J.F.; Habli, M.; Ramos-Gonzales, O.; Owens, K.J.; Hinton, A.C.; Polzin, W.J.; Muglia, L.J.; et al. Hypoplastic left heart syndrome is associated with structural and vascular placental abnormalities and leptin dysregulation. Placenta 2015, 36, 1078–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Y.; Cai, J.; Tong, X.; Chen, R.; Zhu, Y.; Xu, B.; Mo, X. Non-inheritable risk factors during pregnancy for congenital heart defects in offspring: A matched case-control study. Int. J. Cardiol. 2018, 264, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Spero, T.L.; Nolte, C.G.; Garcia, V.C.; Lin, Z.; Romitti, P.A.; Shaw, G.M.; Sheridan, S.C.; Feldkamp, M.L.; Woomert, A.; et al. Projected Changes in Maternal Heat Exposure During Early Pregnancy and the Associated Congenital Heart Defect Burden in the United States. J. Am. Heart Assoc. 2019, 8, e010995. [Google Scholar] [CrossRef] [Green Version]
- Koster, M.P.H.; van Duijn, L.; Krul-Poel, Y.H.M.; Laven, J.S.; Helbing, W.A.; Simsek, S.; Steegers-Theunissen, R.P.M. A compromised maternal vitamin D status is associated with congenital heart defects in offspring. Early Hum. Dev. 2018, 117, 50–56. [Google Scholar] [CrossRef]
- Strzelecka, I.; Karuga, F.F.; Szmyd, B.; Walter, A.; Daszkiewicz, G.; Respondek-Liberska, M. Placental thickness in the 2D prenatal ultrasonographic examination. Arch. Med. Sci. 2021. [Google Scholar] [CrossRef]
- Cronk, C.E.; Pelech, A.N.; Malloy, M.E.; McCarver, D.G. Excess Birth Prevalence of Hypoplastic Left Heart Syndrome in Eastern Wisconsin for Birth Cohorts 1997–1999. Birth Defects Res. Part A Clin. Mol. Teratol. 2004, 70, 114–120. [Google Scholar] [CrossRef]
- Cronk, C.E.; Gangnon, R.; Cossette, S.; McElroy, J.A.; Pelech, A.N. Modeling geographic risk of complex congenital heart defects in Eastern Wisconsin. Birth Defects Res. Part A Clin. Mol. Teratol. 2011, 91, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.K.C.; Zmirou-Navier, D.; Padilla, C.; Deguen, S. Effects of air pollution on the risk of congenital anomalies: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2014, 11, 7642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strickland, M.J.; Klein, M.; Correa, A.; Reller, M.D.; Mahle, W.T.; Riehle-Colarusso, T.J.; Botto, L.D.; Flanders, W.D.; Mulholland, J.A.; Siffel, C.; et al. Ambient air pollution and cardiovascular malformations in Atlanta, Georgia, 1986–2003. Am. J. Epidemiol. 2009, 169, 1004–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vrijheid, M.; Martinez, D.; Manzanares, S.; Dadvand, P.; Schembari, A.; Rankin, J.; Nieuwenhuijsen, M. Ambient air pollution and risk of congenital anomalies: A systematic review and meta-analysis. Environ. Health Perspect. 2011, 119, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Liang, S.; Zhao, J.; Qian, Z.; Bassig, B.A.; Yang, R.; Zhang, Y.; Hu, K.; Xu, S.; Zheng, T.; et al. Maternal exposure to air pollutant PM2.5 and PM10 during pregnancy and risk of congenital heart defects. J. Exp. Sci. Environ. Epidemiol. 2016, 26, 422–427. [Google Scholar] [CrossRef] [Green Version]
- Agay-Shay, K.; Friger, M.; Linn, S.; Peled, A.; Amitai, Y.; Peretz, C. Air pollution and congenital heart defects. Environ. Res. 2013, 124, 28–34. [Google Scholar] [CrossRef]
- Dolk, H.; Armstrong, B.; Lachowycz, K.; Vrijheid, M.; Rankin, J.; Abramsky, L.; Boyd, P.A.; Wellesley, D. Ambient air pollution and risk of congenital anomalies in England, 1991-1999. Occup. Environ. Med. 2010, 67, 223–227. [Google Scholar] [CrossRef]
- Gilboa, S.M.; Mendola, P.; Olshan, A.F.; Langlois, P.H.; Savitz, D.A.; Loomis, D.; Herring, A.H.; Fixler, D.E. Relation between ambient air quality and selected birth defects, seven county study, Texas, 1997-2000. Am. J. Epidemiol. 2005, 162, 238–252. [Google Scholar] [CrossRef]
- Wang, R.; Huang, X.; Ma, C.; Zhang, H. Toxicological Effects of BPDE on Dysfunctions of Female Trophoblast Cells. Adv. Exp. Med. Biol. 2021, 1300, 151–160. [Google Scholar] [CrossRef]
- Ye, Y.; Jiang, S.; Zhang, C.; Cheng, Y.; Zhong, H.; Du, T.; Xu, W.; Azziz, R.; Zhang, H.; Zhao, X. Environmental Pollutant Benzo [a] pyrene Induces Recurrent Pregnancy Loss through Promoting Apoptosis and Suppressing Migration of Extravillous Trophoblast. BioMed Res. Int. 2020, 2020. [Google Scholar] [CrossRef]
- Więckowska, K.; Piątek, K.; Respondek-Liberska, M. Heart Tumors in 33 Fetuses—Review of Twenty-Two Years of the Single-Centre Experience. Prenat. Cardiol. 2016, 6, 22–30. [Google Scholar] [CrossRef] [Green Version]
- Szmyd, B.; Biedrzycka, M.; Karuga, F.F.; Rogut, M.; Strzelecka, I.; Respondek-liberska, M. Interventricular Septal Thickness as a Diagnostic Marker of Fetal Macrosomia. J. Clin. Med. 2021, 10, 949. [Google Scholar] [CrossRef] [PubMed]
- Szmyd, B.; Karuga, F.; Gach, A.; Moszura, T.; Kopala, M.; Respondek-Liberska, M. Complex cardiovascular defects in a male infant with Williams syndrome juxtaposed with results of a preliminary survey illustrating other patients’ experiences. Kardiol. Pol. 2021. [Google Scholar] [CrossRef] [PubMed]
- van Velzen, C.L.; Ket, J.C.F.; van de Ven, P.M.; Blom, N.A.; Haak, M.C. Systematic review and meta-analysis of the performance of second-trimester screening for prenatal detection of congenital heart defects. Int. J. Gynecol. Obstet. 2018, 140, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Sokolowski, L.; Respondek-Liberska, M.; Pietryga, M.; Slodki, M. Prenatally diagnosed foramen ovale restriction in fetuses with hypoplastic left heart syndrome may be a predictor of longer hospitalization, but not of a need for an urgent Rashkind procedure. Ginekol. Pol. 2019, 90, 31–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strzelecka, I.; Moszura, T.; Respondek-Liberska, M. Routine third trimester fetal cardiac evaluation: Time for consideration. Prenat. Cardiol. 2015, 5, 18–23. [Google Scholar] [CrossRef]

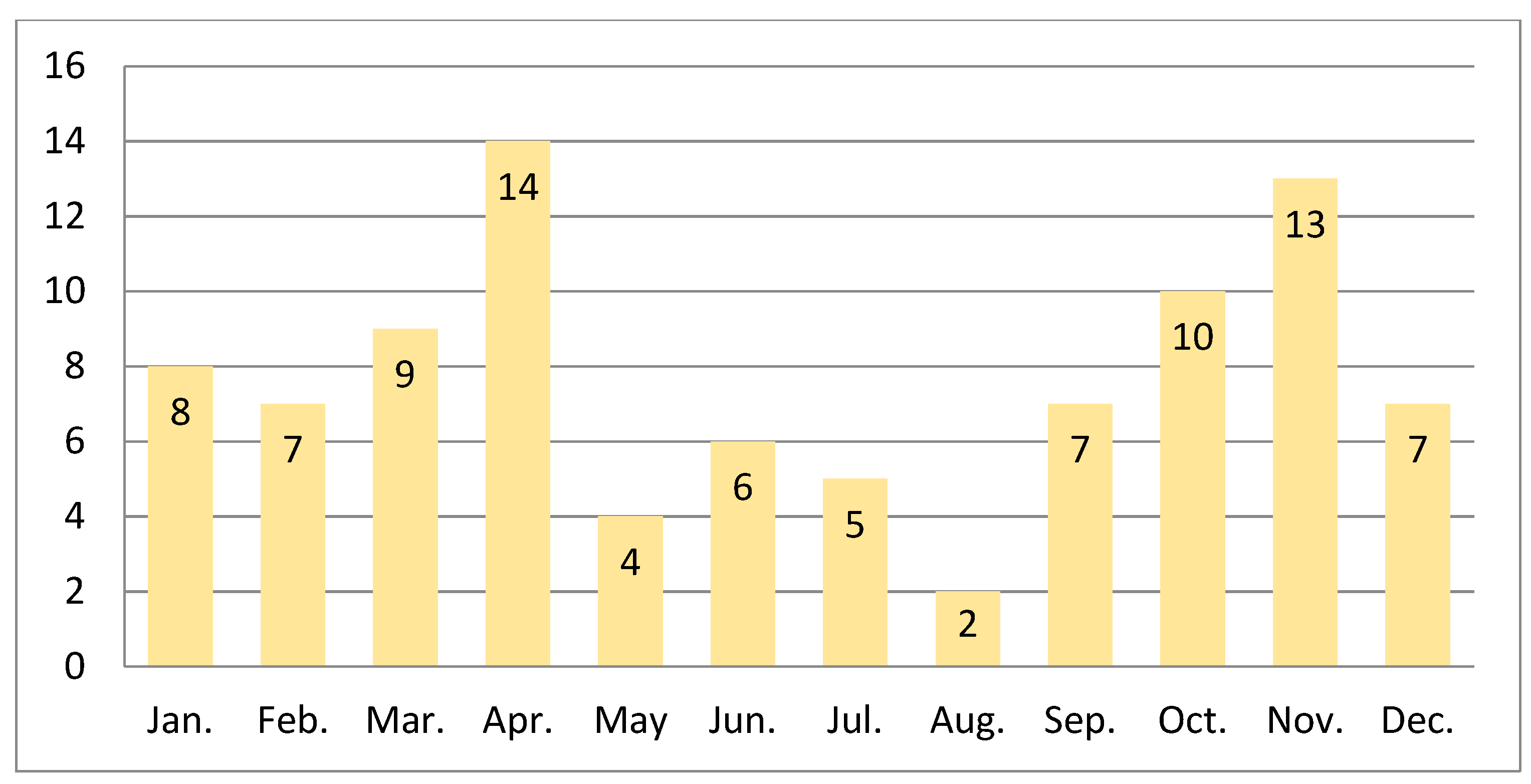
| Jan. | Feb. | Mar. | Apr. | May | Jun. | Jul. | Aug. | Sep. | Oct. | Nov. | Dec. | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HLHS | 21 | 13 | 27 | 15 | 17 | 14 | 15 | 13 | 13 | 16 | 15 | 14 | 193 |
| % | 10.9% | 6.7% | 14.0% | 7.8% | 8.8% | 7.3% | 7.8% | 6.7% | 6.7% | 8.3% | 7.8% | 7.3% | 100.0% |
| SV | 8 | 7 | 9 | 14 | 4 | 6 | 5 | 2 | 7 | 10 | 13 | 7 | 92 |
| % | 8.7% | 7.6% | 9.8% | 15.2% | 4.3% | 6.5% | 5.4% | 2.2% | 7.6% | 10.9% | 14.1% | 7.6% | 100.0% |
| A. Parameters affecting HLHS frequency | |||
| OR | 95% CI | p value | |
| Intercept | 0.000 | 0.000–0.000 | <0.001 |
| Year | 1.163 | 1.095–1.236 | <0.001 |
| B. Parameters affecting SV frequency | |||
| OR | 95% CI | p value | |
| Intercept | 0.006 | 0.004–0.008 | <0.001 |
| Year | 1.739 | 1.132–2.670 | 0.011 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strzelecka, I.; Biedrzycka, M.; Karuga, F.F.; Szmyd, B.; Batarowicz, K.; Respondek-Liberska, M. Seasonality of Hypoplastic Left Heart Syndrome and Single Ventricle Heart in Poland in the Context of Air Pollution. J. Clin. Med. 2021, 10, 3207. https://doi.org/10.3390/jcm10153207
Strzelecka I, Biedrzycka M, Karuga FF, Szmyd B, Batarowicz K, Respondek-Liberska M. Seasonality of Hypoplastic Left Heart Syndrome and Single Ventricle Heart in Poland in the Context of Air Pollution. Journal of Clinical Medicine. 2021; 10(15):3207. https://doi.org/10.3390/jcm10153207
Chicago/Turabian StyleStrzelecka, Iwona, Małgorzata Biedrzycka, Filip Franciszek Karuga, Bartosz Szmyd, Katarzyna Batarowicz, and Maria Respondek-Liberska. 2021. "Seasonality of Hypoplastic Left Heart Syndrome and Single Ventricle Heart in Poland in the Context of Air Pollution" Journal of Clinical Medicine 10, no. 15: 3207. https://doi.org/10.3390/jcm10153207
APA StyleStrzelecka, I., Biedrzycka, M., Karuga, F. F., Szmyd, B., Batarowicz, K., & Respondek-Liberska, M. (2021). Seasonality of Hypoplastic Left Heart Syndrome and Single Ventricle Heart in Poland in the Context of Air Pollution. Journal of Clinical Medicine, 10(15), 3207. https://doi.org/10.3390/jcm10153207








