Ossification and Fusion of the Vertebral Ring Apophysis as an Important Part of Spinal Maturation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. CT-Scan Analysis
- Phase 0: Ring not detectable.
- Phase 1: Ring detectable.
- Phase 2: Fusion not completed.
- Phase 3: Fusion completed.
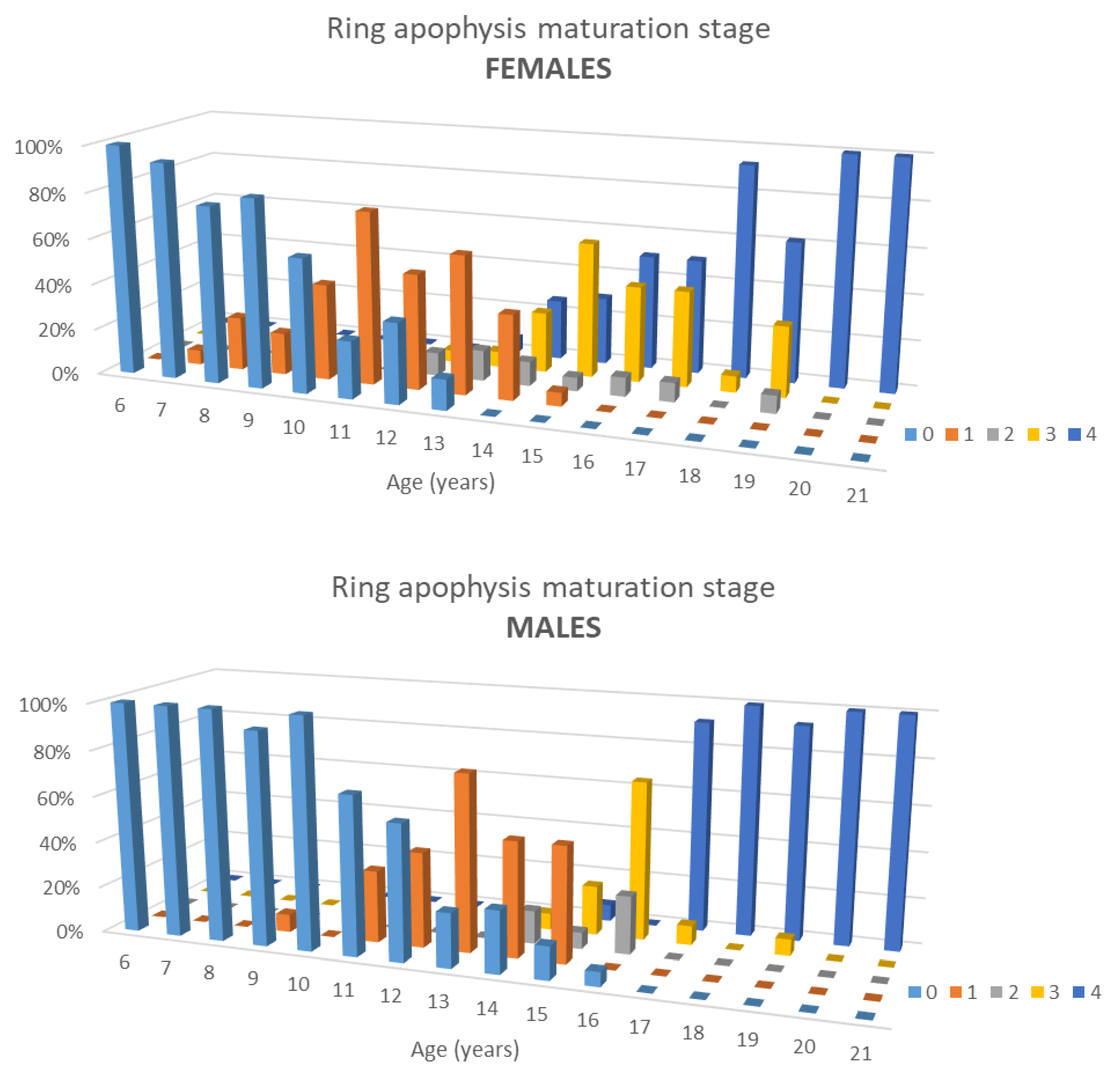
- Stage 0: no ossification (phase 0) in all 4 ROI.
- Stage 1: Beginning of ossification (phase 1 in 1–3 ROI).
- Stage 2: Complete ossification (phase 1 in all 4 ROI).
- Stage 3: Incomplete fusion (phase 3 in 1–3 ROI).
- Stage 4: Complete fusion at all 4 points (phase 3 in all 4 ROI).
2.3. Statistical Analysis
3. Results
3.1. Study Population
3.2. Ring Apophysis Maturation
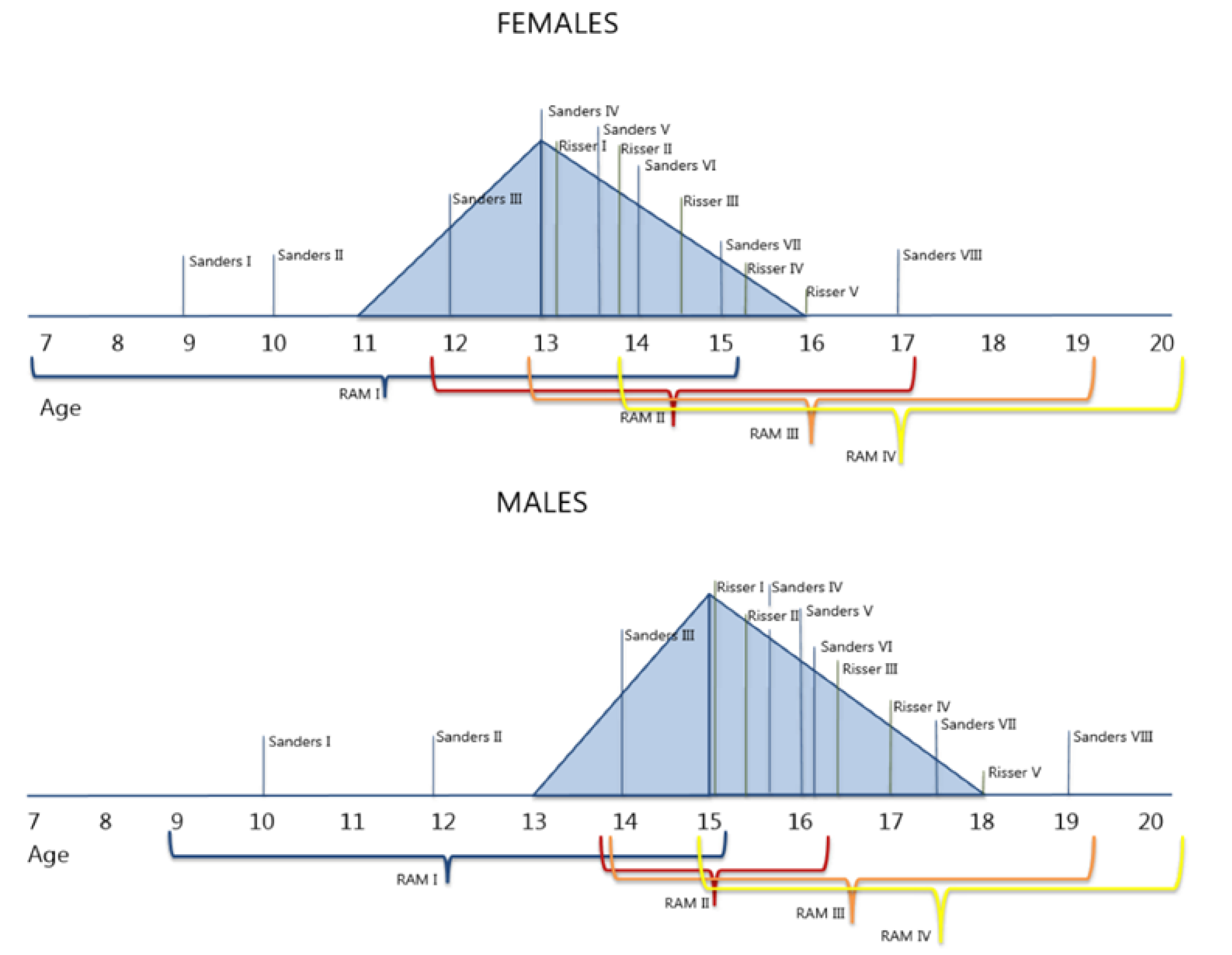
3.3. Correlation with Other Skeletal Maturity Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schlösser, T.P.; Shah, S.A.; Reichard, S.J.; Rogers, K.; Vincken, K.L.; Castelein, R.M. Differences in early sagittal plane alignment between thoracic and lumbar adolescent idiopathic scoliosis. Spine J. 2014, 14, 282–290. [Google Scholar] [CrossRef] [Green Version]
- Will, R.E.; Stokes, I.; Qiu, X.; Walker, M.R.; Sanders, J.O. Cobb Angle Progression in Adolescent Scoliosis Begins at the Intervertebral Disc. Spine 2009, 34, 2782–2786. [Google Scholar] [CrossRef]
- Grivas, T.B.; Vasiliadis, E.; Malakasis, M.; Mouzakis, V.; Segos, D. Intervertebral disc biomechanics in the pathogenesis of idiopathic scoliosis. Stud. Health Technol. Inform. 2006, 123, 80–83. [Google Scholar] [PubMed]
- Castelein, R.M.; Pasha, S.; Cheng, J.C.; Dubousset, J. Idiopathic Scoliosis as a Rotatory Decompensation of the Spine. J. Bone Miner. Res. 2020, 35, 1850–1857. [Google Scholar] [CrossRef]
- Risser, J.C. The Iliac Apophysis:An Invaluable Sign in the Management of Scoliosis. Clin. Orthop. Relat. Res. 1958, 11, 111–119. [Google Scholar]
- Vira, S.; Husain, Q.; Jalai, C.; Paul, J.; Poorman, G.W.; Poorman, C.; Yoon, R.S.; Looze, C.; Lonner, B.; Passias, P.G. The Interobserver and Intraobserver Reliability of the Sanders Classification Versus the Risser Stage. J. Pediatr. Orthop. 2017, 37, e246–e249. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Cui, J.J.; DeVries, S.; Nicholson, A.D.; Li, E.; Petit, L.; Kahan, J.B.; Sanders, J.O.; Liu, R.W.; Cooperman, D.R.; et al. Humeral Head Ossification Predicts Peak Height Velocity Timing and Percentage of Growth Remaining in Children. J. Pediatr. Orthop. 2018, 38, e546–e550. [Google Scholar] [CrossRef] [PubMed]
- Sanders, J.O.; Khoury, J.G.; Kishan, S.; Browne, R.H.; Mooney, J.F.; Arnold, K.D.; McConnell, S.J.; Bauman, J.A.; Finegold, D. Predicting Scoliosis Progression from Skeletal Maturity: A Simplified Classification during Adolescence. J. Bone Jt. Surg. Am. Vol. 2008, 90, 540–553. [Google Scholar] [CrossRef]
- Modi, H.N.; Modi, C.H.; Suh, S.W.; Yang, J.-H.; Hong, J.-Y. Correlation and comparison of Risser sign versus bone age determination (TW3) between children with and without scoliosis in Korean population. J. Orthop. Surg. Res. 2009, 4, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noordeen, M.H.H.; Haddad, F.S.; Edgar, M.A.; Pringle, J. Spinal Growth and a Histologic Evaluation of the Risser Grade in Idiopathic Scoliosis. Spine 1999, 24, 535–538. [Google Scholar] [CrossRef]
- Schlösser, T.P.; Vincken, K.L.; Attrach, H.; Kuijf, H.J.; Viergever, M.A.; Janssen, M.M.; Castelein, R.M. Quantitative analysis of the closure pattern of the neurocentral junction as related to preexistent rotation in the normal immature spine. Spine J. 2013, 13, 756–763. [Google Scholar] [CrossRef] [Green Version]
- Skórzewska, A.; Grzymisławska, M.; Bruska, M.; Łupicka, J.; Woźniak, W. Ossification of the vertebral column in human foetuses: Histological and computed tomography studies. Folia Morphol. 2013, 72, 230–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, T.D.; Tony, G.; Charran, A.; Lalam, R.; Singh, J.; Tyrrell, P.N.M.; Cassar-Pullicino, V.N. Radiographic morphology of normal ring apophyses in the immature cervical spine. Skelet. Radiol. 2018, 47, 1221–1228. [Google Scholar] [CrossRef]
- Taylor, J.R. Growth of human intervertebral discs and vertebral bodies. J. Anat. 1975, 120, 49–68. [Google Scholar] [PubMed]
- Bernick, S.; Cailliet, R.; Levy, B. The Maturation and Aging of the Vertebrae of Marmosets. Spine 1980, 5, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Edelson, J.G.; Nathan, H. Stages in the Natural History of the Vertebral End-Plates. Spine 1988, 13, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Melrose, J.; Smith, S.M.; Little, C.; Moore, R.J.; Vernon-Roberts, B.; Fraser, R.D. Recent advances in annular pathobiology provide insights into rim-lesion mediated intervertebral disc degeneration and potential new approaches to annular repair strategies. Eur. Spine J. 2008, 17, 1131–1148. [Google Scholar] [CrossRef] [Green Version]
- Zaoussis, A.L.; James, J.I.P. The iliac apophysis and the evolution of curves in scoliosis. J. Bone Jt. Surg. Br. Vol. 1958, 40-B, 442–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uys, A.; Bernitz, H.; Pretorius, S.; Steyn, M. Age estimation from anterior cervical vertebral ring apophysis ossification in South Africans. Int. J. Leg. Med. 2019, 133, 1935–1948. [Google Scholar] [CrossRef]
- Makino, T.; Kaito, T.; Sakai, Y.; Kashii, M.; Yoshikawa, H. Asymmetrical ossification in the epiphyseal ring of patients with adolescent idiopathic scoliosis. Bone Jt. J. 2016, 98, 666–671. [Google Scholar] [CrossRef]
- Cheng, J.; Castelein, R.M.; Chu, W.; Danielsson, A.J.; Dobbs, M.B.; Grivas, T.; Gurnett, C.; Luk, K.D.; Moreau, A.; Newton, P.O.; et al. Adolescent idiopathic scoliosis. Nat. Rev. Dis. Prim. 2015, 1, 15030. [Google Scholar] [CrossRef] [Green Version]
- Dimeglio, A.; Bonnel, F.; Canavese, F. Normal Growth of the Spine and Thorax. Eur. Spine J. 2011, 13–42. [Google Scholar] [CrossRef]
- Takeuchi, T.; Abumi, K.; Shono, Y.; Oda, I.; Kaneda, K. Biomechanical Role of the Intervertebral Disc and Costovertebral Joint in Stability of the Thoracic Spine. Spine 1999, 24, 1414–1420. [Google Scholar] [CrossRef]
- Waxenbaum, J.A.; Reddy, V.; Futterman, B.; Williams, C. Anatomy, Back, Intervertebral Discs. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Gerver, W.; De Bruin, R. Growth velocity: A presentation of reference values in Dutch children. Horm. Res. 2003, 60, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Tanner, J.; Davies, P.S. Clinical longitudinal standards for height and height velocity for North American children. J. Pediatr. 1985, 107, 317–329. [Google Scholar] [CrossRef]
- Choudhry, M.N.; Ahmad, Z.; Verma, R. Adolescent Idiopathic Scoliosis. Open Orthop. J. 2016, 10, 143–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanders, J.O.; McConnell, S.J.; Margraf, S.A.; Browne, R.H.; Cooney, T.E.; Finegold, D.N. Maturity Assessment and Curve Progression in Girls with Idiopathic Scoliosis. J. Bone Jt. Surg. Am. Vol. 2007, 89, 64–73. [Google Scholar] [CrossRef]
- Little, D.G.; Sussman, M.D. The Risser Sign. J. Pediatr. Orthop. 1994, 14, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Sanders, J.O.; Karbach, L.E.; Cai, X.; Gao, S.; Liu, R.W.; Cooperman, D.R. Height and Extremity-Length Prediction for Healthy Children Using Age-Based Versus Peak Height Velocity Timing-Based Multipliers. J. Bone Jt. Surg. Am. Vol. 2021, 103, 335–342. [Google Scholar] [CrossRef]
- DiMeglio, A. Growth in Pediatric Orthopaedics. J. Pediatr. Orthop. 2001, 21, 549–555. [Google Scholar] [CrossRef]
- Ron, E. Cancer risks from medical radiation. Health Phys. 2003, 85, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Florkow, M.C.; Zijlstra, F.; Willemsen, K.; Maspero, M.; Berg, C.A.T.V.D.; Kerkmeijer, L.G.W.; Castelein, R.M.; Weinans, H.; Viergever, M.A.; Van Stralen, M.; et al. Deep learning–based MR-to-CT synthesis: The influence of varying gradient echo–based MR images as input channels. Magn. Reson. Med. 2020, 83, 1429–1441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edmondston, S.; Song, S.; Bricknell, R.; Davies, P.; Fersum, K.; Humphries, P.; Wickenden, D.; Singer, K. MRI evaluation of lumbar spine flexion and extension in asymptomatic individuals. Man. Ther. 2000, 5, 158–164. [Google Scholar] [CrossRef] [PubMed]



| Population | ||
|---|---|---|
| Age | Males | Females |
| 6 | 15 | 17 |
| 7 | 18 | 16 |
| 8 | 13 | 13 |
| 9 | 13 | 11 |
| 10 | 11 | 12 |
| 11 | 16 | 16 |
| 12 | 17 | 20 |
| 13 | 17 | 15 |
| 14 | 14 | 19 |
| 15 | 14 | 17 |
| 16 | 16 | 12 |
| 17 | 14 | 13 |
| 18 | 12 | 14 |
| 19 | 14 | 13 |
| 20 | 12 | 11 |
| 21 | 11 | 10 |
| Total (percentage) | 227 (49.7%) | 229 (50.3%) |
| Mean age | 13.22 | 13.15 |
| SD | 4.52 | 4.44 |
| Range | 6–21 | 6–21 |
| CT scans | ||
| Selected CT scans | 456 | |
| Total-body n (%) | 289 (63%) | |
| Thoracic n (%) | 82 (18%) | |
| Abdominal n (%) | 85 (19%) | |
| M | 0 | 1 | 2 | 3 | 4 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | 0 | 1 | 2 | 3 | 4 | ||||||||||||||
| AGE | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | AGE | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| T1 | T1 | ||||||||||||||||||
| T2 | T2 | ||||||||||||||||||
| T3 | T3 | ||||||||||||||||||
| T4 | T4 | ||||||||||||||||||
| T5 | T5 | ||||||||||||||||||
| T6 | T6 | ||||||||||||||||||
| T7 | T7 | ||||||||||||||||||
| T8 | T8 | ||||||||||||||||||
| T9 | T9 | ||||||||||||||||||
| T10 | T10 | ||||||||||||||||||
| T11 | T11 | ||||||||||||||||||
| T12 | T12 | ||||||||||||||||||
| L1 | L1 | ||||||||||||||||||
| L2 | L2 | ||||||||||||||||||
| L3 | L3 | ||||||||||||||||||
| L4 | L4 | ||||||||||||||||||
| L5 | L5 | ||||||||||||||||||
| S1 | S1 | ||||||||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, L.; de Reuver, S.; Kan, L.; Seevinck, P.; Kruyt, M.C.; Schlosser, T.P.C.; Castelein, R.M. Ossification and Fusion of the Vertebral Ring Apophysis as an Important Part of Spinal Maturation. J. Clin. Med. 2021, 10, 3217. https://doi.org/10.3390/jcm10153217
Costa L, de Reuver S, Kan L, Seevinck P, Kruyt MC, Schlosser TPC, Castelein RM. Ossification and Fusion of the Vertebral Ring Apophysis as an Important Part of Spinal Maturation. Journal of Clinical Medicine. 2021; 10(15):3217. https://doi.org/10.3390/jcm10153217
Chicago/Turabian StyleCosta, Lorenzo, Steven de Reuver, Luc Kan, Peter Seevinck, Moyo C. Kruyt, Tom P. C. Schlosser, and René M. Castelein. 2021. "Ossification and Fusion of the Vertebral Ring Apophysis as an Important Part of Spinal Maturation" Journal of Clinical Medicine 10, no. 15: 3217. https://doi.org/10.3390/jcm10153217
APA StyleCosta, L., de Reuver, S., Kan, L., Seevinck, P., Kruyt, M. C., Schlosser, T. P. C., & Castelein, R. M. (2021). Ossification and Fusion of the Vertebral Ring Apophysis as an Important Part of Spinal Maturation. Journal of Clinical Medicine, 10(15), 3217. https://doi.org/10.3390/jcm10153217







