The Impact of Long-Term Antibiotic Therapy of Cutaneous Adverse Reactions to EGFR Inhibitors in Colorectal Cancer Patients
Abstract
:1. Introduction
2. Antibiotic Treatment for Cutaneous Adverse Reactions of EGFR Inhibitors in CRC Patients
2.1. EGFR Inhibitors–Targeted-Therapy for Metastatic CRC Patients
2.2. Dermatological Adverse Reactions of EGFR Inhibitors
2.3. Tetracyclines as Elected Therapy in the Management of Papulo-Pustular Rash to EGFR-Inhibitors
3. The Impact of Long-Term Antibiotherapy on the Intestinal Microbiome in CRC Patients
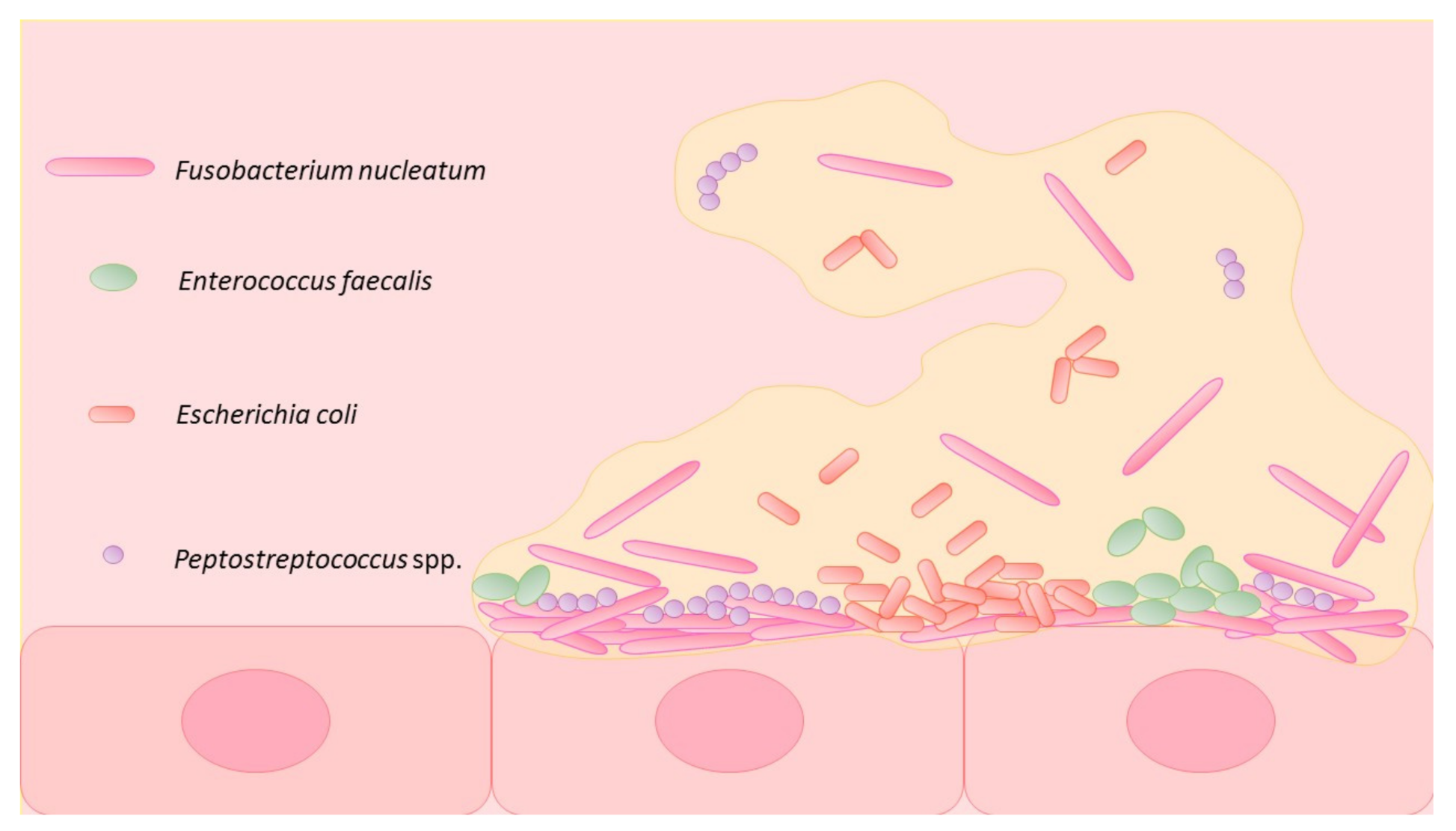
3.1. Intestinal Microbiome and CRC
3.2. Gut Microbiome Manipulation in mCRC Patients
3.3. Impact of Long-Term Tetracyclines on Gut Microbiome in Metastatic CRC Patients
3.4. Impact of Long-Term Antibiotic Therapy on CRC Development
3.5. Long-Term Antibiotherapy May Impact the Response to Oncologic Therapy in Metastatic CRC Patients
4. Alternatives to Antibiotic Treatment
5. Limitations of the Current Knowledge
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Chan, D.L.H.; Segelov, E.; Wong, R.S.; Smith, A.; Herbertson, R.A.; Li, B.T.; Tebbutt, N.; Price, T.; Pavlakis, N. Epidermal growth factor receptor (EGFR) inhibitors for metastatic colorectal cancer. Cochrane Database Syst. Rev. 2017, 6, Cd007047. [Google Scholar] [CrossRef] [PubMed]
- Guggina, L.M.; Choi, A.W.; Choi, J.N. EGFR Inhibitors and Cutaneous Complications: A Practical Approach to Management. Oncol. Ther. 2017, 5, 135–148. [Google Scholar] [CrossRef] [Green Version]
- Bachet, J.B.; Peuvrel, L.; Bachmeyer, C.; Reguiai, Z.; Gourraud, P.A.; Bouché, O.; Ychou, M.; Bensadoun, R.J.; Dreno, B.; André, T. Folliculitis induced by EGFR inhibitors, preventive and curative efficacy of tetracyclines in the management and incidence rates according to the type of EGFR inhibitor administered: A systematic literature review. Oncologist 2012, 17, 555–568. [Google Scholar] [CrossRef] [Green Version]
- Su, X.; Lacouture, M.E.; Jia, Y.; Wu, S. Risk of high-grade skin rash in cancer patients treated with cetuximab--an antibody against epidermal growth factor receptor: Systemic review and meta-analysis. Oncology 2009, 77, 124–133. [Google Scholar] [CrossRef]
- Chanprapaph, K.; Vachiramon, V.; Rattanakaemakorn, P. Epidermal growth factor receptor inhibitors: A review of cutaneous adverse events and management. Dermatol. Res. Pract. 2014, 2014, 734249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burtness, B.; Goldwasser, M.A.; Flood, W.; Mattar, B.; Forastiere, A.A. Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: An Eastern Cooperative Oncology Group study. J. Clin. Oncol. 2005, 23, 8646–8654. [Google Scholar] [CrossRef]
- Cunningham, D.; Humblet, Y.; Siena, S.; Khayat, D.; Bleiberg, H.; Santoro, A.; Bets, D.; Mueser, M.; Harstrick, A.; Verslype, C.; et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N. Engl. J. Med. 2004, 351, 337–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fakih, M.; Vincent, M. Adverse events associated with anti-EGFR therapies for the treatment of metastatic colorectal cancer. Curr. Oncol. 2010, 17 (Suppl. 1), S18–S30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molinari, E.; De Quatrebarbes, J.; André, T.; Aractingi, S. Cetuximab-induced acne. Dermatology 2005, 211, 330–333. [Google Scholar] [CrossRef]
- Lacouture, M.E.; Anadkat, M.J.; Bensadoun, R.J.; Bryce, J.; Chan, A.; Epstein, J.B.; Eaby-Sandy, B.; Murphy, B.A. Clinical practice guidelines for the prevention and treatment of EGFR inhibitor-associated dermatologic toxicities. Support Care Cancer 2011, 19, 1079–1095. [Google Scholar] [CrossRef] [Green Version]
- Cury-Martins, J.; Eris, A.P.M.; Abdalla, C.M.Z.; Silva, G.B.; Moura, V.P.T.; Sanches, J.A. Management of dermatologic adverse events from cancer therapies: Recommendations of an expert panel. An. Bras. Dermatol. 2020, 95, 221–237. [Google Scholar] [CrossRef]
- Rothschild, S.I.; Betticher, D.; Zenhäusern, R.; Anchisi, S.; von Moos, R.; Pless, M.; Moosmann, P.; Popescu, R.A.; Calderoni, A.; Dressler, M.; et al. Prospective, observational practice survey of applied skin care and management of cetuximab-related skin reactions: PROSKIN study. Cancer Chemother. Pharmacol. 2019, 84, 881–889. [Google Scholar] [CrossRef] [Green Version]
- Kripp, M.; Prasnikar, N.; Vehling-Kaiser, U.; Quidde, J.; Al-Batran, S.E.; Stein, A.; Neben, K.; Hannig, C.V.; Tessen, H.W.; Trarbach, T.; et al. AIO LQ-0110: A randomized phase II trial comparing oral doxycycline versus local administration of erythromycin as preemptive treatment strategies of panitumumab-mediated skin toxicity in patients with metastatic colorectal cancer. Oncotarget 2017, 8, 105061–105071. [Google Scholar] [CrossRef] [Green Version]
- Albertha Health Services. Prevention and treatment of acneiform rash in patients treated with EGFR inhibitor therapies. Clin. Pract. Guidel. 2012. Available online: https://www.albertahealthservices.ca/assets/info/hp/cancer/if-hp-cancer-guide-supp003-egfri-rash.pdf (accessed on 23 May 2021).
- Hofheinz, R.D.; Deplanque, G.; Komatsu, Y.; Kobayashi, Y.; Ocvirk, J.; Racca, P.; Guenther, S.; Zhang, J.; Lacouture, M.E.; Jatoi, A. Recommendations for the Prophylactic Management of Skin Reactions Induced by Epidermal Growth Factor Receptor Inhibitors in Patients With Solid Tumors. Oncologist 2016, 21, 1483–1491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yrjänheikki, J.; Keinänen, R.; Pellikka, M.; Hökfelt, T.; Koistinaho, J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc. Natl. Acad. Sci. USA 1998, 95, 15769–15774. [Google Scholar] [CrossRef] [Green Version]
- Tamargo, R.J.; Bok, R.A.; Brem, H. Angiogenesis inhibition by minocycline. Cancer Res. 1991, 51, 672–675. [Google Scholar] [PubMed]
- Sapadin, A.N.; Fleischmajer, R. Tetracyclines: Nonantibiotic properties and their clinical implications. J. Am. Acad. Dermatol. 2006, 54, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Onoda, T.; Ono, T.; Dhar, D.K.; Yamanoi, A.; Fujii, T.; Nagasue, N. Doxycycline inhibits cell proliferation and invasive potential: Combination therapy with cyclooxygenase-2 inhibitor in human colorectal cancer cells. J. Lab. Clin. Med. 2004, 143, 207–216. [Google Scholar] [CrossRef]
- Ciardiello, F.; Tortora, G. Epidermal growth factor receptor (EGFR) as a target in cancer therapy: Understanding the role of receptor expression and other molecular determinants that could influence the response to anti-EGFR drugs. Eur. J. Cancer 2003, 39, 1348–1354. [Google Scholar] [CrossRef]
- Mizukami, T.; Izawa, N.; Nakajima, T.E.; Sunakawa, Y. Targeting EGFR and RAS/RAF Signaling in the Treatment of Metastatic Colorectal Cancer: From Current Treatment Strategies to Future Perspectives. Drugs 2019, 79, 633–645. [Google Scholar] [CrossRef]
- Normanno, N.; De Luca, A.; Bianco, C.; Strizzi, L.; Mancino, M.; Maiello, M.R.; Carotenuto, A.; De Feo, G.; Caponigro, F.; Salomon, D.S. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene 2006, 366, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, V.; von Weikersthal, L.F.; Decker, T.; Kiani, A.; Vehling-Kaiser, U.; Al-Batran, S.E.; Heintges, T.; Lerchenmüller, C.; Kahl, C.; Seipelt, G.; et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): A randomised, open-label, phase 3 trial. Lancet Oncol. 2014, 15, 1065–1075. [Google Scholar] [CrossRef]
- De Leeuw, I. Atherogenic profiles in insulin-dependent diabetic patients and their treatment. Eur. J. Epidemiol. 1992, 8 (Suppl. 1), 125–128. [Google Scholar] [CrossRef]
- Dutta, P.R.; Maity, A. Cellular responses to EGFR inhibitors and their relevance to cancer therapy. Cancer Lett. 2007, 254, 165–177. [Google Scholar] [CrossRef] [Green Version]
- Ciardiello, F.; Tortora, G. EGFR antagonists in cancer treatment. N. Engl. J. Med. 2008, 358, 1160–1174. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.H.; Chen, Y.X.; Fang, J.Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct. Target. Ther. 2020, 5, 22. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Suyama, K.; Baba, H. Recent Advances in Targeting the EGFR Signaling Pathway for the Treatment of Metastatic Colorectal Cancer. Int. J. Mol. Sci. 2017, 18, 752. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Schmitz, K.R.; Jeffrey, P.D.; Wiltzius, J.J.; Kussie, P.; Ferguson, K.M. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell 2005, 7, 301–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snyder, L.C.; Astsaturov, I.; Weiner, L.M. Overview of monoclonal antibodies and small molecules targeting the epidermal growth factor receptor pathway in colorectal cancer. Clin. Colorectal Cancer 2005, 5 (Suppl. 2), S71–S80. [Google Scholar] [CrossRef] [PubMed]
- Douillard, J.Y.; Siena, S.; Cassidy, J.; Tabernero, J.; Burkes, R.; Barugel, M.; Humblet, Y.; Bodoky, G.; Cunningham, D.; Jassem, J.; et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: The PRIME study. J. Clin. Oncol. 2010, 28, 4697–4705. [Google Scholar] [CrossRef]
- Peeters, M.; Price, T.J.; Cervantes, A.; Sobrero, A.F.; Ducreux, M.; Hotko, Y.; André, T.; Chan, E.; Lordick, F.; Punt, C.J.; et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J. Clin. Oncol. 2010, 28, 4706–4713. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Köhne, C.H.; Láng, I.; Folprecht, G.; Nowacki, M.P.; Cascinu, S.; Shchepotin, I.; Maurel, J.; Cunningham, D.; Tejpar, S.; et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: Updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J. Clin. Oncol. 2011, 29, 2011–2019. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, E.; Raghavan, S. Getting under the skin of epidermal morphogenesis. Nat. Rev. Genet. 2002, 3, 199–209. [Google Scholar] [CrossRef]
- Hu, J.C.; Sadeghi, P.; Pinter-Brown, L.C.; Yashar, S.; Chiu, M.W. Cutaneous side effects of epidermal growth factor receptor inhibitors: Clinical presentation, pathogenesis, and management. J. Am. Acad. Dermatol. 2007, 56, 317–326. [Google Scholar] [CrossRef]
- Macdonald, J.B.; Macdonald, B.; Golitz, L.E.; LoRusso, P.; Sekulic, A. Cutaneous adverse effects of targeted therapies: Part I: Inhibitors of the cellular membrane. J. Am. Acad. Dermatol. 2015, 72, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Wnorowski, A.M.; de Souza, A.; Chachoua, A.; Cohen, D.E. The management of EGFR inhibitor adverse events: A case series and treatment paradigm. Int. J. Dermatol. 2012, 51, 223–232. [Google Scholar] [CrossRef]
- Chen, C.B.; Wu, M.Y.; Ng, C.Y.; Lu, C.W.; Wu, J.; Kao, P.H.; Yang, C.K.; Peng, M.T.; Huang, C.Y.; Chang, W.C.; et al. Severe cutaneous adverse reactions induced by targeted anticancer therapies and immunotherapies. Cancer Manag. Res. 2018, 10, 1259–1273. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Perez-Soler, R. Skin toxicities associated with epidermal growth factor receptor inhibitors. Target. Oncol. 2009, 4, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Saltz, L.B.; Meropol, N.J.; Loehrer, P.J., Sr.; Needle, M.N.; Kopit, J.; Mayer, R.J. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J. Clin. Oncol. 2004, 22, 1201–1208. [Google Scholar] [CrossRef]
- Jacot, W.; Bessis, D.; Jorda, E.; Ychou, M.; Fabbro, M.; Pujol, J.L.; Guillot, B. Acneiform eruption induced by epidermal growth factor receptor inhibitors in patients with solid tumours. Br. J. Dermatol. 2004, 151, 238–241. [Google Scholar] [CrossRef]
- Rosen, A.C.; Case, E.C.; Dusza, S.W.; Balagula, Y.; Gordon, J.; West, D.P.; Lacouture, M.E. Impact of dermatologic adverse events on quality of life in 283 cancer patients: A questionnaire study in a dermatology referral clinic. Am. J. Clin. Dermatol. 2013, 14, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Clabbers, J.M.K.; Boers-Doets, C.B.; Gelderblom, H.; Stijnen, T.; Lacouture, M.E.; van der Hoeven, K.J.M.; Kaptein, A.A. Xerosis and pruritus as major EGFRI-associated adverse events. Support Care Cancer 2016, 24, 513–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozuki, T. Skin problems and EGFR-tyrosine kinase inhibitor. Jpn. J. Clin. Oncol. 2016, 46, 291–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Annunziata, M.C.; De Stefano, A.; Fabbrocini, G.; Leo, S.; Marchetti, P.; Romano, M.C.; Romano, I. Current Recommendations and Novel Strategies for the Management of Skin Toxicities Related to Anti-EGFR Therapies in Patients with Metastatic Colorectal Cancer. Clin. Drug Investig. 2019, 39, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Sagar, J.; Sales, K.; Dijk, S.; Taanman, J.; Seifalian, A.; Winslet, M. Does doxycycline work in synergy with cisplatin and oxaliplatin in colorectal cancer? World J. Surg. Oncol. 2009, 7, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, I.; Alfarouk, K.O.; Reshkin, S.J.; Ibrahim, M.E. Doxycycline as Potential Anti-cancer Agent. Anticancer Agents Med. Chem. 2017, 17, 1617–1623. [Google Scholar] [CrossRef] [PubMed]
- Lacouture, M.E.; Mitchell, E.P.; Piperdi, B.; Pillai, M.V.; Shearer, H.; Iannotti, N.; Xu, F.; Yassine, M. Skin toxicity evaluation protocol with panitumumab (STEPP), a phase II, open-label, randomized trial evaluating the impact of a pre-Emptive Skin treatment regimen on skin toxicities and quality of life in patients with metastatic colorectal cancer. J. Clin. Oncol. 2010, 28, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Iihara, H.; Fujii, H.; Ishihara, M.; Matsuhashi, N.; Takahashi, T.; Yoshida, K.; Itoh, Y. Prophylactic Effect of Oral Minocycline in Combination with Topical Steroid and Skin Care Against Panitumumab-induced Acneiform Rash in Metastatic Colorectal Cancer Patients. Anticancer Res. 2015, 35, 6175–6181. [Google Scholar] [PubMed]
- Jatoi, A.; Rowland, K.; Sloan, J.A.; Gross, H.M.; Fishkin, P.A.; Kahanic, S.P.; Novotny, P.J.; Schaefer, P.L.; Johnson, D.B.; Tschetter, L.K.; et al. Tetracycline to prevent epidermal growth factor receptor inhibitor-induced skin rashes: Results of a placebo-controlled trial from the North Central Cancer Treatment Group (N03CB). Cancer 2008, 113, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Komatsu, Y.; Yuki, S.; Fukushima, H.; Sasaki, T.; Iwanaga, I.; Uebayashi, M.; Okuda, H.; Kusumi, T.; Miyagishima, T.; et al. Randomized controlled trial on the skin toxicity of panitumumab in Japanese patients with metastatic colorectal cancer: HGCSG1001 study; J-STEPP. Future Oncol. 2015, 11, 617–627. [Google Scholar] [CrossRef] [Green Version]
- Jatoi, A.; Dakhil, S.R.; Sloan, J.A.; Kugler, J.W.; Rowland, K.M., Jr.; Schaefer, P.L.; Novotny, P.J.; Wender, D.B.; Gross, H.M.; Loprinzi, C.L. Prophylactic tetracycline does not diminish the severity of epidermal growth factor receptor (EGFR) inhibitor-induced rash: Results from the North Central Cancer Treatment Group (Supplementary N03CB). Support Care Cancer 2011, 19, 1601–1607. [Google Scholar] [CrossRef] [Green Version]
- Racca, P.; Fanchini, L.; Caliendo, V.; Ritorto, G.; Evangelista, W.; Volpatto, R.; Milanesi, E.; Ciorba, A.; Paris, M.; Facilissimo, I.; et al. Efficacy and skin toxicity management with cetuximab in metastatic colorectal cancer: Outcomes from an oncologic/dermatologic cooperation. Clin. Colorectal Cancer 2008, 7, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Valentín, S.; Morales, A.; Sánchez, J.L.; Rivera, A. Safety and efficacy of doxycycline in the treatment of rosacea. Clin. Cosmet. Investig. Dermatol. 2009, 2, 129–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segelnick, S.L.; Weinberg, M.A. Recognizing doxycycline-induced esophageal ulcers in dental practice: A case report and review. J. Am. Dent. Assoc. 2008, 139, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; Leyden, J.J. Safety of doxycycline and minocycline: A systematic review. Clin. Ther. 2005, 27, 1329–1342. [Google Scholar] [CrossRef] [PubMed]
- Layton, A.M.; Cunliffe, W.J. Phototoxic eruptions due to doxycycline--a dose-related phenomenon. Clin. Exp. Dermatol. 1993, 18, 425–427. [Google Scholar] [CrossRef]
- Sloan, B.; Scheinfeld, N. The use and safety of doxycycline hyclate and other second-generation tetracyclines. Expert Opin. Drug Saf. 2008, 7, 571–577. [Google Scholar] [CrossRef]
- Simpson, M.B.; Pryzbylik, J.; Innis, B.; Denham, M.A. Hemolytic anemia after tetracycline therapy. N. Engl. J. Med. 1985, 312, 840–842. [Google Scholar] [CrossRef]
- Somech, R.; Arav-Boger, R.; Assia, A.; Spirer, Z.; Jurgenson, U. Complications of minocycline therapy for acne vulgaris: Case reports and review of the literature. Pediatr. Dermatol. 1999, 16, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scepanovic, P.; Hodel, F.; Mondot, S.; Partula, V.; Byrd, A.; Hammer, C.; Alanio, C.; Bergstedt, J.; Patin, E.; Touvier, M.; et al. A comprehensive assessment of demographic, environmental, and host genetic associations with gut microbiome diversity in healthy individuals. Microbiome 2019, 7, 130. [Google Scholar] [CrossRef] [Green Version]
- Nakatsu, G.; Li, X.; Zhou, H.; Sheng, J.; Wong, S.H.; Wu, W.K.; Ng, S.C.; Tsoi, H.; Dong, Y.; Zhang, N.; et al. Gut mucosal microbiome across stages of colorectal carcinogenesis. Nat. Commun. 2015, 6, 8727. [Google Scholar] [CrossRef]
- Watson, A.J.; Collins, P.D. Colon cancer: A civilization disorder. Dig. Dis. 2011, 29, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, M.R.; Wang, X.; Liu, W.; Hao, Y.; Cai, G.; Han, Y.W. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe 2013, 14, 195–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kostic, A.D.; Chun, E.; Robertson, L.; Glickman, J.N.; Gallini, C.A.; Michaud, M.; Clancy, T.E.; Chung, D.C.; Lochhead, P.; Hold, G.L.; et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 2013, 14, 207–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodwin, A.C.; Destefano Shields, C.E.; Wu, S.; Huso, D.L.; Wu, X.; Murray-Stewart, T.R.; Hacker-Prietz, A.; Rabizadeh, S.; Woster, P.M.; Sears, C.L.; et al. Polyamine catabolism contributes to enterotoxigenic Bacteroides fragilis-induced colon tumorigenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 15354–15359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Rhee, K.J.; Albesiano, E.; Rabizadeh, S.; Wu, X.; Yen, H.R.; Huso, D.L.; Brancati, F.L.; Wick, E.; McAllister, F.; et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat. Med. 2009, 15, 1016–1022. [Google Scholar] [CrossRef]
- Yang, Y.; Weng, W.; Peng, J.; Hong, L.; Yang, L.; Toiyama, Y.; Gao, R.; Liu, M.; Yin, M.; Pan, C.; et al. Fusobacterium nucleatum Increases Proliferation of Colorectal Cancer Cells and Tumor Development in Mice by Activating Toll-Like Receptor 4 Signaling to Nuclear Factor-κB, and Up-regulating Expression of MicroRNA-21. Gastroenterology 2017, 152, 851–866.e24. [Google Scholar] [CrossRef] [Green Version]
- Clos-Garcia, M.; Garcia, K.; Alonso, C.; Iruarrizaga-Lejarreta, M.; D’Amato, M.; Crespo, A.; Iglesias, A.; Cubiella, J.; Bujanda, L.; Falcón-Pérez, J.M. Integrative Analysis of Fecal Metagenomics and Metabolomics in Colorectal Cancer. Cancers 2020, 12, 1142. [Google Scholar] [CrossRef]
- Xu, X.; Lv, J.; Guo, F.; Li, J.; Jia, Y.; Jiang, D.; Wang, N.; Zhang, C.; Kong, L.; Liu, Y.; et al. Gut Microbiome Influences the Efficacy of PD-1 Antibody Immunotherapy on MSS-Type Colorectal Cancer via Metabolic Pathway. Front. Microbiol. 2020, 11, 814. [Google Scholar] [CrossRef]
- Gagnière, J.; Raisch, J.; Veziant, J.; Barnich, N.; Bonnet, R.; Buc, E.; Bringer, M.A.; Pezet, D.; Bonnet, M. Gut microbiota imbalance and colorectal cancer. World J. Gastroenterol. 2016, 22, 501–518. [Google Scholar] [CrossRef]
- Alhinai, E.A.; Walton, G.E.; Commane, D.M. The Role of the Gut Microbiota in Colorectal Cancer Causation. Int. J. Mol. Sci. 2019, 20, 5295. [Google Scholar] [CrossRef] [Green Version]
- Yu, T.; Guo, F.; Yu, Y.; Sun, T.; Ma, D.; Han, J.; Qian, Y.; Kryczek, I.; Sun, D.; Nagarsheth, N.; et al. Fusobacterium nucleatum Promotes Chemoresistance to Colorectal Cancer by Modulating Autophagy. Cell 2017, 170, 548–563.e16. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Peng, Y.; Yu, J.; Chen, T.; Wu, Y.; Shi, L.; Li, Q.; Wu, J.; Fu, X. Invasive Fusobacterium nucleatum activates beta-catenin signaling in colorectal cancer via a TLR4/P-PAK1 cascade. Oncotarget 2017, 8, 31802–31814. [Google Scholar] [CrossRef]
- Gur, C.; Ibrahim, Y.; Isaacson, B.; Yamin, R.; Abed, J.; Gamliel, M.; Enk, J.; Bar-On, Y.; Stanietsky-Kaynan, N.; Coppenhagen-Glazer, S.; et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity 2015, 42, 344–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kostic, A.D.; Gevers, D.; Pedamallu, C.S.; Michaud, M.; Duke, F.; Earl, A.M.; Ojesina, A.I.; Jung, J.; Bass, A.J.; Tabernero, J.; et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012, 22, 292–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Feng, Q.; Wong, S.H.; Zhang, D.; Liang, Q.Y.; Qin, Y.; Tang, L.; Zhao, H.; Stenvang, J.; Li, Y.; et al. Metagenomic analysis of faecal microbiome as a tool towards targeted non-invasive biomarkers for colorectal cancer. Gut 2017, 66, 70–78. [Google Scholar] [CrossRef]
- Bullman, S.; Pedamallu, C.S.; Sicinska, E.; Clancy, T.E.; Zhang, X.; Cai, D.; Neuberg, D.; Huang, K.; Guevara, F.; Nelson, T.; et al. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science 2017, 358, 1443–1448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Lu, Y.; Ke, Y.; Li, Y. Prognostic impact of the Fusobacterium nucleatum status in colorectal cancers. Medicine 2019, 98, e17221. [Google Scholar] [CrossRef]
- Liu, Y.; Baba, Y.; Ishimoto, T.; Tsutsuki, H.; Zhang, T.; Nomoto, D.; Okadome, K.; Yamamura, K.; Harada, K.; Eto, K.; et al. Fusobacterium nucleatum confers chemoresistance by modulating autophagy in oesophageal squamous cell carcinoma. Br. J. Cancer 2021, 124, 963–974. [Google Scholar] [CrossRef]
- Gilbert, D.N.; Chambers, H.F.; Saag, M.S.; Pavia, A.T.; Black, D.; Boucher, H.W.; Freedman, D.O.; Kim, K.; Scwartz, B.S. The Sanford Guide to Antimicrobial Therapy 2020-Pocket Edition, 50th ed.; Antimicrobial Therapy, Inc.: Sperryville, VA, USA, 2020. [Google Scholar]
- Lee, W.S.; Jean, S.S.; Chen, F.L.; Hsieh, S.M.; Hsueh, P.R. Lemierre’s syndrome: A forgotten and re-emerging infection. J. Microbiol. Immunol. Infect. 2020, 53, 513–517. [Google Scholar] [CrossRef]
- Kaźmierczak-Siedlecka, K.; Daca, A.; Fic, M.; van de Wetering, T.; Folwarski, M.; Makarewicz, W. Therapeutic methods of gut microbiota modification in colorectal cancer management-fecal microbiota transplantation, prebiotics, probiotics, and synbiotics. Gut Microbes 2020, 11, 1518–1530. [Google Scholar] [CrossRef]
- Sizentsov, A.N.; Kvan, O.V.; Bykov, A.V.; Zamana, S.P.; Torshkov, A.A.; Sizentsov, Y.A. A technology of experimental studies on the xenobiotic element sorption characteristics of representatives of the intestinal normal flora. Bioint. Res. App. Chem. 2019, 9, 4131–4135. [Google Scholar] [CrossRef]
- Sizentsov, A.; Mindolina, Y.; Barysheva, E.; Ponomareva, P.; Kunavina, E.; Levenets, T.; Dudko, A.; Kvan, O. Effectiveness of combined use of antibiotics, essential metals and probiotic bacterial strain complexes against multidrug resistant pathogens. Bioint. Res. App. Chem. 2020, 10, 4830–4836. [Google Scholar] [CrossRef]
- Thakur, A.K.; Singh, I. Formulation strategies for the oral delivery of probiotics: A review. Bioint. Res. App. Chem. 2019, 9, 4327–4333. [Google Scholar] [CrossRef]
- Kotrsová, V.; Kushkevych, I. Possible methods for evaluation of hydrogen sulfide toxicity against lactic acid bacteria. Bioint. Res. App. Chem. 2019, 9, 4066–4069. [Google Scholar] [CrossRef]
- Hooshdar, P.; Kermanshahi, R.K.; Ghadam, P.; Khosravi-Darani, K. A review on production of exopolysaccharide and biofilm in probiotics like lactobacilli and methods of analysis. Bioint. Res. App. Chem. 2020, 10, 6058–6075. [Google Scholar] [CrossRef]
- Mehta, R.S.; Nishihara, R.; Cao, Y.; Song, M.; Mima, K.; Qian, Z.R.; Nowak, J.A.; Kosumi, K.; Hamada, T.; Masugi, Y.; et al. Association of Dietary Patterns With Risk of Colorectal Cancer Subtypes Classified by Fusobacterium nucleatum in Tumor Tissue. JAMA Oncol. 2017, 3, 921–927. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Xie, C.; Lu, S.; Nichols, R.G.; Tian, Y.; Li, L.; Patel, D.; Ma, Y.; Brocker, C.N.; Yan, T.; et al. Intermittent Fasting Promotes White Adipose Browning and Decreases Obesity by Shaping the Gut Microbiota. Cell Metab. 2017, 26, 672–685.e74. [Google Scholar] [CrossRef] [Green Version]
- Hao, G.W.; Chen, Y.S.; He, D.M.; Wang, H.Y.; Wu, G.H.; Zhang, B. Growth of human colon cancer cells in nude mice is delayed by ketogenic diet with or without omega-3 fatty acids and medium-chain triglycerides. APJCP 2015, 16, 2061–2068. [Google Scholar] [CrossRef] [Green Version]
- Rafter, J. The effects of probiotics on colon cancer development. Nutr. Res. Rev. 2004, 17, 277–284. [Google Scholar] [CrossRef]
- Calu, V.; Toma, E.A.; Enciu, O.; Miron, A. Clostridium difficile Infection and Colorectal Surgery: Is There Any Risk? Medicina 2019, 55, 683. [Google Scholar] [CrossRef] [Green Version]
- Miron, A.; Giulea, C.; Tudose, I.; Petrache, D.; Giurcaneanu, C. Pyoderma gangrenosum, rare parietal complication after colorectal surgery. Chirurgia 2014, 109, 248–253. [Google Scholar]
- Uccello, M.; Malaguarnera, G.; Basile, F.; D’Agata, V.; Malaguarnera, M.; Bertino, G.; Vacante, M.; Drago, F.; Biondi, A. Potential role of probiotics on colorectal cancer prevention. BMC Surg. 2012, 12 (Suppl. 1), S35. [Google Scholar] [CrossRef] [Green Version]
- Ma, E.L.; Choi, Y.J.; Choi, J.; Pothoulakis, C.; Rhee, S.H.; Im, E. The anticancer effect of probiotic Bacillus polyfermenticus on human colon cancer cells is mediated through ErbB2 and ErbB3 inhibition. Int. J. Cancer 2010, 127, 780–790. [Google Scholar] [CrossRef] [Green Version]
- Kotzampassi, K.; Stavrou, G.; Damoraki, G.; Georgitsi, M.; Basdanis, G.; Tsaousi, G.; Giamarellos-Bourboulis, E.J. A Four-Probiotics Regimen Reduces Postoperative Complications After Colorectal Surgery: A Randomized, Double-Blind, Placebo-Controlled Study. World J. Surg. 2015, 39, 2776–2783. [Google Scholar] [CrossRef]
- Bruno-Barcena, J.M.; Azcarate-Peril, M.A. Galacto-oligosaccharides and Colorectal Cancer: Feeding our Intestinal Probiome. J. Funct. Foods 2015, 12, 92–108. [Google Scholar] [CrossRef]
- Chen, D.; Wu, J.; Jin, D.; Wang, B.; Cao, H. Fecal microbiota transplantation in cancer management: Current status and perspectives. Int. J. Cancer 2019, 145, 2021–2031. [Google Scholar] [CrossRef] [Green Version]
- Angelakis, E.; Million, M.; Kankoe, S.; Lagier, J.C.; Armougom, F.; Giorgi, R.; Raoult, D. Abnormal weight gain and gut microbiota modifications are side effects of long-term doxycycline and hydroxychloroquine treatment. Antimicrob. Agents Chemother. 2014, 58, 3342–3347. [Google Scholar] [CrossRef] [Green Version]
- Elvers, K.T.; Wilson, V.J.; Hammond, A.; Duncan, L.; Huntley, A.L.; Hay, A.D.; van der Werf, E.T. Antibiotic-induced changes in the human gut microbiota for the most commonly prescribed antibiotics in primary care in the UK: A systematic review. BMJ Open 2020, 10, e035677. [Google Scholar] [CrossRef]
- Thompson, K.G.; Rainer, B.M.; Antonescu, C.; Florea, L.; Mongodin, E.F.; Kang, S.; Chien, A.L. Minocycline and Its Impact on Microbial Dysbiosis in the Skin and Gastrointestinal Tract of Acne Patients. Ann. Dermatol. 2020, 32, 21–30. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. The effect of antibiotics on the composition of the intestinal microbiota-a systematic review. J. Infect. 2019, 79, 471–489. [Google Scholar] [CrossRef] [PubMed]
- Zaura, E.; Brandt, B.W.; Teixeira de Mattos, M.J.; Buijs, M.J.; Caspers, M.P.; Rashid, M.U.; Weintraub, A.; Nord, C.E.; Savell, A.; Hu, Y.; et al. Same Exposure but Two Radically Different Responses to Antibiotics: Resilience of the Salivary Microbiome versus Long-Term Microbial Shifts in Feces. mBio 2015, 6, e01693-15. [Google Scholar] [CrossRef] [Green Version]
- Laurent, F.; Lelièvre, H.; Cornu, M.; Vandenesch, F.; Carret, G.; Etienne, J.; Flandrois, J.P. Fitness and competitive growth advantage of new gentamicin-susceptible MRSA clones spreading in French hospitals. J. Antimicrob. Chemother. 2001, 47, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Podgoreanu, P.; Negrea, S.M.; Buia, R.; Delcaru, C.; Trusca, S.B.; Lazar, V.; Chifiriuc, M.C. Alternative strategies for fighting multidrug resistant bacterial infections. Bioint. Res. App. Chem. 2019, 9, 3834–3841. [Google Scholar] [CrossRef]
- Ducu, R.; Gheorghe, I.; Chifiriuc, M.C.; Mihăescu, G.; Sârbu, I. Prevalence of vancomycin resistance phenotypes among Enterococcus species isolated from clinical samples in a Romanian hospital. Bioint. Res. App. Chem. 2019, 9, 4699–4704. [Google Scholar] [CrossRef]
- Zhu, Z.; Surujon, D.; Ortiz-Marquez, J.C.; Huo, W.; Isberg, R.R.; Bento, J.; van Opijnen, T. Entropy of a bacterial stress response is a generalizable predictor for fitness and antibiotic sensitivity. Nat. Commun. 2020, 11, 4365. [Google Scholar] [CrossRef]
- Bhattacharyya, R.P.; Bandyopadhyay, N.; Ma, P.; Son, S.S.; Liu, J.; He, L.L.; Wu, L.; Khafizov, R.; Boykin, R.; Cerqueira, G.C.; et al. Simultaneous detection of genotype and phenotype enables rapid and accurate antibiotic susceptibility determination. Nat. Med. 2019, 25, 1858–1864. [Google Scholar] [CrossRef]
- Khazaei, T.; Barlow, J.T.; Schoepp, N.G.; Ismagilov, R.F. RNA markers enable phenotypic test of antibiotic susceptibility in Neisseria gonorrhoeae after 10 minutes of ciprofloxacin exposure. Sci. Rep. 2018, 8, 11606. [Google Scholar] [CrossRef] [Green Version]
- Kilkkinen, A.; Rissanen, H.; Klaukka, T.; Pukkala, E.; Heliövaara, M.; Huovinen, P.; Männistö, S.; Aromaa, A.; Knekt, P. Antibiotic use predicts an increased risk of cancer. Int. J. Cancer 2008, 123, 2152–2155. [Google Scholar] [CrossRef]
- Dik, V.K.; van Oijen, M.G.; Smeets, H.M.; Siersema, P.D. Frequent Use of Antibiotics Is Associated with Colorectal Cancer Risk: Results of a Nested Case-Control Study. Dig. Dis. Sci. 2016, 61, 255–264. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Haines, C.; Watson, A.J.M.; Hart, A.R.; Platt, M.J.; Pardoll, D.M.; Cosgrove, S.E.; Gebo, K.A.; Sears, C.L. Oral antibiotic use and risk of colorectal cancer in the United Kingdom, 1989-2012: A matched case-control study. Gut 2019, 68, 1971–1978. [Google Scholar] [CrossRef]
- Lu, L.; Zhuang, T.; Shao, E.; Liu, Y.; He, H.; Shu, Z.; Huang, Y.; Yao, Y.; Lin, S.; Lin, S.; et al. Association of antibiotic exposure with the mortality in metastatic colorectal cancer patients treated with bevacizumab-containing chemotherapy: A hospital-based retrospective cohort study. PLoS ONE 2019, 14, e0221964. [Google Scholar] [CrossRef]
- Nenclares, P.; Bhide, S.A.; Sandoval-Insausti, H.; Pialat, P.; Gunn, L.; Melcher, A.; Newbold, K.; Nutting, C.M.; Harrington, K.J. Impact of antibiotic use during curative treatment of locally advanced head and neck cancers with chemotherapy and radiotherapy. Eur. J. Cancer 2020, 131, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Pennock, G.K.; Chow, L.Q. The Evolving Role of Immune Checkpoint Inhibitors in Cancer Treatment. Oncologist 2015, 20, 812–822. [Google Scholar] [CrossRef] [Green Version]
- Overman, M.J.; Ernstoff, M.S.; Morse, M.A. Where We Stand With Immunotherapy in Colorectal Cancer: Deficient Mismatch Repair, Proficient Mismatch Repair, and Toxicity Management. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 239–247. [Google Scholar] [CrossRef]
- Scope, A.; Agero, A.L.; Dusza, S.W.; Myskowski, P.L.; Lieb, J.A.; Saltz, L.; Kemeny, N.E.; Halpern, A.C. Randomized double-blind trial of prophylactic oral minocycline and topical tazarotene for cetuximab-associated acne-like eruption. J. Clin. Oncol. 2007, 25, 5390–5396. [Google Scholar] [CrossRef]
- Nikolaou, V.; Stratigos, A.; Antoniou, C.; Kiagia, M.; Nikolaou, C.; Katsambas, A.; Syrigos, K. Pimecrolimus cream 1% for the treatment of papulopustular eruption related to epidermal growth factor receptor inhibitors: A case series and a literature review of therapeutic approaches. Dermatology 2010, 220, 243–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scope, A.; Lieb, J.A.; Dusza, S.W.; Phelan, D.L.; Myskowski, P.L.; Saltz, L.; Halpern, A.C. A prospective randomized trial of topical pimecrolimus for cetuximab-associated acnelike eruption. J. Am. Acad. Dermatol. 2009, 61, 614–620. [Google Scholar] [CrossRef]
- Pinta, F.; Ponzetti, A.; Spadi, R.; Fanchini, L.; Zanini, M.; Mecca, C.; Sonetto, C.; Ciuffreda, L.; Racca, P. Pilot clinical trial on the efficacy of prophylactic use of vitamin K1-based cream (Vigorskin) to prevent cetuximab-induced skin rash in patients with metastatic colorectal cancer. Clin. Colorectal Cancer 2014, 13, 62–67. [Google Scholar] [CrossRef] [Green Version]
- Jo, J.C.; Hong, Y.S.; Kim, K.P.; Lee, J.L.; Kim, H.J.; Lee, M.W.; Lim, S.B.; Yu, C.S.; Kim, J.C.; Kim, J.H.; et al. Topical vitamin K1 may not be effective in preventing acneiform rash during cetuximab treatment in patients with metastatic colorectal cancer. Eur. J. Dermatol. 2013, 23, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.J.; Brownell, I. BRAF Inhibitors for the Treatment of Papulopustular Eruptions from MAPK Pathway Inhibitors. Am. J. Clin. Dermatol. 2020, 21, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Lacouture, M.E.; Wainberg, Z.A.; Patel, A.B.; Anadkat, M.J.; Stemmer, S.M.; Shacham-Shmueli, E.; Medina, E.; Zelinger, G.; Shelach, N.; Ribas, A. Reducing skin toxicities from EGFR inhibitors with topical BRAF inhibitor therapy. Cancer Discov. 2021. [Google Scholar] [CrossRef]
- Costello, C.M.; Hill, H.E.; Brumfiel, C.M.; Yang, Y.W.; Swanson, D.L. Choosing between isotretinoin and acitretin for epidermal growth factor receptor inhibitor and small molecule tyrosine kinase inhibitor acneiform eruptions. J. Am. Acad. Dermatol. 2021, 84, 840–841. [Google Scholar] [CrossRef]
- Andrews, E.D.; Garg, N.; Patel, A.B. A retrospective chart review on oral retinoids as a treatment for epidermal growth factor receptor inhibitor- and mitogen-activated protein kinase kinase inhibitor-induced acneiform eruptions. J. Am. Acad. Dermatol. 2020, 82, 998–1000. [Google Scholar] [CrossRef] [Green Version]
- Caruana, M.; Hatami, A.; Marcoux, D.; Perreault, S.; McCuaig, C.C. Isotretinoin for the treatment of severe acneiform eruptions associated with the MEK inhibitor trametinib. JAAD Case Rep. 2020, 6, 1056–1058. [Google Scholar] [CrossRef]
- Mima, K.; Sukawa, Y.; Nishihara, R.; Qian, Z.R.; Yamauchi, M.; Inamura, K.; Kim, S.A.; Masuda, A.; Nowak, J.A.; Nosho, K.; et al. Fusobacterium nucleatum and T Cells in Colorectal Carcinoma. JAMA Oncol. 2015, 1, 653–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biagi, E.; Nylund, L.; Candela, M.; Ostan, R.; Bucci, L.; Pini, E.; Nikkïla, J.; Monti, D.; Satokari, R.; Franceschi, C.; et al. Through ageing, and beyond: Gut microbiota and inflammatory status in seniors and centenarians. PLoS ONE 2010, 5, e10667. [Google Scholar] [CrossRef]
- Walker, A.W.; Ince, J.; Duncan, S.H.; Webster, L.M.; Holtrop, G.; Ze, X.; Brown, D.; Stares, M.D.; Scott, P.; Bergerat, A.; et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011, 5, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.J.; Vangay, P.; Al-Ghalith, G.A.; Hillmann, B.M.; Ward, T.L.; Shields-Cutler, R.R.; Kim, A.D.; Shmagel, A.K.; Syed, A.N.; Walter, J.; et al. Daily Sampling Reveals Personalized Diet-Microbiome Associations in Humans. Cell Host Microbe 2019, 25, 789–802.e85. [Google Scholar] [CrossRef] [PubMed]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Eilers, R.E., Jr.; Gandhi, M.; Patel, J.D.; Mulcahy, M.F.; Agulnik, M.; Hensing, T.; Lacouture, M.E. Dermatologic infections in cancer patients treated with epidermal growth factor receptor inhibitor therapy. J. Natl. Cancer Inst. 2010, 102, 47–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomková, H.; Kohoutek, M.; Zábojníková, M.; Pospísková, M.; Ostrízková, L.; Gharibyar, M. Cetuximab-induced cutaneous toxicity. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 692–696. [Google Scholar] [CrossRef]
- Wang, J.; Li, S.; Liu, Y.; Zhang, C.; Li, H.; Lai, B. Metastatic patterns and survival outcomes in patients with stage IV colon cancer: A population-based analysis. Cancer Med. 2020, 9, 361–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihai, M.M.; Ion, A.; Giurcăneanu, C.; Nițipir, C.; Popa, A.-M.; Chifiriuc, M.-C.; Popa, M.I.; Říčař, J.; Popa, L.G.; Sârbu, I.; et al. The Impact of Long-Term Antibiotic Therapy of Cutaneous Adverse Reactions to EGFR Inhibitors in Colorectal Cancer Patients. J. Clin. Med. 2021, 10, 3219. https://doi.org/10.3390/jcm10153219
Mihai MM, Ion A, Giurcăneanu C, Nițipir C, Popa A-M, Chifiriuc M-C, Popa MI, Říčař J, Popa LG, Sârbu I, et al. The Impact of Long-Term Antibiotic Therapy of Cutaneous Adverse Reactions to EGFR Inhibitors in Colorectal Cancer Patients. Journal of Clinical Medicine. 2021; 10(15):3219. https://doi.org/10.3390/jcm10153219
Chicago/Turabian StyleMihai, Mara Mădălina, Ana Ion, Călin Giurcăneanu, Cornelia Nițipir, Ana-Maria Popa, Mariana-Carmen Chifiriuc, Mircea Ioan Popa, Jan Říčař, Liliana Gabriela Popa, Ionela Sârbu, and et al. 2021. "The Impact of Long-Term Antibiotic Therapy of Cutaneous Adverse Reactions to EGFR Inhibitors in Colorectal Cancer Patients" Journal of Clinical Medicine 10, no. 15: 3219. https://doi.org/10.3390/jcm10153219










