Oral Antioxidant Treatment of Men Significantly Improves the Reproductive Outcome of IVF Cycles
Abstract
:1. Introduction
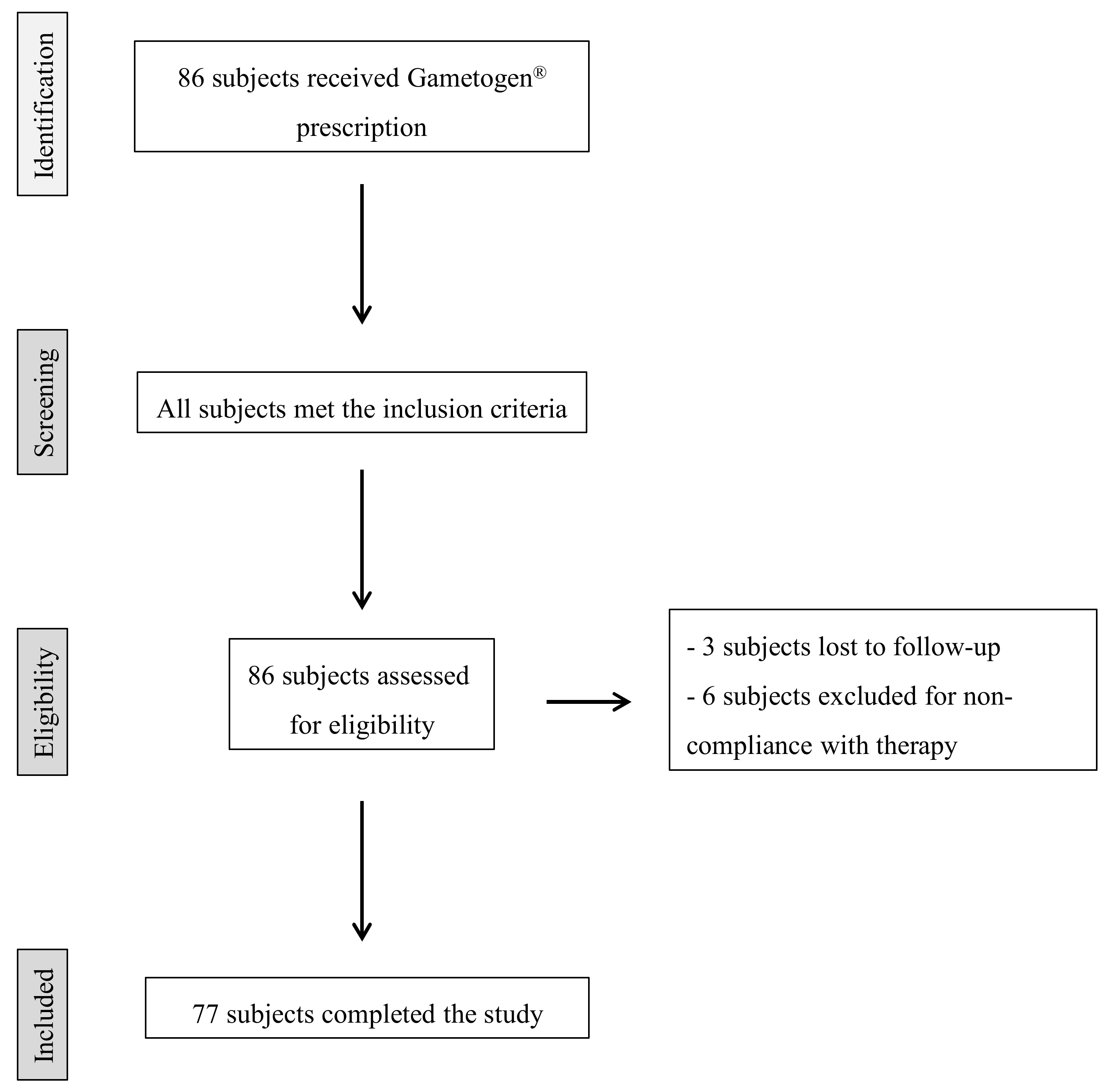
2. Materials and Methods
2.1. Study Design and Participants
2.2. Outcomes Measures
2.3. Sperm Analysis
2.4. Evaluation of Sperm DNA Fragmentation
2.5. Assisted Reproduction Techniques
2.6. Vitrification and Warming of Blastocysts
2.7. Data Collection and Statistical Analyses
3. Results
3.1. Semen Parameters before and after Oral Antioxidant Treatment
3.2. Embryological Outcomes of Cycles with Ejaculated Spermatozoa before and after Oral Antioxidant Treatment
3.3. Clinical Outcomes of Cycles with Ejaculated Spermatozoa before and after Oral Antioxidant Treatment
3.4. Perinatal Characteristics of Babies from Cycles with Ejaculated Spermatozoa after Oral Antioxidant Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, N.; Singh, A.K. Trends of male factor infertility, an important cause of infertility: A review of literature. J. Hum. Reprod. Sci. 2015, 8, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Majzoub, A. Role of Antioxidants in Assisted Reproductive Techniques. World J. Mens Health 2017, 35, 77–93. [Google Scholar] [CrossRef]
- Tremellen, K. Oxidative stress and male infertility—A clinical perspective. Hum. Reprod. Update 2008, 14, 243–258. [Google Scholar] [CrossRef]
- Pourmasumi, S.; Sabeti, P.; Rahiminia, T.; Mangoli, E.; Tabibnejad, N.; Talebi, A.R. The etiologies of DNA abnormalities in male infertility: An assessment and review. Int. J. Reprod. Biomed. 2017, 15, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Opuwari, C.S.; Henkel, R.R. An update on oxidative damage to spermatozoa and oocytes. Biomed. Res. Int. 2016, 2016, 9540142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammadeh, M.E.; Al Hasani, S.; Rosenbaum, P.; Schmidt, W.; Fischer Hammadeh, C. Reactive oxygen species, total antioxidant concentration of seminal plasma and their effect on sperm parameters and outcome of IVF/ICSI patients. Arch. Gynecol. Obstet. 2008, 277, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Majzoub, A.; Agarwal, A. Systematic review of antioxidant types and doses in male infertility: Benefits on semen parameters, advanced sperm function, assisted reproduction and live-birth rate. Arab. J. Urol. 2018, 16, 113–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smits, R.M.; Mackenzie-Proctor, R.; Yazdani, A.; Stankiewicz, M.T.; Jordan, V.; Showell, M.G. Antioxidants for male subfertility. Cochrane Database Syst. Rev. 2019, 3, CD007411. [Google Scholar] [CrossRef]
- Geva, E.; Bartoov, B.; Zabludovsky, N.; Lessing, J.B.; Lerner-Geva, L.; Amit, A. The effect of antioxidant treatment on human spermatozoa and fertilization rate in an in vitro fertilization program. Fertil Steril 1996, 66, 430–434. [Google Scholar] [CrossRef]
- Greco, E.; Romano, S.; Iacobelli, M.; Ferrero, S.; Baroni, E.; Minasi, M.G.; Ubaldi, F.; Rienzi, L.; Tesarik, J. ICSI in cases of sperm DNA damage: Beneficial effect of oral antioxidant treatment. Hum. Reprod. 2005, 20, 2590–2594. [Google Scholar] [CrossRef] [Green Version]
- Korosi, T.; Barta, C.; Rokob, K.; Torok, T. Physiological Intra-Cytoplasmic Sperm Injection (PICSI) outcomes after oral pretreatment and semen incubation with myo-inositol in oligoasthenoteratozoospermic men: Results from a prospective, randomized controlled trial. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 66–72. [Google Scholar]
- Rago, R.; Gallo, M.; Dal Lago, A.; Licata, E.; Paciotti, G.; Amodei, M.; Meneghini, C.; Fabiani, C.; Dani, G.; Liberanome, C.; et al. Controlled, prospective, observational study on the efficiency and tolerability of a combination of potential Nrf2-inducing antioxidants and micronutrients as pre-treatment for ICSI in dyspermic patients with previous failure. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1645–1652. [Google Scholar]
- Tremellen, K.; Miari, G.; Froiland, D.; Thompson, J. A randomised control trial examining the effect of an antioxidant (Menevit) on pregnancy outcome during IVF-ICSI treatment. Aust. N. Z. J. Obstet. Gynaecol. 2007, 47, 216–221. [Google Scholar] [CrossRef]
- Gambera, L.; Stendardi, A.; Ghelardi, C.; Fineschi, B.; Aini, R. Effects of antioxidant treatment on seminal parameters in patients undergoing in vitro fertilization. Arch. Ital. Urol. Androl. 2019, 91, 3. [Google Scholar] [CrossRef]
- Canepa, P.; Dal Lago, A.; De Leo, C.; Gallo, M.; Rizzo, C.; Licata, E.; Anserini, P.; Rago, R.; Scaruffi, P. Combined treatment with myo-inositol, alpha-lipoic acid, folic acid and vitamins significantly improves sperm parameters of sub-fertile men: A multi-centric study. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7078–7085. [Google Scholar] [CrossRef]
- World Health Organization, Department of Reproductive Health and Research. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; WHO Press: Geneva, Switzerland, 2010. [Google Scholar]
- Bertino, E.; Spada, E.; Occhi, L.; Coscia, A.; Giuliani, F.; Gagliardi, L.; Gilli, G.; Bona, G.; Fabris, C.; De Curtis, M.; et al. Neonatal anthropometric charts: The Italian neonatal study compared with other European studies. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 353–361. [Google Scholar] [CrossRef] [Green Version]
- Massarotti, C.; Stigliani, S.; Ramone, A.; Bovis, F.; Sozzi, F.; Remorgida, V.; Cagnacci, A.; Anserini, P.; Scaruffi, P. Occurrence of smooth endoplasmic reticulum aggregates in metaphase II oocytes: Relationship with stimulation protocols and outcome of ICSI and IVF cycles. Hum. Reprod. 2021, 36, 907–917. [Google Scholar] [CrossRef]
- Kuwayama, M. Highly efficient vitrification for cryopreservation of human oocytes and embryos: The Cryotop method. Theriogenology 2007, 67, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Cardona Barberán, A.; Boel, A.; Vanden Meerschaut, F.; Stoop, D.; Heindryckx, B. Diagnosis and Treatment of Male Infertility-Related Fertilization Failure. J. Clin. Med. 2020, 9, 3899. [Google Scholar] [CrossRef]
- Zorn, B.; Vidmar, G.; Meden-Vrtovec, H. Seminal reactive oxygen species as predictors of fertilization, embryo quality and pregnancy rates after conventional in vitro fertilization and intracytoplasmic sperm injection. Int. J. Androl. 2003, 26, 279–285. [Google Scholar] [CrossRef]
- Oumaima, A.; Tesnim, A.; Zohra, H.; Amira, S.; Ines, Z.; Sana, C.; Intissar, G.; Lobna, E.; Ali, J.; Meriem, M. Investigation on the origin of sperm morphological defects: Oxidative attacks, chromatin immaturity, and DNA fragmentation. Environ. Sci. Pollut. Res. Int. 2018, 25, 13775–13786. [Google Scholar] [CrossRef]
- Tesarik, J. Paternal effects on cell division in the human preimplantation embryo. Reprod. Biomed. Online 2005, 10, 370–375. [Google Scholar] [CrossRef]
- Tesařík, J.; Kopečný, V.; Plachot, M.; Mandelbaum, J. Activation of nucleolar and extranucleolar RNA synthesis and changes in the ribosomal content of human embryos developing in vitro. J. Reprod. Fertil. 1986, 78, 463–470. [Google Scholar] [CrossRef] [Green Version]
- Kuroda, S.; Takeshima, T.; Takeshima, K.; Usui, K.; Yasuda, K.; Sanjo, H.; Kawahara, T.; Uemura, H.; Murase, M.; Yumura, Y. Early and late paternal effects of reactive oxygen species in semen on embryo development after intracytoplasmic sperm injection. Syst. Biol. Reprod. Med. 2020, 66, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Simon, L.; Murphy, K.; Shamsi, M.B.; Liu, L.; Emery, B.; Aston, K.I.; Carrell, D.T. Paternal influence of sperm DNA integrity on early embryonic development. Hum. Reprod. 2014, 29, 2402–2412. [Google Scholar] [CrossRef] [PubMed]
- Amorini, A.M.; Listorti, I.; Bilotta, G.; Pallisco, R.; Saab, M.W.; Mangione, R.; Manca, B.; Lazzarino, G.; Tavazzi, B.; Lazzarino, G.; et al. Antioxidant-Based Therapies in Male Infertility: Do We Have Sufficient Evidence Supporting Their Effectiveness? Antioxidants 2021, 10, 220. [Google Scholar] [CrossRef]
- Ghanem, H.; Shaeer, O.; El-Segini, A. Combination clomiphene citrate and antioxidant therapy for idiopathic male infertility: A randomized controlled trial. Fertil Steril 2010, 93, 2232–2235. [Google Scholar] [CrossRef]
- Barekat, F.; Tavalaee, M.; Deemeh, M.R.; Bahreinian, M.; Azadi, L.; Abbasi, H.; Rozbahani, S.; Nasr-Esfahani, M.H. A Preliminary Study: N-acetyl-L-cysteine Improves Semen Quality following Varicocelectomy. Int. J. Fertil. Steril. 2016, 10, 120–126. [Google Scholar] [CrossRef]
| Female age (years; mean ± SD, range) | 35.3 ± 3.2, 20–38 |
| Male age (years; mean ± SD, range) | 38.6 ± 4.8, 30–53 |
| Etiology of infertility (N, %) | |
| Idiopathic | 31/77, 40% |
| Female | 10/77, 13% |
| Male | 26/77, 34% |
| Combined | 10/77, 13% |
| Male BMI (kg/m2, mean ± SD, range) | 25.5 ± 3.1, 19.8–35.9 |
| Male smoking habit (N, %) | 26/77, 34% |
| T0 | T90 | p-Value | |
|---|---|---|---|
| Sperm concentration (×106/mL) | 27.2 ± 32.7 | 27.5 ± 26.9 | 0.027 |
| Number of spermatozoa (×106/mL) | 58.3 ± 64.1 | 63.5 ± 59.2 | 0.040 |
| Progressive motility (%) | 27.6 ± 17.2 | 34.6 ± 16.7 | <0.0001 |
| Total motile sperm count (×106) | 26.8 ± 35.3 | 33.1 ± 38.9 | 0.003 |
| Sperm DNA Fragmentation 1 (%) | 28.3 ± 25.1 | 16.3 ± 7.9 | 0.078 |
| T0 | T90 | Unadjusted p-Value | Adjusted p-Value 1 | |
|---|---|---|---|---|
| Patient’s age at pick-up (years) | 35.3 ± 3.2 | 35.4 ± 3.1 | 0.777 | 0.232 |
| N retrieved oocytes | 10.1 ± 3.6 | 9.7 ± 4.9 | 0.016 | 0.082 |
| N MII oocytes | 7.9 ± 2.9 | 7.9 ± 3.7 | 0.329 | 0.329 |
| N inseminated oocytes | 7.1 ± 2.2 | 7.2 ± 3.8 | 0.233 | 0.329 |
| Fertilization rate (%) | 39.8 ± 16.2 | 72.9 ± 19.9 | <0.0001 | <0.0001 |
| Cleavage rate (%) | 66.9 ± 24.7 | 71.5 ± 25.6 | 0.022 | 0.088 |
| N cleavage-stage embryos | 2.1 ± 1.1 | 3.3 ± 2.3 | <0.0001 | <0.0001 |
| Top quality embryos (%) | 64.8 ± 37.4 | 82.7 ± 27.6 | <0.0001 | <0.0001 |
| Patients who developed at least one blastocyst (N, %) | 4/77 (5%) | 36/77 (47%) | <0.0001 | <0.0001 |
| N developed blastocysts | 0.2 ± 0.6 | 1.1 ± 1.4 | 0.0001 | 0.001 |
| T0 | T90 | Unadjusted p-Value | Adjusted p-Value 1 | |
|---|---|---|---|---|
| Cancelled embryo transfer (%) | 9/77 (12) | 3/77 (4) | 0.068 | 0.614 |
| Absence of fertilized oocytes (%) | 4/77 (5) | 0 | 0.048 | 0.476 |
| Absence of viable embryos (%) | 3/77 (4) | 0 | 0.077 | 0.618 |
| Delayed embryo transfer (freeze-all) (%) | 2/77 (3) | 3/77 (4) | 0.736 | 0.974 |
| N transferred embryos | 1.8 ± 0.6 | 1.5 ± 0.6 | 0.022 | 0.240 |
| Cycles with day 2–3 ET | 65/68 (96%) | 45/74 (61%) | <0.0001 | <0.0001 |
| Implantation (%) | 2/124 (2%) | 15/85 (18%) | <0.0001 | <0.0001 |
| Pregnancy (%) | 2/65 (3%) | 15/45 (33%) | 0.0001 | 0.001 |
| Live-birth rate (%) | 0/124 (0%) | 13/83 (16%) 2 | <0.0001 | <0.0001 |
| Miscarriage (%) | 2/2 (100%) | 0/15 (0%) | <0.0001 | <0.0001 |
| Cycles with day 5 ET | 3/68 (4%) | 29/73 (40%) | <0.0001 | <0.0001 |
| Implantation (%) | 0/4 (0%) | 10/32 (31%) | 0.475 | 0.974 |
| Pregnancy (%) | 0/3 (0%) | 10/29 (34%) | 0.577 | 0.974 |
| Live-birth rate (%) | 0/4 (0%) | 10/32 (31%) | 0.475 | 0.974 |
| Miscarriage (%) | 0% | 0/10 (0%) | - | |
| N ET in freeze–thaw cycles | 3 | 9 | ||
| Implantation (%) | 1/4 (25%) | 4/9 (44%) | 0.974 | 0.974 |
| Pregnancy (%) | 1/3 (3%) | 4/9 (44%) | 0.551 | 0.974 |
| Live-birth (%) | 0/1 (0%) | -/9 3 | - | |
| Miscarriage (%) | 1/1 (100%) | 1/4 (25%) | 0.819 | 0.974 |
| Cumulative pregnancy rate per cycle (%) | 1/71 (2%) | 29/83 (35%) | <0.0001 | |
| Cumulative live-birth rate per cycle (%) | 0/132 (0%) | 23/124 (19%) 4 | <0.0001 | <0.0001 |
| Cumulative miscarriage (%) | 3/3 (100%) | 1/29 (3%) | 0.0001 | 0.001 |
| Cumulative pregnancy rate per couple (%) | 1/77 (1%) | 29/77 (38%) | <0.0001 | <0.0001 |
| Cumulative live-birth rate per couple (%) | 0/77 (0%) | 23/77 (30%) 4 | <0.0001 | <0.0001 |
| T90 Cycles | |
|---|---|
| N live births | 23 |
| N lost follow-up | 1 |
| N ongoing pregnancies | 5 |
| Birthweight (grams) | 3147.7 ± 321.5 |
| N birthweight < 2500 g | 0 |
| Gestational age (weeks) | 39.0 ± 1.1 |
| N prematurity < 37 weeks | 0 |
| Birthweight centiles | 34.7 ± 22.9 |
| SDS-score | −0.4 ± 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scaruffi, P.; Licata, E.; Maccarini, E.; Massarotti, C.; Bovis, F.; Sozzi, F.; Stigliani, S.; Dal Lago, A.; Casciano, I.; Rago, R.; et al. Oral Antioxidant Treatment of Men Significantly Improves the Reproductive Outcome of IVF Cycles. J. Clin. Med. 2021, 10, 3254. https://doi.org/10.3390/jcm10153254
Scaruffi P, Licata E, Maccarini E, Massarotti C, Bovis F, Sozzi F, Stigliani S, Dal Lago A, Casciano I, Rago R, et al. Oral Antioxidant Treatment of Men Significantly Improves the Reproductive Outcome of IVF Cycles. Journal of Clinical Medicine. 2021; 10(15):3254. https://doi.org/10.3390/jcm10153254
Chicago/Turabian StyleScaruffi, Paola, Emanuele Licata, Elena Maccarini, Claudia Massarotti, Francesca Bovis, Fausta Sozzi, Sara Stigliani, Alessandro Dal Lago, Ida Casciano, Rocco Rago, and et al. 2021. "Oral Antioxidant Treatment of Men Significantly Improves the Reproductive Outcome of IVF Cycles" Journal of Clinical Medicine 10, no. 15: 3254. https://doi.org/10.3390/jcm10153254






