New Closure Method Using Loop and Open–Close Clips after Endoscopic Submucosal Dissection of Stomach and Colon Lesions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
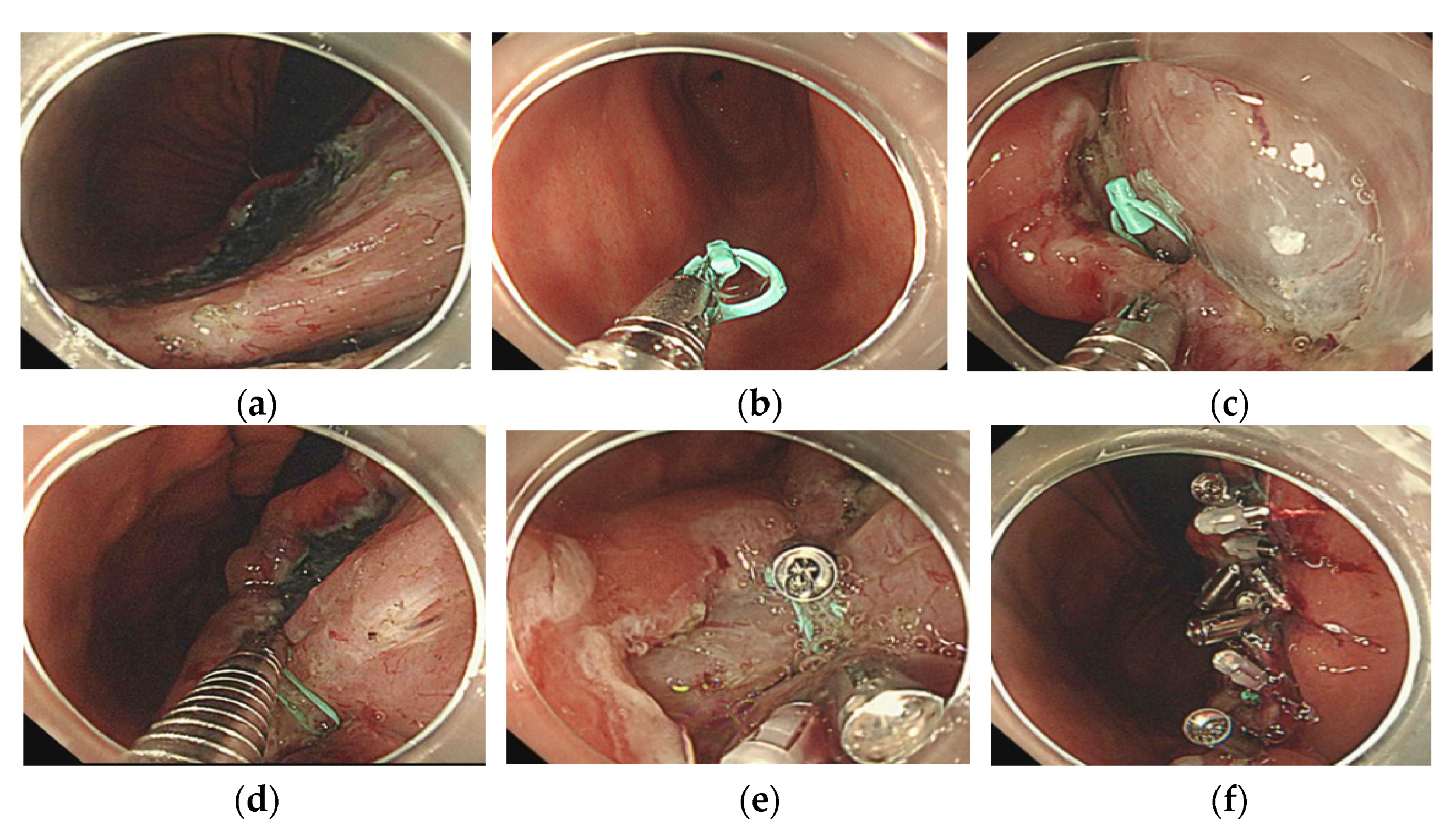
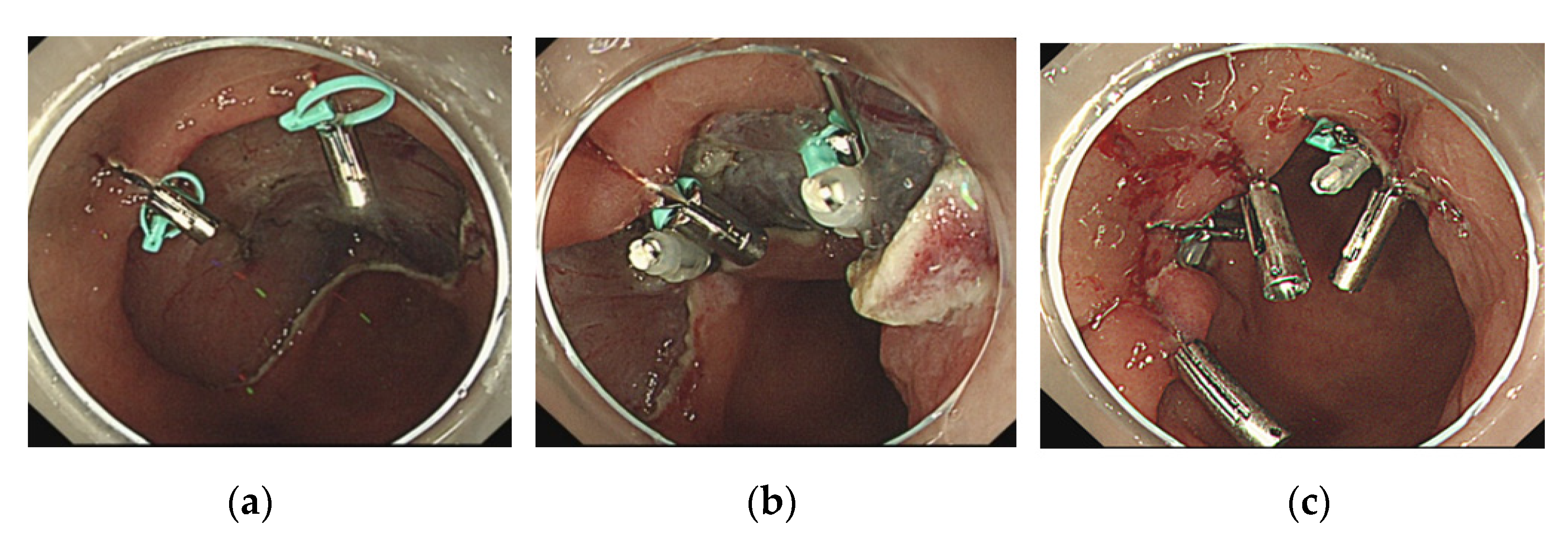
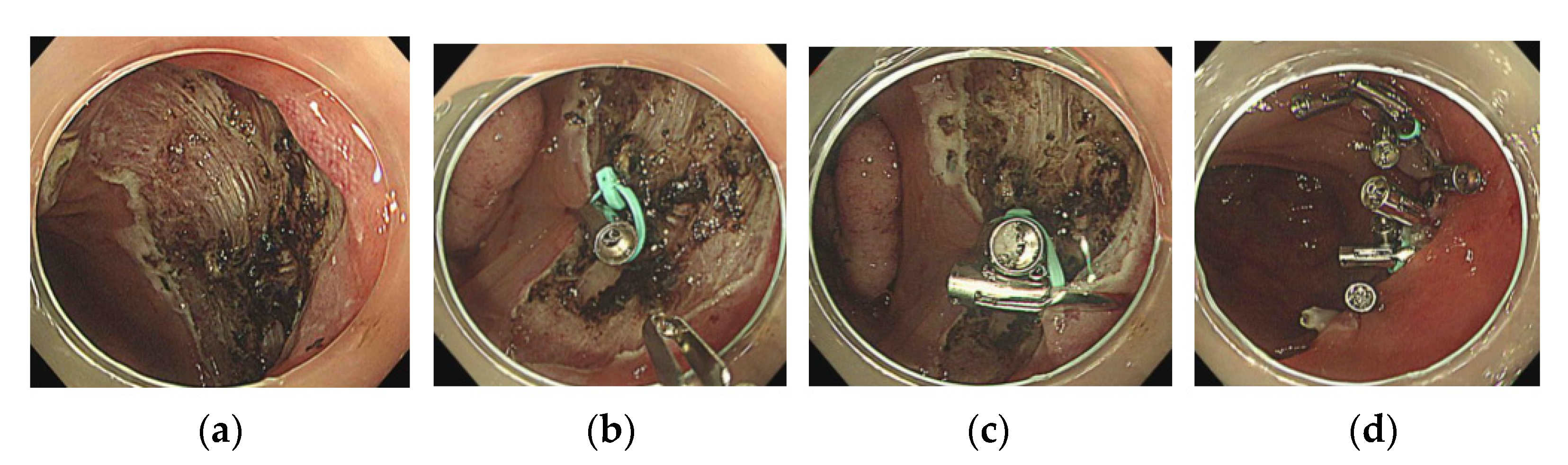
2.2. The Loop and Open–Close Clip Closure Method
- 1.
- An open–close clip (SureClip) and loop were prepared;
- 2.
- After removal of the forceps plug from the endoscope, the SureClip was passed through the forceps plug and then opened to grasp the loop;
- 3.
- The forceps plug was reattached to the endoscope, and the clip was advanced with the loop to bring it to the edge of the post-ESD mucosal defect;
- 4.
- The first clip with a loop was used to grasp the center of the mucosal defect;
- 5.
- The next clip was used to drag the loop and grasp the distal side of the mucosal defect.
2.3. Evaluation of Procedural Outcomes
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ono, H.; Kondo, H.; Gotoda, T.; Shirao, K.; Yamaguchi, H.; Saito, D.; Hosokawa, K.; Shimoda, T.; Yoshida, S. Endoscopic mucosal resection for treatment of early gastric cancer. Gut 2001, 48, 225–229. [Google Scholar] [CrossRef] [Green Version]
- Isomoto, H.; Shikuwa, S.; Yamaguchi, N.; Fukuda, E.; Ikeda, K.; Nishiyama, H.; Ohnita, K.; Mizuta, Y.; Shiozawa, J.; Kohno, S. Endoscopic submucosal dissection for early gastric cancer: A large-scale feasibility study. Gut 2009, 58, 331–336. [Google Scholar] [CrossRef]
- Nishizawa, T.; Yahagi, N. Long-term outcomes of using endoscopic submucosal dissection to treat early gastric cancer. Gut Liver 2018, 12, 119–124. [Google Scholar] [CrossRef] [Green Version]
- Hayee, B.; Inoue, H.; Sato, H.; Santi, E.G.; Yoshida, A.; Onimaru, M.; Ikeda, H.; Kudo, S. Magnification narrow-band imaging for the diagnosis of early gastric cancer: A review of the Japanese literature for the Western endoscopist. Gastrointest. Endosc. 2013, 78, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Y.; Othman, M. EMR/ESD: Techniques, complications, and evidence. Curr. Gastroenterol. Rep. 2020, 22, 39. [Google Scholar] [CrossRef]
- Saito, Y.; Uraoka, T.; Yamaguchi, Y.; Hotta, K.; Sakamoto, N.; Ikematsu, H.; Fukuzawa, M.; Kobayashi, N.; Nasu, J.; Michida, T.; et al. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest. Endosc. 2010, 72, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Isomoto, H.; Nishiyama, H.; Yamaguchi, N.; Fukuda, E.; Ishii, H.; Ikeda, K.; Ohnita, K.; Nakao, K.; Kohno, S.; Shikuwa, S. Clinicopathological factors associated with clinical outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms. Endoscopy 2009, 41, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, X.; Ge, J.; Yang, C.; Liu, J.; Zhao, S. Endoscopic submucosal dissection vs. endoscopic mucosal resection for colorectal tumors: A meta-analysis. World J. Gastroenterol. 2014, 20, 8282–8287. [Google Scholar] [CrossRef]
- Eun, Y.; Kang, C.; Kim, J. The effectiveness and safety of endoscopic submucosal dissection compared with endoscopic mucosal resection for early gastric cancer: A systematic review and metaanalysis. Surg. Endosc. 2011, 25, 2666–2677. [Google Scholar] [CrossRef]
- Hanaoka, N.; Uedo, N.; Ishihara, R.; Higashino, K.; Takeuchi, Y.; Inoue, T.; Chatani, R.; Hanafusa, M.; Tsujii, Y.; Kanzaki, H.; et al. Clinical features and outcomes of delayed perforation after endoscopic submucosal dissection for early gastric cancer. Endoscopy 2010, 42, 1112–1115. [Google Scholar] [CrossRef]
- Kawata, N.; Ono, H.; Takizawa, K.; Kakushima, N.; Tanaka, M.; Igarashi, K.; Yoshida, M.; Kishida, Y.; Iwai, T.; Ito, S.; et al. Efficacy of polyglycolic acid sheets and fibrin glue for prevention of bleeding after gastric endoscopic submucosal dissection in patients under continued antithrombotic agents. Gastric Cancer 2018, 21, 696–702. [Google Scholar] [CrossRef] [Green Version]
- Osada, T.; Sakamoto, N.; Ritsuno, H.; Murakami, T.; Ueyama, H.; Matsumoto, K.; Shibuya, T.; Ogihara, T.; Watanabe, S. Closure with clips to accelerate healing of mucosal defects caused by colorectal endoscopic submucosal dissection. Surg. Endosc. 2016, 30, 4438–4444. [Google Scholar] [CrossRef]
- Harada, H.; Suehiro, S.; Murakami, D.; Nakahara, R.; Ujihara, T.; Shimizu, T.; Miyama, Y.; Katsuyama, Y.; Hayasaka, K.; Tounou, S. Clinical impact of prophylactic clip closure of mucosal defects after colorectal endoscopic submucosal dissection. Endosc. Int. Open 2017, 5, e1165–e1171. [Google Scholar] [CrossRef] [Green Version]
- Albéniz, E.; Álvarez, M.; Espinós, J.; Nogales, O.; Guarner, C.; Alonso, P.; Rodríguez-Téllez, M.; de Tejada, A.H.; Santiago, J.; Bustamante-Balén, M.; et al. Clip closure after resection of large colorectal lesions with substantial risk of bleeding. Gastroenterology 2019, 157, 1213–1221. [Google Scholar] [CrossRef] [Green Version]
- Nomura, T.; Matsuzaki, I.; Sugimoto, S.; Oyamda, J.; Kamei, A.; Kobayashi, M. Clip-on-clip closure method for a mucosal defect after colorectal endoscopic submucosal dissection: A prospective feasibility study. Surg. Endosc. 2020, 34, 1412–1416. [Google Scholar] [CrossRef]
- Goto, O.; Sasaki, M.; Akimoto, T.; Ochiai, Y.; Kiguchi, Y.; Mitsunaga, Y.; Fujimoto, A.; Maehata, T.; Nishizawa, T.; Takeuchi, H.; et al. Endoscopic hand-suturing for defect closure after gastric endoscopic submucosal dissection: A pilot study in animals and in humans. Endoscopy 2017, 49, 792–797. [Google Scholar] [CrossRef]
- Yahagi, N.; Nishizawa, T.; Akimoto, T.; Ochiai, Y.; Goto, O. New endoscopic suturing method: String clip suturing method. Gastrointest. Endosc. 2016, 84, 1064–1106. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, S.; Nishizawa, T.; Fujimoto, A.; Mori, H.; Nakazato, Y.; Kikuchi, M.; Uraoka, T. Efficacy of mucosa-submucosa clip closure method after gastric endoscopic submucosal dissection. World J. Gastrointest. Endosc. 2020, 12, 17–22. [Google Scholar] [CrossRef]
- Akimoto, T.; Goto, O.; Sasaki, M.; Ochiai, Y.; Maehata, T.; Fujimoto, A.; Nishizawa, T.; Yahagi, N. “Hold-and-drag” closure technique using repositionable clips for large mucosal defects after colonic endoscopic submucosal dissection. Endosc. Int. Open 2016, 4, e1068–e1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, B.; Kim, B.; Kim, H.; Choi, H.; Ji, J.; Hwang, S.; Cho, Y.; Chae, H.; Choi, K. Routine mucosal closure with a detachable snare and clips after endoscopic submucosal dissection for gastric epithelial neoplasms: A randomized controlled trial. Gut Liver 2011, 5, 454–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maekawa, S.; Nomura, R.; Murase, T.; Ann, Y.; Hard, M. Complete closure of artificial gastric ulcer after endoscopic submucosal dissection by combined use of a single over-the-scope clip and through-the-scope clips (with videos). Surg. Endosc. 2015, 29, 500–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakamoto, N.; Osada, T.; Shibuya, T.; Beppu, K.; Matsumoto, K.; Mori, H.; Kawabe, M.; Nagahara, A.; Otaka, M.; Ogihara, T.; et al. Endoscopic submucosal dissection of large colorectal tumors by using a novel spring-action S-O clip for traction (with video). Gastrointest. Endosc. 2009, 69, 1370–1374. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Aihara, H.; Thompson, A.; Schulman, A.; Thompson, C.; Ryou, M. Choosing the right through-the-scope clip: A rigorous comparison of rotatability, whip, open/close precision, and closure strength (with videos). Gastrointest. Endosc. 2019, 89, 77–86. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.; Wei, K.; Deng, J.; Zhou, X.; Huang, X. Effects of antithrombotic therapy on bleeding after endoscopic submucosal dissection. Gastrointest. Endosc. 2017, 86, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Ogiyama, H.; Inoue, T.; Maekawa, A.; Yoshii, S.; Yamaguchi, S.; Nagai, K.; Yamamoto, M.; Egawa, S.; Horimoto, M.; Ogawa, H.; et al. Effect of anticoagulants on the risk of delayed bleeding after colorectal endoscopic submucosal dissection. Endosc. Int. Open 2020, 8, e1654–e1663. [Google Scholar] [CrossRef]

| Patient Characteristics | Value | |
|---|---|---|
| Patient information | ||
| Age (years) | 71.4 ± 6.5 | |
| Stomach | 72.9 ± 6.7 | |
| Colon | 69.7 ± 5.9 | |
| Sex | 15/19 (78.9) | |
| Stomach Male | 7/10 (70.0) | |
| Colon Male | 8/9 (88.9) | |
| Antiplatelet therapy | 3 (15.8) | |
| Anticoagulant therapy | 5 (26.3) | |
| Antiplatelet and/or coagulant therapy | 6 (31.6) | |
| Antiplatelet and coagulant therapy | 3 (15.8) | |
| Lesion characteristics | ||
| Stomach location | 10 (52.6) | |
| Lower third | 2 | |
| Middle third | 6 | |
| Upper third | 1 | |
| Remnant | 1 | |
| Stomach circumference | ||
| Anterior wall | 1 | |
| Posterior wall | 3 | |
| Lesser curvature | 5 | |
| Greater curvature | 1 | |
| Colon location | 9 (47.3) | |
| Cecum | 2 | |
| Ascending colon | 3 | |
| Transverse colon | 3 (hepatic flexure: 2) | |
| Descending colon | 0 | |
| Sigmoid colon | 1 | |
| Rectum | 0 | |
| ESD factors | ||
| Procedural time (min ± SD) | Stomach | 99.7 ± 48.5 |
| Colon | 71.2 ± 47.4 | |
| En bloc resection | 19 (100) | |
| R0 resection | 19 (100) | |
| Perforation | 0 (0) | |
| Lesion size (mm) | ||
| Stomach/colon resection size | 40.6 ± 7.1 | |
| Stomach/colon tumor size | 25.2 ± 9.4 | |
| Stomach resection size | 40.4 ± 5.9 | |
| Colon resection size | 40.9 ± 8.2 | |
| Stomach tumor size | 22.1 ± 8.7 | |
| Colon tumor size | 28.8 ± 8.8 | |
| Outcomes | Location | Value |
|---|---|---|
| Complete closure | Stomach and colon | 17 (89) |
| Stomach | 8 (80) | |
| Colon | 9 (100) | |
| Semi-complete closure | Stomach | 2 (10) |
| Colon | 0 (0) | |
| Number of loops (n ± SD) | Stomach | 2.6 ± 0.7 |
| Colon | 1.4 ± 0.7 | |
| Number of clips used (n ± SD) | Stomach | 9.8 ± 2.3 |
| Colon | 8.2 ± 1.7 | |
| LOCCM time (min ± SD) | Stomach | 24.9 ± 10.9 |
| Colon | 18.7 ± 8.0 | |
| Lesion pathology | ||
| Adenocarcinoma | Stomach | 9 (90) |
| Colon | 3 (33) | |
| Adenoma with high-grade dysplasia | Stomach | 1 (10) |
| Colon | 6 (67) | |
| Delayed bleeding | 0 (0) | |
| Delayed perforation | 0 (0) | |
| Discharge after ESD (days ± SD) | Stomach | 4.8 ± 1.1 |
| Colon | 3.8 ± 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, A.; Kurumi, H.; Ikebuchi, Y.; Kawaguchi, K.; Yashima, K.; Kamitani, Y.; Yasui, S.; Nakada, Y.; Kanda, T.; Takata, T.; et al. New Closure Method Using Loop and Open–Close Clips after Endoscopic Submucosal Dissection of Stomach and Colon Lesions. J. Clin. Med. 2021, 10, 3260. https://doi.org/10.3390/jcm10153260
Yoshida A, Kurumi H, Ikebuchi Y, Kawaguchi K, Yashima K, Kamitani Y, Yasui S, Nakada Y, Kanda T, Takata T, et al. New Closure Method Using Loop and Open–Close Clips after Endoscopic Submucosal Dissection of Stomach and Colon Lesions. Journal of Clinical Medicine. 2021; 10(15):3260. https://doi.org/10.3390/jcm10153260
Chicago/Turabian StyleYoshida, Akira, Hiroki Kurumi, Yuichiro Ikebuchi, Koichiro Kawaguchi, Kazuo Yashima, Yu Kamitani, Sho Yasui, Yusuke Nakada, Tsutomu Kanda, Tomoaki Takata, and et al. 2021. "New Closure Method Using Loop and Open–Close Clips after Endoscopic Submucosal Dissection of Stomach and Colon Lesions" Journal of Clinical Medicine 10, no. 15: 3260. https://doi.org/10.3390/jcm10153260







