Prognosis of Atrial Fibrillation Patients Undergoing PCI According to Anticoagulants and Antiplatelet Agents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Definitions
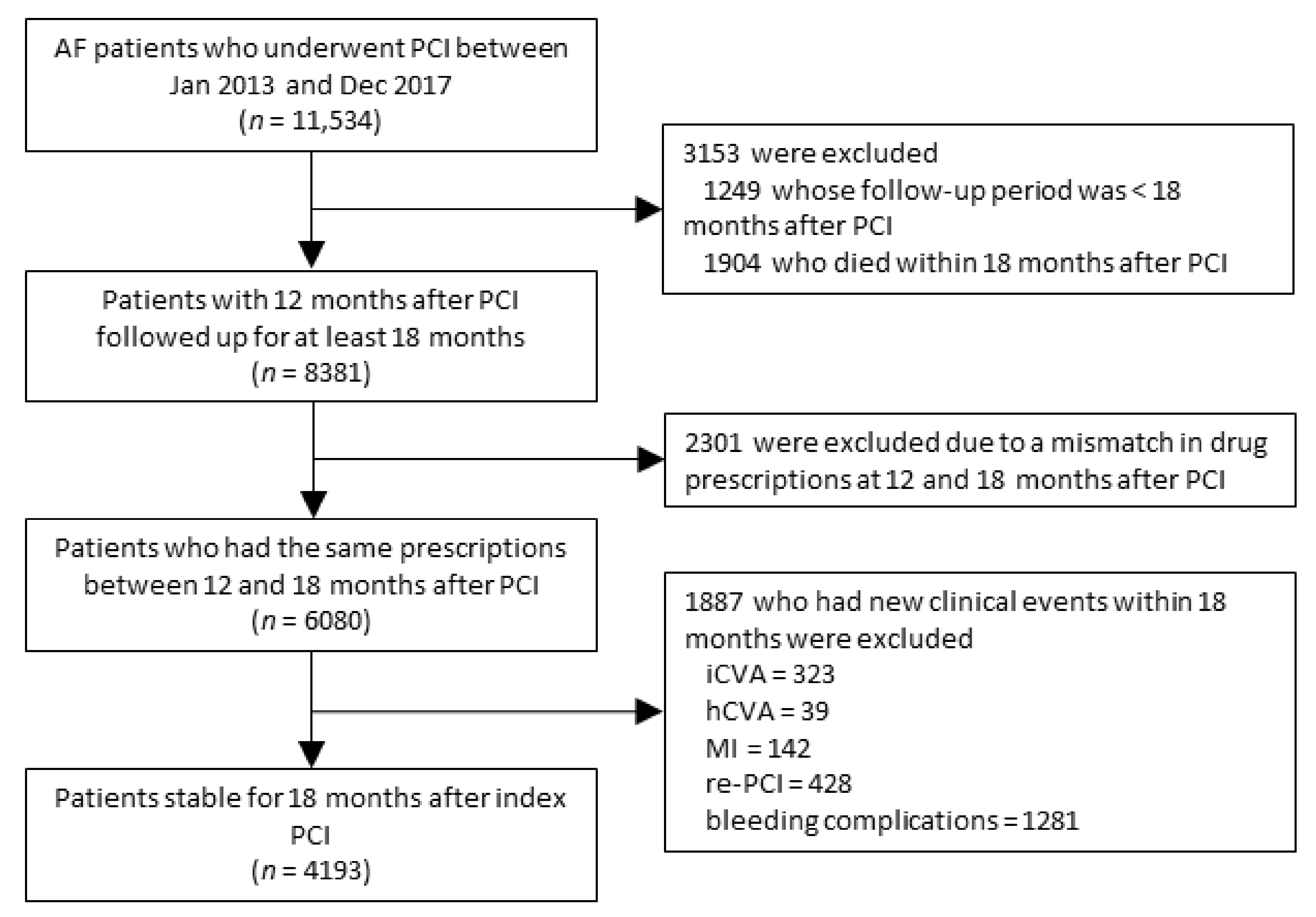
2.3. Study Population
2.4. Clinical Outcomes
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
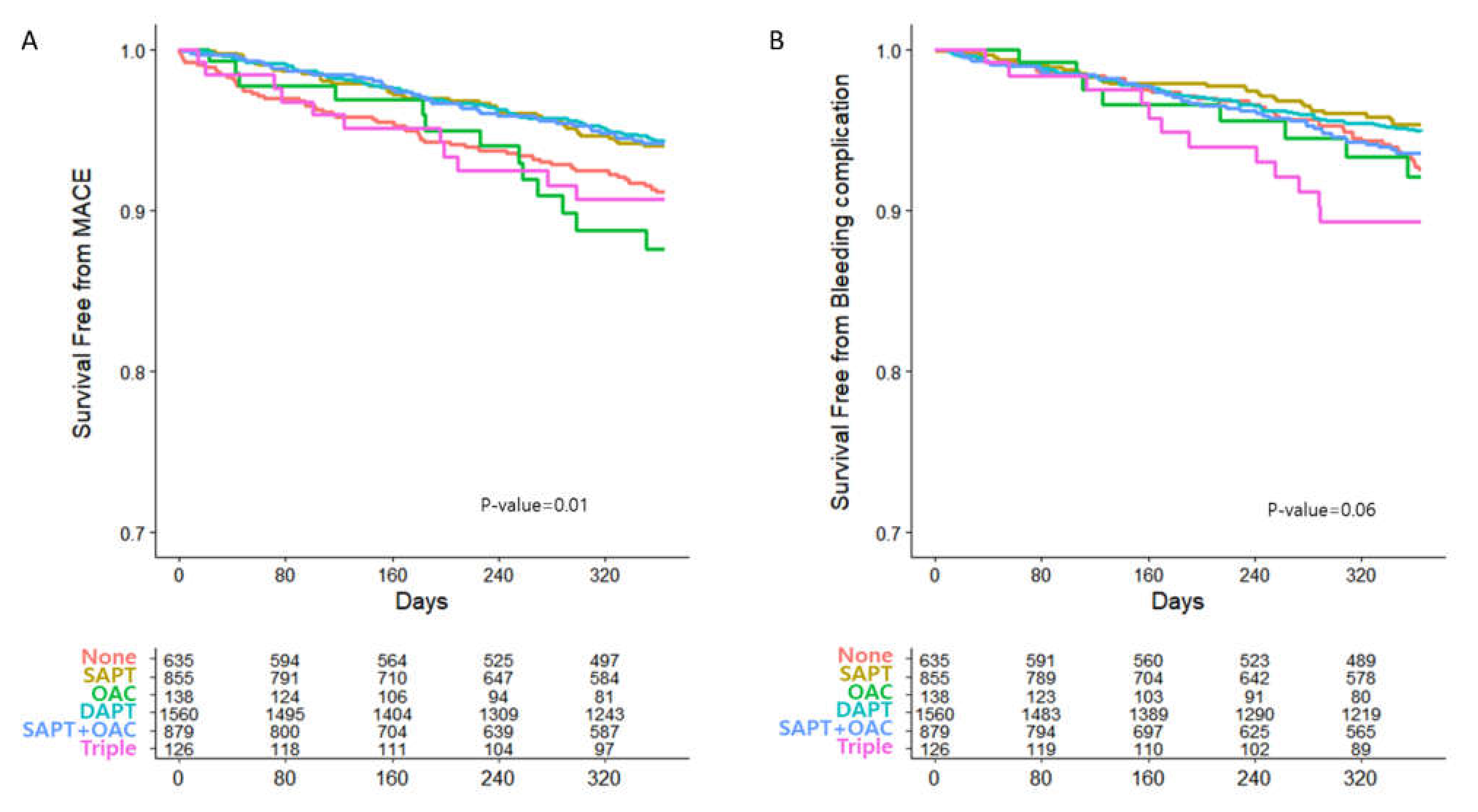
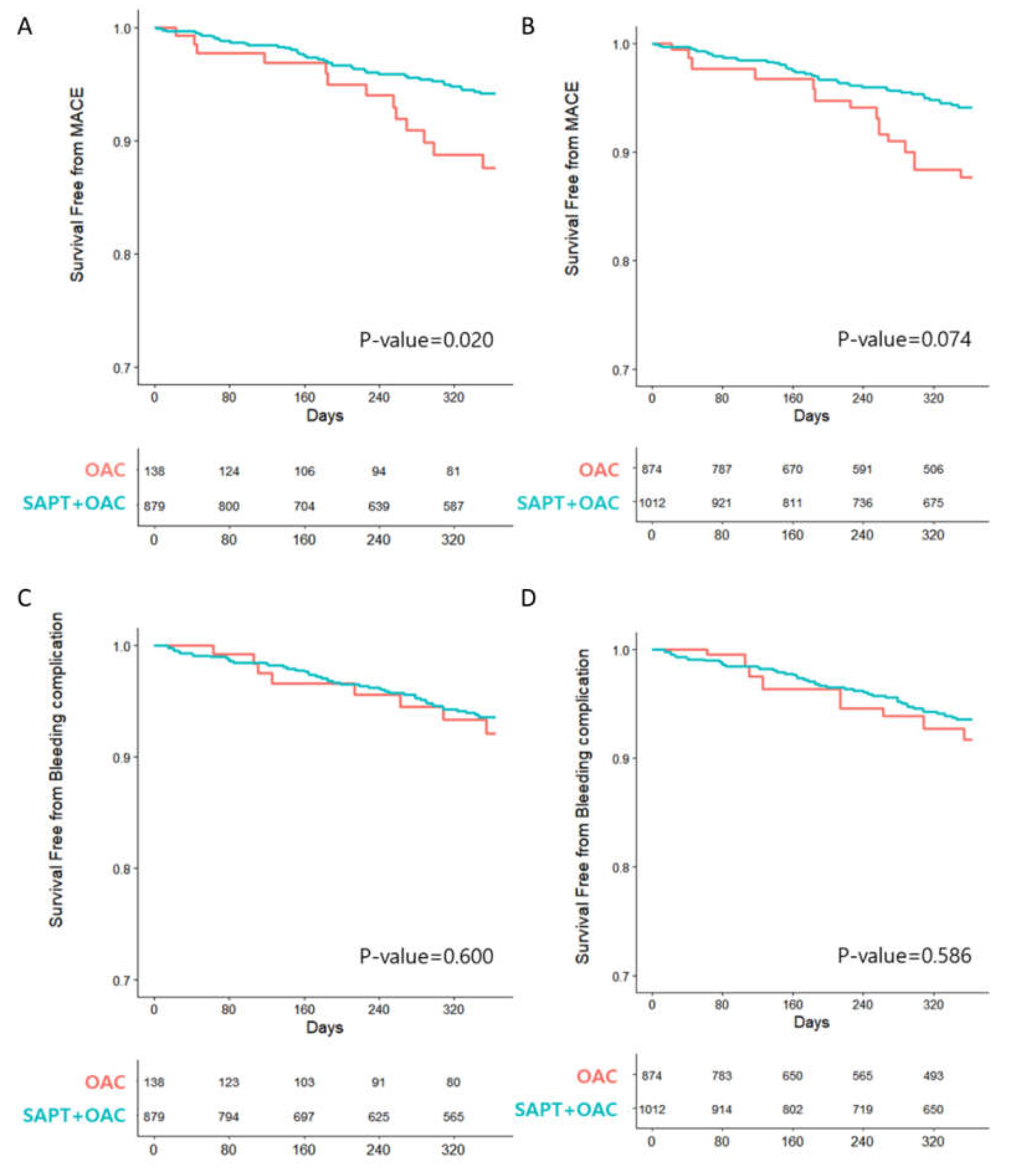
3.2. Clinical Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Angiolillo, D.J.; Goodman, S.G.; Bhatt, D.L.; Eikelboom, J.W.; Price, M.J.; Moliterno, D.J.; Cannon, C.P.; Tanguay, J.F.; Granger, C.B.; Mauri, L.; et al. Antithrombotic Therapy in Patients with Atrial Fibrillation Undergoing Percutaneous Coronary Intervention: A North American Perspective—2016 Update. Circ. Cardiovasc. Interv. 2016, 9, e004395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kralev, S.; Schneider, K.; Lang, S.; Suselbeck, T.; Borggrefe, M. Incidence and severity of coronary artery disease in patients with atrial fibrillation undergoing first-time coronary angiography. PLoS ONE 2011, 6, e24964. [Google Scholar] [CrossRef]
- Michniewicz, E.; Mlodawska, E.; Lopatowska, P.; Tomaszuk-Kazberuk, A.; Malyszko, J. Patients with atrial fibrillation and coronary artery disease—Double trouble. Adv. Med. Sci. 2018, 63, 30–35. [Google Scholar] [CrossRef]
- Lip, G.; Freedman, B.; De Caterina, R.; Potpara, T.S. Stroke prevention in atrial fibrillation: Past, present and future. Comparing the guidelines and practical decision-making. Thromb. Haemost. 2017, 117, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, M.; Bueno, H.; Byrne, R.A.; Collet, J.P.; Costa, F.; Jeppsson, A.; Juni, P.; Kastrati, A.; Kolh, P.; Mauri, L.; et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2018, 39, 213–260. [Google Scholar] [PubMed]
- Hindricks, G.; Potpara, T.; Nikolaos, D.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [PubMed]
- Angiolillo, D.J.; Bhatt, D.L.; Cannon, C.P.; Eikelboom, J.W.; Gibson, C.M.; Goodman, S.G.; Granger, C.B.; Holmes, D.R.; Lopes, R.D.; Mehran, R.; et al. Antithrombotic Therapy in Patients with Atrial Fibrillation Treated with Oral Anticoagulation Undergoing Percutaneous Coronary Intervention: A North American Perspective: 2021 Update. Circulation 2021, 143, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Lam, D.H.; Bell, S.M.; Hira, R.S. Concomitant Use of Antiplatelets and Anticoagulants in Patients with Coronary Heart Disease and Atrial Fibrillation: What Do Recent Clinical Trials Teach Us? Curr. Atheroscler. Rep. 2018, 20, 4. [Google Scholar] [CrossRef] [PubMed]
- Dans, A.L.; Connolly, S.J.; Wallentin, L.; Yang, S.; Nakamya, J.; Brueckmann, M.; Ezekowitz, M.; Oldgren, J.; Eikelboom, J.W.; Reilly, P.A.; et al. Concomitant use of antiplatelet therapy with dabigatran or warfarin in the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial. Circulation 2013, 127, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.H.; Wojdyla, D.; Vora, A.N.; Thomas, L.; Granger, C.B.; Goodman, S.G.; Aronson, R.; Windecker, S.; Mehran, R.; Lopes, R.D. Risk/Benefit Tradeoff of Antithrombotic Therapy in Patients with Atrial Fibrillation Early and Late after an Acute Coronary Syndrome or Percutaneous Coronary Intervention. Circulation 2020, 141, 1618–1627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andò, G.; Costa, F. Double or triple antithrombotic therapy after coronary stenting and atrial fibrillation: A systematic review and meta-analysis of randomized clinical trials. Int. J. Cardiol. 2020, 302, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Ancedy, Y.; Lecoq, C.; Saint Etienne, C.; Ivanes, F.; Angoulvant, D.; Babuty, D.; Lip, G.Y.; Fauchier, L. Antithrombotic management in patients with atrial fibrillation undergoing coronary stent implantation: What is the impact of guideline adherence? Int. J. Cardiol. 2016, 203, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Ono, F.; Tanaka, S.; Nakao, Y.M.; Kawakami, K. Utilization of Anticoagulant and Antiplatelet Agents Among Patients with Atrial Fibrillation Undergoing Percutaneous Coronary Intervention- Retrospective Cohort Study Using a Nationwide Claims Database in Japan. Circ. J. 2018, 82, 361–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Choi, E.K.; Han, K.D.; Choi, Y.J.; Lee, S.R.; Cha, M.J.; Kang, J.; Park, K.W.; Oh, S.; Lip, G.Y.H. Antithrombotic Therapy in Patients with Atrial Fibrillation after Percutaneous Coronary Intervention During 2-Year Follow-Up, from a Nationwide Population Study. Am. J. Circ. 2019, 123, 1921–1926. [Google Scholar] [CrossRef] [PubMed]
- Matsumura-Nakano, Y.; Shizuta, S.; Komasa, A.; Morimoto, T.; Masuda, H.; Shiomi, H.; Goto, K.; Nakai, K.; Ogawa, H.; Kobori, A.; et al. Open-Label Randomized Trial Comparing Oral Anticoagulation with and without Single Antiplatelet Therapy in Patients with Atrial Fibrillation and Stable Coronary Artery Disease beyond 1 Year after Coronary Stent Implantation. Circulation 2019, 139, 604–616. [Google Scholar] [CrossRef] [PubMed]
- Fischer, Q.; Georges, J.L.; Feuvre, C.L.; Sharma, A.; Hammoudi, N.; Berman, E.; Cohen, S.; Jolivet, I.; Silvain, J.; Helft, G. Optimal long-term antithrombotic treatment of patients with stable coronary artery disease and atrial fibrillation: “OLTAT registry”. Int. J. Cardiol. 2018, 264, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, S.; Kaikita, K.; Akao, M.; Ako, J.; Matoba, T.; Nakamura, M.; Miyauchi, K.; Hagiwara, N.; Kimura, K.; Hirayama, A.; et al. Antithrombotic Therapy for Atrial Fibrillation with Stable Coronary Disease. N. Eng. J. Med. 2019, 381, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Gragnano, F.; Spirito, A.; Corpataux, N.; Vaisnora, L.; Galea, R.; Gargiulo, G.; Siontis, G.C.M.; Praz, F.; Lanz, J.; Billinger, M.; et al. Impact of Clinical Presentation on Bleeding Risk after Percutaneous Coronary Intervention and Implications for the ARC-HBR Definition. EuroIntervention 2021. [Google Scholar] [CrossRef]
| Variables | Total | No Treatment | SAPT | OAC Monotherapy | DAPT | OAC with SAPT | OAC with DAPT | p-Value |
|---|---|---|---|---|---|---|---|---|
| (n = 4193) | (n = 635) | (n = 855) | (n = 138) | (n = 1560) | (n = 879) | (n = 126) | ||
| Age, years | 69.8 ± 10.6 | 70.8 ± 10.8 | 69.0 ± 10.9 | 72.3 ± 8.4 | 68.9 ± 11.1 | 71.0 ± 9.2 | 68.5 ± 10.2 | <0.001 |
| Male | 2874 (68.5) | 443 (69.7) | 560 (65.5) | 77 (55.8) | 1090 (69.8) | 611 (69.5) | 93 (73.8) | 0.003 |
| Baseline risk factors | ||||||||
| Hypertension | 3595 (85.7) | 541 (85.2) | 720 (84.2) | 125 (90.5) | 1302 (83.4) | 797 (90.6) | 110 (87.3) | <0.001 |
| Diabetes mellitus | 1494 (35.6) | 243 (38.2) | 280 (32.7) | 49 (35.5) | 556 (35.6) | 312 (35.4) | 54 (42.8) | 0.162 |
| Renal disease | 398 (9.4) | 121 (19.0) | 65 (7.6) | 12 (8.7) | 121 (7.7) | 70 (7.9) | 9 (7.1) | <0.001 |
| Liver disease | 108 (2.5) | 20 (3.1) | 19 (2.2) | 6 (4.3) | 43 (2.7) | 17 (1.9) | 3 (2.3) | 0.460 |
| Dyslipidemia | 4060 (96.8) | 621 (97.8) | 829 (96.9) | 133 (96.3) | 1506 (96.5) | 848 (96.4) | 123 (97.6) | 0.682 |
| Pre-existing cardiovascular disease | ||||||||
| Ischemic heart disease | 4186 (99.8) | 634 (99.8) | 854 (99.8) | 137 (99.2) | 1559 (99.9) | 877 (99.7) | 125 (99.2) | 0.231 |
| Prior myocardial infarction | 1810 (43.1) | 287 (45.2) | 364 (42.5) | 45 (32.6) | 695 (44.5) | 358 (40.7) | 61 (48.4) | 0.032 |
| Peripheral artery disease | 1134 (27.0) | 207 (32.6) | 216 (25.2) | 41 (29.7) | 414 (26.5) | 227 (25.8) | 29 (23.0) | 0.018 |
| Prior stroke | 1498 (35.7) | 260 (40.9) | 247 (28.8) | 67 (48.5) | 515 (33.0) | 360 (40.9) | 49 (38.8) | <0.001 |
| Heart failure | 2396 (57.1) | 384 (60.4) | 427 (49.9) | 92 (66.6) | 854 (54.7) | 562 (63.9) | 77 (61.1) | <0.001 |
| Atrial fibrillation | 3479 (82.9) | 539 (84.8) | 692 (80.9) | 120 (86.9) | 1227 (78.6) | 795 (90.4) | 106 (84.1) | <0.001 |
| CHA2DS2-VASc score | 4.90 (1.9) | 5.10 (1.8) | 4.63 (2.0) | 5.62 (1.8) | 4.72 (2.0) | 5.21 (1.7) | 4.95 (1.9) | <0.001 |
| Medications use | ||||||||
| Aspirin | 2395 (57.1) | 0 (0) | 462 (54.0) | 0 (0) | 1560 (100) | 247 (28.1) | 126 (100) | |
| P2Y12 inhibitors | 2711 (64.6) | 0 (0) | 393 (45.9) | 0 (0) | 1560 (100) | 632 (71.9) | 126 (100) | |
| Warfarin | 507 (12.0) | 0 (0) | 0 (0) | 42 (30.4) | 0 (0) | 372 (42.3) | 93 (73.8) | |
| OAC | 636 (15.1) | 0 (0) | 0 (0) | 96 (69.5) | 0 (0) | 507 (57.6) | 33 (26.1) |
| (A) | ||||||||
| Outcomes | Total | No Treatment | SAPT | OAC Monotherapy | DAPT | OAC with SAPT | OAC with DAPT | p-Value |
| (n = 4193) | (n = 635) | (n = 855) | (n = 138) | (n = 1560) | (n = 879) | (n = 126) | ||
| Primary efficacy end point | ||||||||
| MACE | 242 (5.7) | 52 (8.1) | 43 (5.0) | 13 (9.4) | 81 (5.1) | 42 (4.7) | 11 (8.7) | 0.008 |
| All-cause of death | 172 (4.1) | 40 (6.3) | 31 (3.6) | 8 (5.8) | 55 (3.5) | 32 (3.6) | 6 (4.7) | 0.047 |
| MI | 23 (0.5) | 4 (0.6) | 6 (0.7) | 1 (0.7) | 6 (0.3) | 5 (0.5) | 1 (0.7) | 0.92 |
| Stroke | 77 (1.8) | 11 (1.7) | 14 (1.6) | 6 (4.3) | 29 (1.8) | 12 (1.3) | 5 (3.9) | 0.097 |
| Primary safety end point | ||||||||
| Bleeding complication | 207 (4.9) | 41 (6.4) | 32 (3.7) | 8 (5.8) | 70 (4.4) | 44 (5.0) | 12 (9.5) | 0.031 |
| (B) | ||||||||
| Outcomes | OAC Monotherapy | OAC with SAPT | p-Value | |||||
| (n = 138) | (n = 879) | |||||||
| Primary efficacy end point | ||||||||
| MACE | 13 (9.4) | 42 (4.7) | 0.017 | |||||
| All-cause of death | 8 (5.8) | 32 (3.6) | 0.175 | |||||
| MI | 1 (0.7) | 5 (0.5) | 0.798 | |||||
| Stroke | 6 (4.3) | 12 (1.3) | 0.009 | |||||
| Primary safety end point | ||||||||
| Bleeding complication | 8 (5.8) | 44 (5.0) | 0.587 | |||||
| Outcomes | Hazard Ratio | 95% CI | p-Value |
|---|---|---|---|
| Univariate | |||
| MACE | 0.48 | 0.26–0.89 | 0.019 |
| Bleeding complications | 0.83 | 0.39–1.76 | 0.630 |
| Adjusted by age, sex | |||
| MACE | 0.48 | 0.25–0.89 | 0.020 |
| Bleeding complications | 0.84 | 0.39–1.79 | 0.651 |
| Adjusted by CHA2DS2-VASc | |||
| MACE | 0.52 | 0.28–0.97 | 0.040 |
| Bleeding complications | 0.87 | 0.41–1.86 | 0.726 |
| Adjusted by all covariates | |||
| MACE | 0.52 | 0.28–0.98 | 0.043 |
| Bleeding complications | 0.86 | 0.40–1.84 | 0.697 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, G.-S.; Kim, S.-H.; Kang, S.-H.; Yoon, C.-H.; Cho, Y.-S.; Youn, T.-J.; Chae, I.-H. Prognosis of Atrial Fibrillation Patients Undergoing PCI According to Anticoagulants and Antiplatelet Agents. J. Clin. Med. 2021, 10, 3370. https://doi.org/10.3390/jcm10153370
Yoon G-S, Kim S-H, Kang S-H, Yoon C-H, Cho Y-S, Youn T-J, Chae I-H. Prognosis of Atrial Fibrillation Patients Undergoing PCI According to Anticoagulants and Antiplatelet Agents. Journal of Clinical Medicine. 2021; 10(15):3370. https://doi.org/10.3390/jcm10153370
Chicago/Turabian StyleYoon, Gwang-Seok, Sun-Hwa Kim, Si-Hyuck Kang, Chang-Hwan Yoon, Young-Seok Cho, Tae-Jin Youn, and In-Ho Chae. 2021. "Prognosis of Atrial Fibrillation Patients Undergoing PCI According to Anticoagulants and Antiplatelet Agents" Journal of Clinical Medicine 10, no. 15: 3370. https://doi.org/10.3390/jcm10153370
APA StyleYoon, G.-S., Kim, S.-H., Kang, S.-H., Yoon, C.-H., Cho, Y.-S., Youn, T.-J., & Chae, I.-H. (2021). Prognosis of Atrial Fibrillation Patients Undergoing PCI According to Anticoagulants and Antiplatelet Agents. Journal of Clinical Medicine, 10(15), 3370. https://doi.org/10.3390/jcm10153370






