Can VA-ECMO Be Used as an Adequate Treatment in Massive Pulmonary Embolism?
Abstract
1. Introduction
2. Material and Method
2.1. Patients
2.2. Criteria for VA-ECMO Implantation
2.3. VA-ECMO Cannulation and Management
2.4. Data Collection
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Competency in Medical Knowledge
Translational Outlook
References
- Kucher, N.; Rossi, E.; De Rosa, M.; Goldhaber, S.Z. Massive pulmonary embolism. Circulation 2006, 113, 577–582. [Google Scholar] [CrossRef]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jimenez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef] [PubMed]
- Meneveau, N.; Guillon, B.; Planquette, B.; Piton, G.; Kimmoun, A.; Gaide-Chevronnay, L.; Aissaoui, N.; Neuschwander, A.; Zogheib, E.; Dupont, H.; et al. Outcomes after extracorporeal membrane oxygenation for the treatment of high-risk pulmonary embolism: A multicentre series of 52 cases. Eur. Heart J. 2018, 39, 4196–4204. [Google Scholar] [CrossRef] [PubMed]
- Pineton de Chambrun, M.; Brechot, N.; Combes, A. The place of extracorporeal life support in cardiogenic shock. Curr. Opin. Crit. Care 2020, 26, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Kalra, R.; Kosmopoulos, M.; Goslar, T.; Raveendran, G.; Bartos, J.A.; Yannopoulos, D. Extracorporeal cardiopulmonary resuscitation for cardiac arrest. Curr. Opin. Crit. Care 2020, 26, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Brunner, M.E.; Siegenthaler, N.; Shah, D.; Licker, M.J.; Cikirikcioglu, M.; Brochard, L.; Bendjelid, K.; Giraud, R. Extracorporeal membrane oxygenation support as bridge to recovery in a patient with electrical storm related cardiogenic shock. Am. J. Emerg. Med. 2013, 31, e461–e466. [Google Scholar] [CrossRef]
- Pozzi, M.; Banfi, C.; Grinberg, D.; Koffel, C.; Bendjelid, K.; Robin, J.; Giraud, R.; Obadia, J.F. Veno-arterial extracorporeal membrane oxygenation for cardiogenic shock due to myocarditis in adult patients. J. Thorac. Dis. 2016, 8, E495–E502. [Google Scholar] [CrossRef]
- Maggio, P.; Hemmila, M.; Haft, J.; Bartlett, R. Extracorporeal life support for massive pulmonary embolism. J. Trauma 2007, 62, 570–576. [Google Scholar] [CrossRef]
- Corsi, F.; Lebreton, G.; Brechot, N.; Hekimian, G.; Nieszkowska, A.; Trouillet, J.L.; Luyt, C.E.; Leprince, P.; Chastre, J.; Combes, A.; et al. Life-threatening massive pulmonary embolism rescued by venoarterial-extracorporeal membrane oxygenation. Crit. Care 2017, 21, 76. [Google Scholar] [CrossRef]
- Guliani, S.; Das Gupta, J.; Osofsky, R.; Kraai, E.P.; Mitchell, J.A.; Dettmer, T.S.; Wray, T.C.; Tawil, I.; Rana, M.A.; Marinaro, J. Venoarterial extracorporeal membrane oxygenation is an effective management strategy for massive pulmonary embolism patients. J. Vasc. Surg. Venous Lymphat Disord. 2020, 9, 307–314. [Google Scholar] [CrossRef]
- George, B.; Parazino, M.; Omar, H.R.; Davis, G.; Guglin, M.; Gurley, J.; Smyth, S. A retrospective comparison of survivors and non-survivors of massive pulmonary embolism receiving veno-arterial extracorporeal membrane oxygenation support. Resuscitation 2018, 122, 1–5. [Google Scholar] [CrossRef]
- Yusuff, H.O.; Zochios, V.; Vuylsteke, A. Extracorporeal membrane oxygenation in acute massive pulmonary embolism: A systematic review. Perfusion 2015, 30, 611–616. [Google Scholar] [CrossRef]
- Abraham, P.; Arroyo, D.A.; Giraud, R.; Bounameaux, H.; Bendjelid, K. Understanding haemorrhagic risk following thrombolytic therapy in patients with intermediate-risk and high-risk pulmonary embolism: A hypothesis paper. Open Heart 2018, 5, e000735. [Google Scholar] [CrossRef]
- Giraud, R.; Banfi, C.; Siegenthaler, N.; Bendjelid, K. Massive pulmonary embolism leading to cardiac arrest: One pathology, two different ECMO modes to assist patients. J. Clin. Monit. Comput. 2015, 30, 933–937. [Google Scholar] [CrossRef]
- Pavlovic, G.; Banfi, C.; Tassaux, D.; Peter, R.E.; Licker, M.J.; Bendjelid, K.; Giraud, R. Peri-operative massive pulmonary embolism management: Is veno-arterial ECMO a therapeutic option? Acta Anaesthesiol. Scand. 2014, 58, 1280–1286. [Google Scholar] [CrossRef]
- Konstantinides, S.V.; Torbicki, A.; Agnelli, G.; Danchin, N.; Fitzmaurice, D.; Galie, N.; Gibbs, J.S.; Huisman, M.V.; Humbert, M.; Kucher, N.; et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur. Heart J. 2014, 35, 3033–3069. [Google Scholar] [CrossRef] [PubMed]
- Banfi, C.; Pozzi, M.; Brunner, M.E.; Rigamonti, F.; Murith, N.; Mugnai, D.; Obadia, J.F.; Bendjelid, K.; Giraud, R. Veno-arterial extracorporeal membrane oxygenation: An overview of different cannulation techniques. J. Thorac. Dis. 2016, 8, E875–E885. [Google Scholar] [CrossRef] [PubMed]
- Moret, M.; Banfi, C.; Sartorius, D.; Fumeaux, T.; Leeman-Refondini, C.; Sologashvili, T.; Reuse, J.; Nowicki, B.; Mamode-Premdjee, J.; Tassaux, D.; et al. [“Mobile” ECMO]. Rev. Med. Suisse 2014, 10, 2368–2370. [Google Scholar] [PubMed]
- Investigators, G.A. The effects of tissue plasminogen activator, streptokinase, or both on coronary-artery patency, ventricular function, and survival after acute myocardial infarction. N. Engl. J. Med. 1993, 329, 1615–1622. [Google Scholar] [CrossRef]
- Bendjelid, K. Right atrial pressure: Determinant or result of change in venous return? Chest 2005, 128, 3639–3640. [Google Scholar] [CrossRef][Green Version]
- Jaff, M.R.; McMurtry, M.S.; Archer, S.L.; Cushman, M.; Goldenberg, N.; Goldhaber, S.Z.; Jenkins, J.S.; Kline, J.A.; Michaels, A.D.; Thistlethwaite, P.; et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: A scientific statement from the American Heart Association. Circulation 2011, 123, 1788–1830. [Google Scholar] [CrossRef] [PubMed]
- Burrowes, K.S.; Clark, A.R.; Tawhai, M.H. Blood flow redistribution and ventilation-perfusion mismatch during embolic pulmonary arterial occlusion. Pulm. Circ. 2011, 1, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Tahir, U.A.; Carroll, B.; Pinto, D.S. Massive pulmonary embolism: Embolectomy or extracorporeal membrane oxygenation? Curr. Opin. Crit. Care 2019, 25, 630–637. [Google Scholar] [CrossRef]
- Weinberg, A.; Tapson, V.F.; Ramzy, D. Massive Pulmonary Embolism: Extracorporeal Membrane Oxygenation and Surgical Pulmonary Embolectomy. Semin. Respir. Crit. Care Med. 2017, 38, 66–72. [Google Scholar] [CrossRef]
- Dolmatova, E.V.; Moazzami, K.; Cocke, T.P.; Elmann, E.; Vaidya, P.; Ng, A.F.; Satya, K.; Narayan, R.L. Extracorporeal Membrane Oxygenation in Massive Pulmonary Embolism. Heart Lung 2017, 46, 106–109. [Google Scholar] [CrossRef]
- Swol, J.; Buchwald, D.; Strauch, J.; Schildhauer, T.A. Extracorporeal life support (ECLS) for cardiopulmonary resuscitation (CPR) with pulmonary embolism in surgical—A case series. Perfusion 2016, 31, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Goldhaber, S.Z.; Visani, L.; De Rosa, M. Acute pulmonary embolism: Clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet 1999, 353, 1386–1389. [Google Scholar] [CrossRef]
- Meyer, G.; Gisselbrecht, M.; Diehl, J.L.; Journois, D.; Sors, H. Incidence and predictors of major hemorrhagic complications from thrombolytic therapy in patients with massive pulmonary embolism. Am. J. Med. 1998, 105, 472–477. [Google Scholar] [CrossRef]
- Marti, C.; John, G.; Konstantinides, S.; Combescure, C.; Sanchez, O.; Lankeit, M.; Meyer, G.; Perrier, A. Systemic thrombolytic therapy for acute pulmonary embolism: A systematic review and meta-analysis. Eur. Heart J 2015, 36, 605–614. [Google Scholar] [CrossRef]
- Jerjes-Sanchez, C.; Ramirez-Rivera, A.; de Lourdes Garcia, M.; Arriaga-Nava, R.; Valencia, S.; Rosado-Buzzo, A.; Pierzo, J.A.; Rosas, E. Streptokinase and Heparin versus Heparin Alone in Massive Pulmonary Embolism: A Randomized Controlled Trial. J. Thromb. Thrombolysis 1995, 2, 227–229. [Google Scholar] [CrossRef]
- Scott, J.H.; Gordon, M.; Vender, R.; Pettigrew, S.; Desai, P.; Marchetti, N.; Mamary, A.J.; Panaro, J.; Cohen, G.; Bashir, R.; et al. Venoarterial Extracorporeal Membrane Oxygenation in Massive Pulmonary Embolism-Related Cardiac Arrest: A Systematic Review. Crit. Care Med. 2021, 49, 760–769. [Google Scholar] [CrossRef]
- Kabrhel, C.; Jaff, M.R.; Channick, R.N.; Baker, J.N.; Rosenfield, K. A multidisciplinary pulmonary embolism response team. Chest 2013, 144, 1738–1739. [Google Scholar] [CrossRef] [PubMed]
- Chaudhury, P.; Gadre, S.K.; Schneider, E.; Renapurkar, R.D.; Gomes, M.; Haddadin, I.; Heresi, G.A.; Tong, M.Z.; Bartholomew, J.R. Impact of Multidisciplinary Pulmonary Embolism Response Team Availability on Management and Outcomes. Am. J. Cardiol. 2019, 124, 1465–1469. [Google Scholar] [CrossRef] [PubMed]
- Al-Bawardy, R.; Rosenfield, K.; Borges, J.; Young, M.N.; Albaghdadi, M.; Rosovsky, R.; Kabrhel, C. Extracorporeal membrane oxygenation in acute massive pulmonary embolism: A case series and review of the literature. Perfusion 2019, 34, 22–28. [Google Scholar] [CrossRef]
- Soar, J.; Nolan, J.P.; Bottiger, B.W.; Perkins, G.D.; Lott, C.; Carli, P.; Pellis, T.; Sandroni, C.; Skrifvars, M.B.; Smith, G.B.; et al. European Resuscitation Council Guidelines for Resuscitation 2015: Section 3. Adult advanced life support. Resuscitation 2015, 95, 100–147. [Google Scholar] [CrossRef] [PubMed]
- Pasrija, C.; Kronfli, A.; George, P.; Raithel, M.; Boulos, F.; Herr, D.L.; Gammie, J.S.; Pham, S.M.; Griffith, B.P.; Kon, Z.N. Utilization of Veno-Arterial Extracorporeal Membrane Oxygenation for Massive Pulmonary Embolism. Ann. Thorac. Surg. 2018, 105, 498–504. [Google Scholar] [CrossRef]
| Variables | n = 36 |
|---|---|
| Age, years, median ± interquartile range (IQR) | 57 (23) |
| Male, n (%) | 27 (75) |
| BMI, kg/m2, median (IQR) | 27.8 (6.3) |
| Comorbidities, n (%) | |
| None | 5 (14) |
| Postoperative (orthopaedic/visceral/other surgery/polytraumatism) | 18 (50) |
| Medical (HBP, CKI, obesity) | 5 (13.9) |
| Recent stroke (ischemic or haemorrhagic) | 3 (8.3) |
| Other | 5 (13.9) |
| Pre-ECMO | |
| Cardiac arrest, n (%) | 22 (61.1) |
| Systemic fibrinolytic therapy, n (%) | 16 (44.4) |
| Catheter-directed thromboaspiration, n (%) | 5 (13.9) |
| No-flow time, min, median (IQR) | 0 (0) |
| Low-flow time, min, median (IQR) | 17.5 (52) |
| Systolic blood pressure, mmHg, median (IQR) | 76 (54) |
| Mean blood pressure, mmHg, median (IQR) | 59 (35) |
| Heart rate, bpm, median (IQR) | 108 (47) |
| pH, median (IQR) | 7.08 (0.38) |
| Blood lactate, mmol/L, median (IQR) | 8.3 (11.1) |
| Bicarbonate, mmol/L, median (IQR) | 15.2 (8) |
| PaO2/FiO2 ratio, mmHg, median (IQR) | 69 (28) |
| Creatinine, μmol/L, median (IQR) | 127 (62) |
| ASAT, U/L, median (IQR) | 219 (416) |
| ALAT, U/L, median (IQR) | 171 (377) |
| Quick, %, median (IQR) | 52 (47) |
| Cardiac echocardiography, n (%) | 36 (100) |
| RV dilation, n (%) | 36 (100) |
| RV/LV dimensions ratio, cm, median (IQR) | 2.1 (0.6) |
| TAPSE, mm, median (IQR) | 8 (5.2) |
| Chest CT-Scan, n (%) | 9 (25) |
| Surgical thrombectomy, n (%) | 0 (0) |
| ECMO during cardiopulmonary resuscitation n (%) | 13 (36.1) |
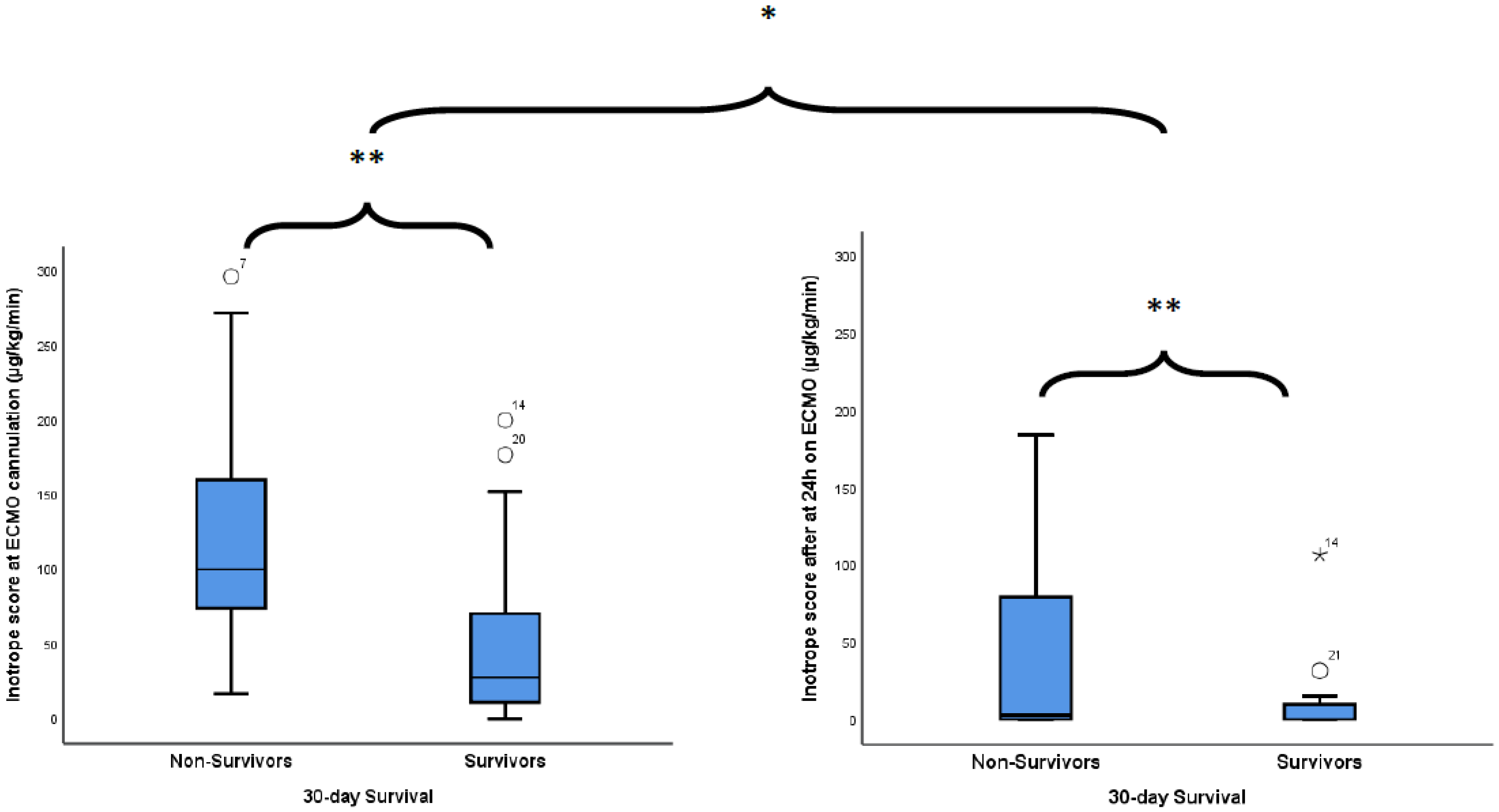
| Inotrope score at ECMO cannulation, µg/kg/min, median (IQR) | 49 (98) |
| Shock onset-to-ECMO interval, hours, median (IQR) | 1 (1.5) |
| Femoral–femoral VA-ECMO, n (%) | 36 (100) |
| Percutaneous VA-ECMO, n (%) | 25 (69.4) |
| Thrombolysis contraindication, n (%) | 21 (58.3) |
| SAPS II at ICU admission, median (IQR) | 68 (38) |
| One year follow-up | |
| Chronic dyspnoea n (%) | 1 (2.8) |
| Chronic thromboembolic pulmonary hypertension n (%) | 0 (0) |
| Pulmonary angioplasty, n (%) | 1 (2.8) |
| Surgical endarterectomy, n (%) | 1 (2.8) |
| Timing | Pre-ECMO | After 24 h on ECMO | p-Value | |
|---|---|---|---|---|
| Variables | ||||
| pH, median (IQR) | 7.08 (0.38) | 7.43 (0.1) | <0.001 1 | |
| Blood lactate, mmol/L, median (IQR) | 8.3 (11.1) | 1.1 (0.9) | <0.001 1 | |
| Inotrope score, μg/kg/min, median (IQR) | 49 (98) | 0 (10) | <0.001 1 | |
| Variables | All Patients (n = 36) | Non-Survivors (n = 13) | Survivors (n = 23) | p-Value |
|---|---|---|---|---|
| Pre-ECMO | ||||
| Inotrope score, μg/kg/min, median (IQR) | 49 (98) | 100 (86) | 28 (60) | 0.004 1 |
| Thrombolysis, n (%) | 13 (36.1) | 10 (76.9) | 6 (26.1) | 0.005 |
| pH, median (IQR) | 7.08 (0.38) | 6.99 (0.31) | 7.12 (0.42) | 0.055 1 |
| Blood lactate, mmol/L, median (IQR) | 8.3 (11.1) | 13.8 (8.5) | 4.2 (8.6) | 0.008 1 |
| Bicarbonate, mmol/L, median (IQR) | 15.2 (8) | 11 (7.1) | 17.1 (7.2) | 0.018 1 |
| PaO2/FiO2, mmHg, median (IQR) | 69 (28) | 61 (19) | 73 (37) | 0.415 1 |
| Creatinine, µmol/L, median (IQR) | 127 (62) | 137 (77) | 124 (47) | 0.214 1 |
| ASAT, U/L, median (IQR) | 219 (416) | 342 (538) | 125 (305) | 0.015 1 |
| ALAT, U/L, median (IQR) | 171 (377) | 309 (398) | 102 (243) | 0.026 1 |
| Quick, %, median (IQR) | 52 (47) | 43 (27) | 57 (52) | 0.034 1 |
| TAPSE, mm, median (IQR) | 8 (5.2) | 8 (5) | 8.1 (4.5) | 0.922 1 |
| RV/LV dimension ratio, median (IQR) | 2.1 (0.6) | 2.32 (0.84) | 2.1 (0.36) | 0.626 1 |
| No-flow time, min,median (IQR) | 0 (0) | 0 (0) | 0 (0) | 1.000 |
| Low-flow time, min,median (IQR) | 17.5 (52) | 50 (30) | 0 (25) | 0.002 1 |
| ECMO during cardiopulmonary resuscitation (vs. not), n (%) | 13 (36.1) | 8 (61.5) | 5 (21.7) | 0.030 3 |
| SAPS II at ICU admission, median (IQR) | 68 (38) | 75 (19) | 55 (42) | 0.020 1 |
| Inotrope score after 24 h of ECMO, μg/kg/min, median (IQR) | 49 (98) | 3 (44) | 0 (10) | 0.004 1 |
| In-ICU complications, n (%) | ||||
| Haemorrhage | 18 (50) | 12 (92.3) | 6 (26.1) | <0.001 3 |
| Stroke | 4 (11.1) | 1 (7.7) | 3 (13) | 0.541 2 |
| Infection | 3 (8.33) | 1 (7.7) | 2 (8.7) | 0.709 2 |
| Packed red-cell units transfused, n, median (IQR) | 4.5 (8.5) | 8 (7) | 0 (5) | 0.003 1 |
| Fresh-frozen plasma units transfused, n, mean ± SD | 1 (4) | 5 (11) | 0 (2) | 0.012 1 |
| Platelets transfused, n, mean ± SD | 1 (5) | 0 (1) | 0 (0) | 0.253 1 |
| ECMO duration, days, mean ± SD | 3.2 (3.2) | 1 (1.3) | 4 (2.4) | <0.001 1 |
| MV duration, days, median (IQR) | 2.4 (10.5) | 1 (1.3) | 8 (14) | 0.149 1 |
| ICU LOS, days, median (IQR) | 8.7 (16) | 1.2 (1.9) | 15.8 (13.4) | <0.001 1 |
| Hospital LOS, days, median (IQR) | 15 (36) | 1.3 (2) | 30 (44.5) | <0.001 1 |
| Variables | OR | 95%CI | p-Value 1 |
|---|---|---|---|
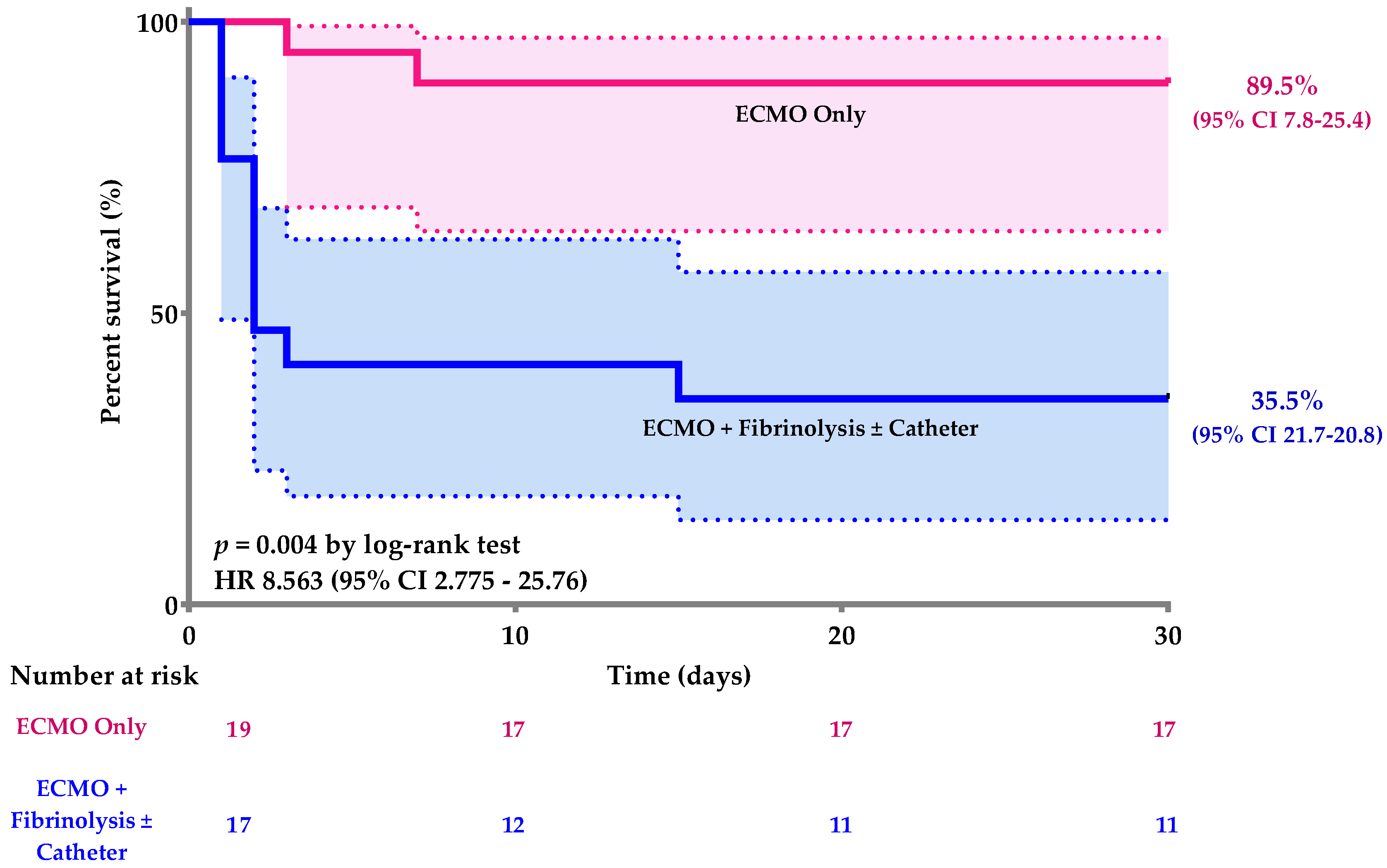
| ECMO only (vs ECMO + thrombolysis or catheter directed thromboaspiration) | 15.583 | 2.652–91.572 | 0.002 |
| Thrombolysis failure | 0.106 | 0.022–0.520 | 0.006 |
| ECMO during cardiopulmonary resuscitation (vs. not) | 0.174 | 0.039–0.773 | 0.022 |
| Pre-ECMO cardia arrest (vs. not) | 0.064 | 0.007–0.579 | 0.014 |
| Exposure | ECMO Only (n = 19) | ECMO + Fibrinolytic or Catheter-Directed Thromboaspiration (n = 17) | p-Value | |
|---|---|---|---|---|
| Outcomes | ||||
| In-ICU complications, n (%) | ||||
| Haemorrhage | 1 (5.3) | 17 (100) | <0.001 1 | |
| Stroke | 2 (10.5) | 2 (11.8) | 1.000 2 | |
| Infection | 1 (5.3) | 2 (11.8) | 0.593 2 | |
| Anoxic encephalopathy | 4 (28.6) | 10 (71.4) | 0.039 2 | |
| 30-day death status, n (%) | 2 (10.5) | 11 (64.7) | 0.001 1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giraud, R.; Laurencet, M.; Assouline, B.; De Charrière, A.; Banfi, C.; Bendjelid, K. Can VA-ECMO Be Used as an Adequate Treatment in Massive Pulmonary Embolism? J. Clin. Med. 2021, 10, 3376. https://doi.org/10.3390/jcm10153376
Giraud R, Laurencet M, Assouline B, De Charrière A, Banfi C, Bendjelid K. Can VA-ECMO Be Used as an Adequate Treatment in Massive Pulmonary Embolism? Journal of Clinical Medicine. 2021; 10(15):3376. https://doi.org/10.3390/jcm10153376
Chicago/Turabian StyleGiraud, Raphaël, Matthieu Laurencet, Benjamin Assouline, Amandine De Charrière, Carlo Banfi, and Karim Bendjelid. 2021. "Can VA-ECMO Be Used as an Adequate Treatment in Massive Pulmonary Embolism?" Journal of Clinical Medicine 10, no. 15: 3376. https://doi.org/10.3390/jcm10153376
APA StyleGiraud, R., Laurencet, M., Assouline, B., De Charrière, A., Banfi, C., & Bendjelid, K. (2021). Can VA-ECMO Be Used as an Adequate Treatment in Massive Pulmonary Embolism? Journal of Clinical Medicine, 10(15), 3376. https://doi.org/10.3390/jcm10153376







