Increased Pericardial Adipose Tissue in Smokers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Protocol
2.2. Risk Factors
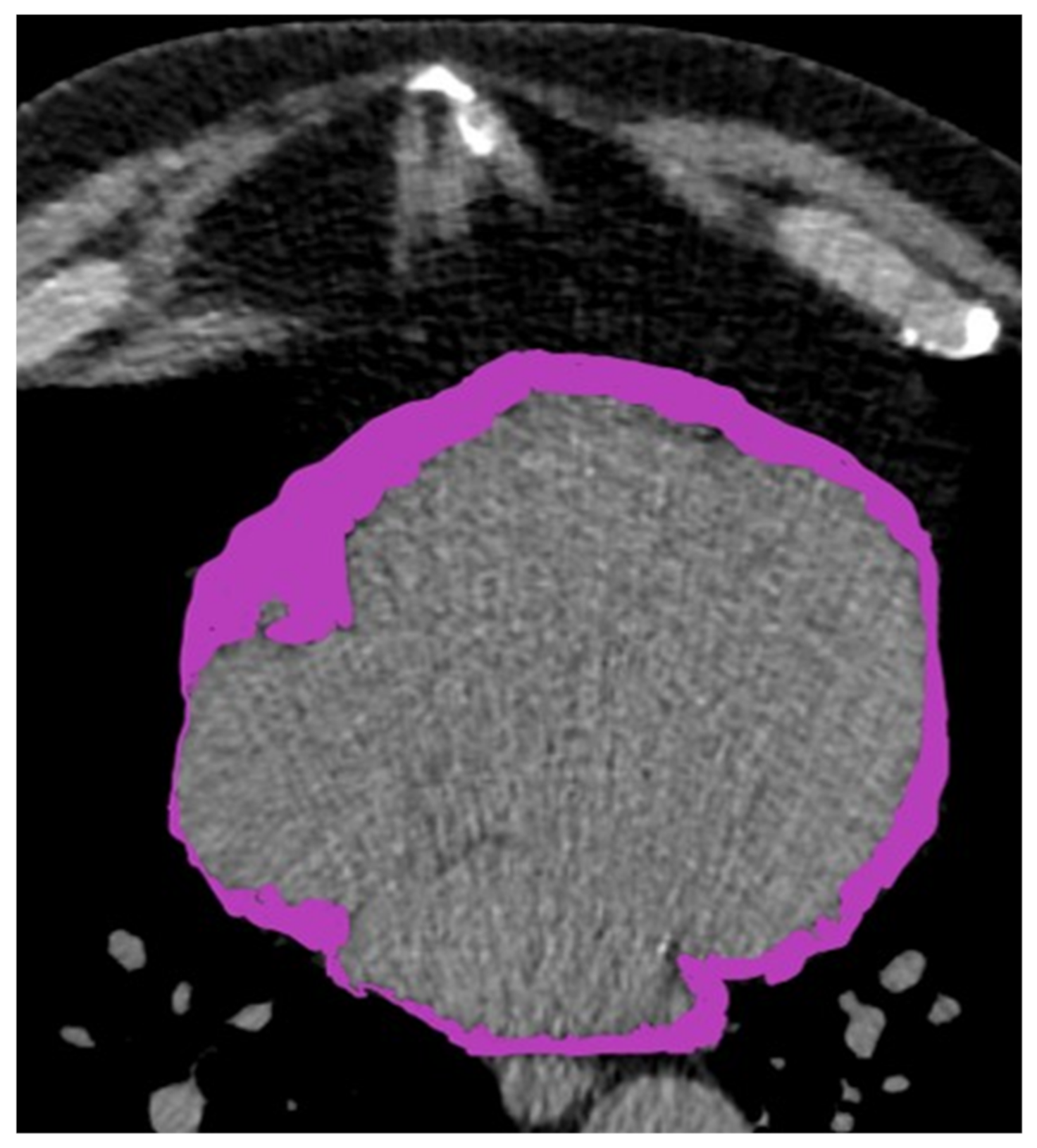
2.3. Pericardial Fat Assessment Protocol
2.4. Statistical Analysis
3. Results
3.1. Study Population
3.2. PAT Volume and CAC
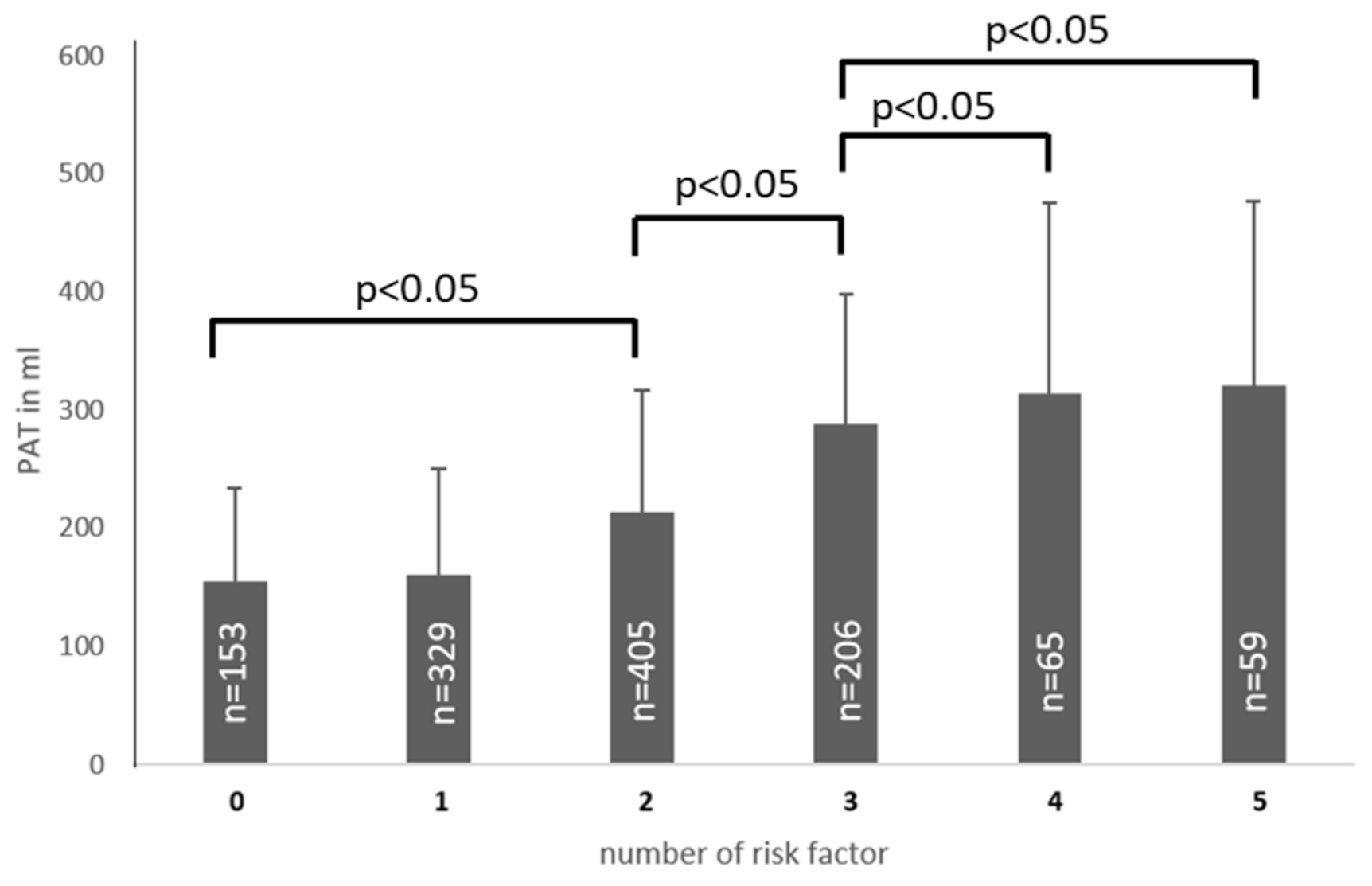
3.3. PAT Volume and Risk Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| CAC | Coronary Calcium |
| CAD | Coronary Artery Disease |
| CT | Computed Tomography |
| ECG | Electrocardiogram |
| HDL | High Density Lipoprotein |
| hs-CRP | high-sensitivity C-Reactive Protein |
| LDL | Low Density Lipoprotein |
| MRI | Magnetic Resonance Imaging |
| PAT | Pericardial Adipose Tissue |
| TNF-α | Tumor Necrosis Factor alpha |
References
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics—2017 Update: A Report From the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, J.; Henschke, C.I.; Shaham, D.; Yip, R.; Farooqi, A.O.; Cham, M.D.; McCauley, D.I.; Chen, M.; Smith, J.P.; Libby, D.M.; et al. Ordinal scoring of coronary artery calcifications on low-dose CT scans of the chest is predictive of death from cardiovascular disease. Radiology 2010, 257, 541–548. [Google Scholar] [CrossRef]
- Raggi, P.; Gongora, M.C.; Gopal, A.; Callister, T.Q.; Budoff, M.; Shaw, L.J. Coronary artery calcium to predict all-cause mortality in elderly men and women. J. Am. Coll. Cardiol. 2008, 52, 17–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmermann, G.S.; Ruther, T.; Ziegler, F.V.; Greif, M.; Becker, C.; Becker, A. Predictive value of coronary calcifications for future cardiac events in asymptomatic patients: Underestimation of risk in asymptomatic smokers. Int. J. Cardiovasc. Imaging 2019, 35, 1387–1393. [Google Scholar] [CrossRef]
- Achenbach, S.; Nomayo, A.; Couturier, G.; Ropers, D.; Pohle, K.; Schlundt, C.; Schmermund, A.; Matarazzo, T.J.; Hoffmann, U.; Daniel, W.G.; et al. Relation between coronary calcium and 10-year risk scores in primary prevention patients. Am. J. Cardiol. 2003, 92, 1471–1475. [Google Scholar] [CrossRef]
- Jayawardena, E.; Li, D.; Nakanishi, R.; Dey, D.; Dailing, C.; Qureshi, A.; Dickens, B.; Hathiramani, N.; Kim, M.; Flores, F.; et al. Non-contrast cardiac CT-based quantitative evaluation of epicardial and intra-thoracic fat in healthy, recently menopausal women: Reproducibility data from the Kronos Early Estrogen Prevention Study. J. Cardiovasc. Comput. Tomogr. 2020, 14, 55–59. [Google Scholar] [CrossRef]
- Greif, M.; Becker, A.; von Ziegler, F.; Lebherz, C.; Lehrke, M.; Broedl, U.C.; Tittus, J.; Parhofer, K.; Becker, C.; Reiser, M.; et al. Pericardial adipose tissue determined by dual source CT is a risk factor for coronary atherosclerosis. Arter. Thromb. Vasc. Biol. 2009, 29, 781–786. [Google Scholar] [CrossRef] [Green Version]
- Mancio, J.; Azevedo, D.; Saraiva, F.; Azevedo, A.I.; Pires-Morais, G.; Leite-Moreira, A.; Falcao-Pires, I.; Lunet, N.; Bettencourt, N. Epicardial adipose tissue volume assessed by computed tomography and coronary artery disease: A systematic review and meta-analysis. Eur. Heart J.Cardiovasc. Imaging 2018, 19, 490–497. [Google Scholar] [CrossRef] [Green Version]
- Spearman, J.V.; Renker, M.; Schoepf, U.J.; Krazinski, A.W.; Herbert, T.L.; De Cecco, C.N.; Nietert, P.J.; Meinel, F.G. Prognostic value of epicardial fat volume measurements by computed tomography: A systematic review of the literature. Eur. Radiol. 2015, 25, 3372–3381. [Google Scholar] [CrossRef] [PubMed]
- Greif, M.; von Ziegler, F.; Wakili, R.; Tittus, J.; Becker, C.; Helbig, S.; Laubender, R.P.; Schwarz, W.; D’Anastasi, M.; Schenzle, J.; et al. Increased pericardial adipose tissue is correlated with atrial fibrillation and left atrial dilatation. Clin. Res. Cardiol. 2013, 102, 555–562. [Google Scholar] [CrossRef]
- Rosito, G.A.; Massaro, J.M.; Hoffmann, U.; Ruberg, F.L.; Mahabadi, A.A.; Vasan, R.S.; O’Donnell, C.J.; Fox, C.S. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: The Framingham Heart Study. Circulation 2008, 117, 605–613. [Google Scholar] [CrossRef] [Green Version]
- Cheng, K.H.; Chu, C.S.; Lee, K.T.; Lin, T.H.; Hsieh, C.C.; Chiu, C.C.; Voon, W.C.; Sheu, S.H.; Lai, W.T. Adipocytokines and proinflammatory mediators from abdominal and epicardial adipose tissue in patients with coronary artery disease. Int. J. Obes. 2008, 32, 268–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iacobellis, G. Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat. Rev. Endocrinol. 2015, 11, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Yin, X.; Hoffmann, U.; Fox, C.S.; Benjamin, E.J. Relation of Pericardial Fat, Intrathoracic Fat, and Abdominal Visceral Fat With Incident Atrial Fibrillation (from the Framingham Heart Study). Am. J. Cardiol. 2016, 118, 1486–1492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancio, J.; Oikonomou, E.K.; Antoniades, C. Perivascular adipose tissue and coronary atherosclerosis. Heart 2018, 104, 1654–1662. [Google Scholar] [CrossRef]
- Franssens, B.T.; Nathoe, H.M.; Leiner, T.; van der Graaf, Y.; Visseren, F.L.; SMART Study Group. Relation between cardiovascular disease risk factors and epicardial adipose tissue density on cardiac computed tomography in patients at high risk of cardiovascular events. Eur. J. Prev. Cardiol. 2017, 24, 660–670. [Google Scholar] [CrossRef]
- Mazurek, T.; Zhang, L.; Zalewski, A.; Mannion, J.D.; Diehl, J.T.; Arafat, H.; Sarov-Blat, L.; O’Brien, S.; Keiper, E.A.; Johnson, A.G.; et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 2003, 108, 2460–2466. [Google Scholar] [CrossRef] [Green Version]
- Aeddula, N.R.; Cheungpasitporn, W.; Thongprayoon, C.; Pathireddy, S. Epicardial Adipose Tissue and Renal Disease. J. Clin. Med. 2019, 8, 299. [Google Scholar] [CrossRef] [Green Version]
- Greif, M.; Leber, A.W.; Saam, T.; Uebleis, C.; von Ziegler, F.; Rummler, J.; D’Anastasi, M.; Arias-Herrera, V.; Becker, C.; Steinbeck, G.; et al. Determination of pericardial adipose tissue increases the prognostic accuracy of coronary artery calcification for future cardiovascular events. Cardiology 2012, 121, 220–227. [Google Scholar] [CrossRef] [Green Version]
- Mahabadi, A.A.; Berg, M.H.; Lehmann, N.; Kalsch, H.; Bauer, M.; Kara, K.; Dragano, N.; Moebus, S.; Jockel, K.H.; Erbel, R.; et al. Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population: The Heinz Nixdorf Recall Study. J. Am. Coll. Cardiol. 2013, 61, 1388–1395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tibuakuu, M.; Kamimura, D.; Kianoush, S.; DeFilippis, A.P.; Al Rifai, M.; Reynolds, L.M.; White, W.B.; Butler, K.R.; Mosley, T.H.; Turner, S.T.; et al. The association between cigarette smoking and inflammation: The Genetic Epidemiology Network of Arteriopathy (GENOA) study. PLoS ONE 2017, 12, e0184914. [Google Scholar] [CrossRef]
- Levitzky, Y.S.; Guo, C.Y.; Rong, J.; Larson, M.G.; Walter, R.E.; Keaney, J.F., Jr.; Sutherland, P.A.; Vasan, A.; Lipinska, I.; Evans, J.C.; et al. Relation of smoking status to a panel of inflammatory markers: The framingham offspring. Atherosclerosis 2008, 201, 217–224. [Google Scholar] [CrossRef] [Green Version]
- Delgado, G.E.; Kramer, B.K.; Marz, W.; Hellstern, P.; Kleber, M.E.; Leipe, J. Immune Status and Mortality in Smokers, ex-Smokers and never-Smokers: The Ludwigshafen Risk and Cardiovascular Health Study. Nicotine Tob. Res. 2021, 23, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Tibuakuu, M.; Kianoush, S.; DeFilippis, A.P.; McEvoy, J.W.; Zhao, D.; Guallar, E.; Ballantyne, C.M.; Hoogeveen, R.C.; Blaha, M.J.; Michos, E.D. Usefulness of Lipoprotein-Associated Phospholipase A2 Activity and C-Reactive Protein in Identifying High-Risk Smokers for Atherosclerotic Cardiovascular Disease (from the Atherosclerosis Risk in Communities Study). Am. J. Cardiol. 2018, 121, 1056–1064. [Google Scholar] [CrossRef]
- Lavi, S.; Prasad, A.; Yang, E.H.; Mathew, V.; Simari, R.D.; Rihal, C.S.; Lerman, L.O.; Lerman, A. Smoking is associated with epicardial coronary endothelial dysfunction and elevated white blood cell count in patients with chest pain and early coronary artery disease. Circulation 2007, 115, 2621–2627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Csordas, A.; Bernhard, D. The biology behind the atherothrombotic effects of cigarette smoke. Nat. Rev. Cardiol. 2013, 10, 219–230. [Google Scholar] [CrossRef]
- Messner, B.; Bernhard, D. Smoking and cardiovascular disease: Mechanisms of endothelial dysfunction and early atherogenesis. Arter. Thromb. Vasc. Biol. 2014, 34, 509–515. [Google Scholar] [CrossRef] [Green Version]
- Becker, A.; Leber, A.W.; Becker, C.; von Ziegler, F.; Tittus, J.; Schroeder, I.; Steinbeck, G.; Knez, A. Predictive value of coronary calcifications for future cardiac events in asymptomatic patients with diabetes mellitus: A prospective study in 716 patients over 8 years. BMC Cardiovasc. Disord. 2008, 8, 27. [Google Scholar] [CrossRef] [Green Version]
- Iacobellis, G.; Ribaudo, M.C.; Assael, F.; Vecci, E.; Tiberti, C.; Zappaterreno, A.; Di Mario, U.; Leonetti, F. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: A new indicator of cardiovascular risk. J. Clin. Endocrinol. Metab. 2003, 88, 5163–5168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorter, P.M.; van Lindert, A.S.; de Vos, A.M.; Meijs, M.F.; van der Graaf, Y.; Doevendans, P.A.; Prokop, M.; Visseren, F.L. Quantification of epicardial and peri-coronary fat using cardiac computed tomography; reproducibility and relation with obesity and metabolic syndrome in patients suspected of coronary artery disease. Atherosclerosis 2008, 197, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.H.; Joo, H.J.; Kim, M.N.; Lim, D.S.; Shim, W.J.; Park, S.M. Association between epicardial adipose tissue, high-sensitivity C-reactive protein and myocardial dysfunction in middle-aged men with suspected metabolic syndrome. Cardiovasc. Diabetol. 2018, 17, 95. [Google Scholar] [CrossRef]
- Morris, P.B.; Ference, B.A.; Jahangir, E.; Feldman, D.N.; Ryan, J.J.; Bahrami, H.; El-Chami, M.F.; Bhakta, S.; Winchester, D.E.; Al-Mallah, M.H.; et al. Cardiovascular Effects of Exposure to Cigarette Smoke and Electronic Cigarettes: Clinical Perspectives From the Prevention of Cardiovascular Disease Section Leadership Council and Early Career Councils of the American College of Cardiology. J. Am. Coll. Cardiol. 2015, 66, 1378–1391. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.J.; Han, J.M.; Kang, J.G.; Kim, B.S.; Kang, J.H. The association between self-reported versus nicotine metabolite-confirmed smoking status and coronary artery calcification. Coron. Artery Dis. 2018, 29, 254–261. [Google Scholar] [CrossRef]
- Mach, L.; Bedanova, H.; Soucek, M.; Karpisek, M.; Nemec, P.; Orban, M. Tobacco smoking and cytokine levels in human epicardial adipose tissue: Impact of smoking cessation. Atherosclerosis 2016, 255, 37–42. [Google Scholar] [CrossRef]
- Ryden, M.; Dicker, A.; van Harmelen, V.; Hauner, H.; Brunnberg, M.; Perbeck, L.; Lonnqvist, F.; Arner, P. Mapping of early signaling events in tumor necrosis factor-alpha-mediated lipolysis in human fat cells. J. Biol. Chem. 2002, 277, 1085–1091. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Wang, D.; Wang, Y. Cigarette Smoking and Adipose Tissue: The Emerging Role in Progression of Atherosclerosis. Mediat. Inflamm. 2017, 2017, 3102737. [Google Scholar] [CrossRef] [Green Version]
- Iwano, S.; Nukaya, M.; Saito, T.; Asanuma, F.; Kamataki, T. A possible mechanism for atherosclerosis induced by polycyclic aromatic hydrocarbons. Biochem. Biophys. Res. Commun. 2005, 335, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Song, P.; Zhang, W.; Liu, J.; Dai, X.; Liu, Z.; Lu, Q.; Ouyang, C.; Xie, Z.; Zhao, Z.; et al. Activation of AMPKalpha2 in adipocytes is essential for nicotine-induced insulin resistance in vivo. Nat. Med. 2015, 21, 373–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kroll, L.; Nassenstein, K.; Jochims, M.; Koitka, S.; Nensa, F. Assessing the Role of Pericardial Fat as a Biomarker Connected to Coronary Calcification-A Deep Learning Based Approach Using Fully Automated Body Composition Analysis. J. Clin. Med. 2021, 10, 356. [Google Scholar] [CrossRef]
- Oka, T.; Yamamoto, H.; Ohashi, N.; Kitagawa, T.; Kunita, E.; Utsunomiya, H.; Yamazato, R.; Urabe, Y.; Horiguchi, J.; Awai, K.; et al. Association between epicardial adipose tissue volume and characteristics of non-calcified plaques assessed by coronary computed tomographic angiography. Int. J. Cardiol. 2012, 161, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, I.; Ohkubo, T.; Kadowaki, S.; Fujiyoshi, A.; Hisamatsu, T.; Kadota, A.; Arima, H.; Budoff, M.; Murata, K.; Miura, K.; et al. Change in Pericardial Fat Volume and Cardiovascular Risk Factors in a General Population of Japanese Men. Circ. J. 2018, 82, 2542–2548. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| All Patients | Non-Smokers | Smokers | p-Value | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| patients | 1217 | 644 | 52.9 | 573 | 47.1 | ||
| male | 727 | 59.7 | 374 | 30.7 | 353 | 29 | 0.24 |
| female | 490 | 40.3 | 270 | 22.2 | 220 | 18.1 | 0.29 |
| age | 58.3 ± 8.3 | 57 ± 7.9 | 59.9 ± 8 | 0.41 | |||
| BMI | 27.2 ± 4.8 | 27 ± 4.5 | 27.5 ± 4.9 | 0.28 | |||
| BMI > 30 kg/m2 | 389 | 32.0 | 215 | 33.4 | 174 | 30.5 | 0.27 |
| arterial hypertension | 602 | 49.5 | 312 | 25.6 | 290 | 23.8 | 0.24 |
| hyperlipidemia | 465 | 38.2 | 245 | 20.1 | 220 | 18.1 | 0.31 |
| diabetes | 171 | 14.1 | 91 | 7.5 | 80 | 6.6 | 0.29 |
| family history of CAD | 584 | 48 | 301 | 24.7 | 283 | 23.3 | 0.15 |
| average number of risk factors | 1.9 | 1.4 | 2.5 | <0.001 | |||
| PAT | 215 ± 107 | 201 ± 99 | 231 ± 104 | 0.03 | |||
| OR [95% CI] | p-Value | |
|---|---|---|
| age | 1.10 [1.06, 1.14] | <0.001 |
| BMI | 1.19 [1.09, 1.32] | <0.001 |
| male sex | 1.20 [1.15, 1.30] | <0.001 |
| hypertension | 1.80 [1.60, 2.04] | <0.001 |
| hyperlipidemia | 2.84 [2.31, 3.39] | <0.001 |
| diabetes | 2.31 [2.04, 2.61] | <0.001 |
| smoking | 2.92 [2.31, 3.61] | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimmermann, G.S.; Ruether, T.; von Ziegler, F.; Greif, M.; Tittus, J.; Schenzle, J.; Becker, C.; Becker, A. Increased Pericardial Adipose Tissue in Smokers. J. Clin. Med. 2021, 10, 3382. https://doi.org/10.3390/jcm10153382
Zimmermann GS, Ruether T, von Ziegler F, Greif M, Tittus J, Schenzle J, Becker C, Becker A. Increased Pericardial Adipose Tissue in Smokers. Journal of Clinical Medicine. 2021; 10(15):3382. https://doi.org/10.3390/jcm10153382
Chicago/Turabian StyleZimmermann, Gregor S., Tobias Ruether, Franz von Ziegler, Martin Greif, Janine Tittus, Jan Schenzle, Christoph Becker, and Alexander Becker. 2021. "Increased Pericardial Adipose Tissue in Smokers" Journal of Clinical Medicine 10, no. 15: 3382. https://doi.org/10.3390/jcm10153382
APA StyleZimmermann, G. S., Ruether, T., von Ziegler, F., Greif, M., Tittus, J., Schenzle, J., Becker, C., & Becker, A. (2021). Increased Pericardial Adipose Tissue in Smokers. Journal of Clinical Medicine, 10(15), 3382. https://doi.org/10.3390/jcm10153382






