The Role of the 3Rs for Understanding and Modeling the Human Placenta
Abstract
:1. Introduction
2. In Vivo Placental Models
In Vivo Metrics—Impact on 3Rs
3. In Vitro Placental Models
3.1. Placental Explants
3.2. Primary Cultures and Cell Lines
3.3. Classical In Vitro Systems: Transwells and Cocultures
3.4. Advanced In Vitro Systems
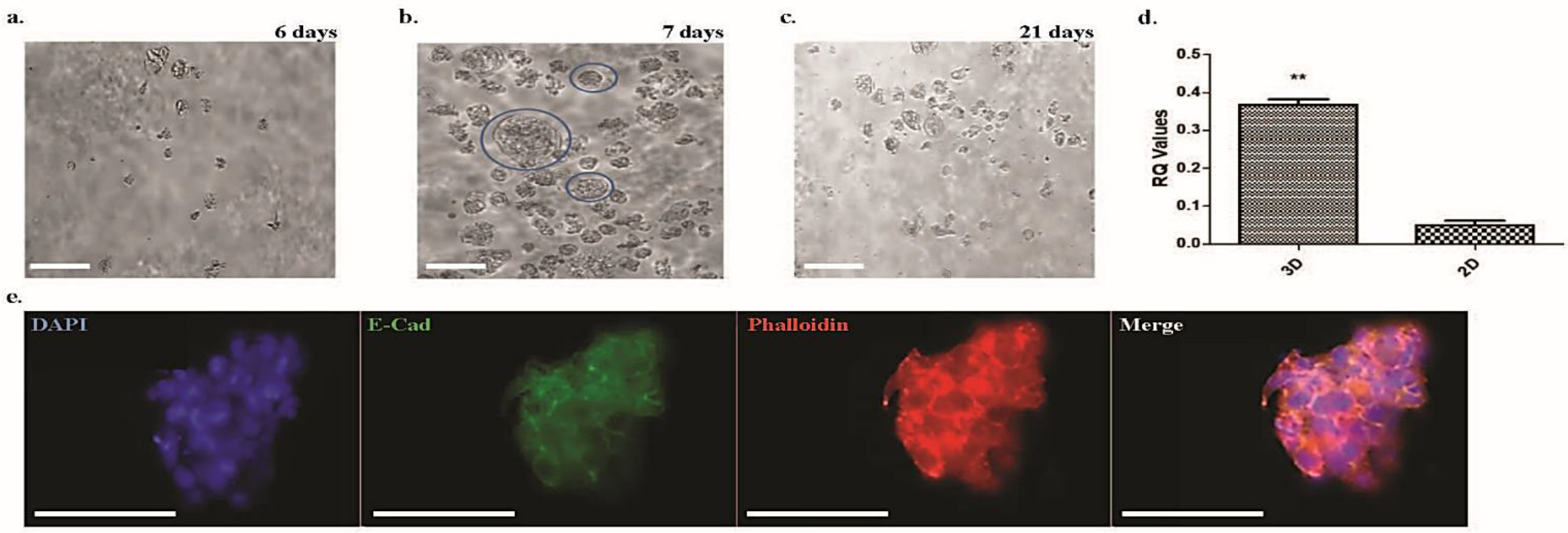
3.4.1. Spheroids and Organoids
3.4.2. Planar and Organ-on-a-Chip Models
4. In Silico Placental Models
4.1. Fluid and Structure Interaction Models
4.2. Models for the Transfer of Xenobiotics
5. Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Zakowski, M.I.; Herman, N.L. The Placenta: Anatomy, Physiology, and Transfer of Drugs. In Part II Maternal and Fetal Physiology; Elsevier: Amsterdam, NL, USA, 2009; pp. 55–72. [Google Scholar]
- Donnelly, L.; Campling, G. Functions of the Placenta. Anaesth. Intensive Care Med. 2014, 15, 136–139. [Google Scholar] [CrossRef]
- Ramsey, E.M. Biology of Gestation. Vol. 1, The Maternal Organism, 1st ed.; Academic Press: New York, NY, USA, 1968; Volume 162. [Google Scholar]
- Firth, J.A.; Leach, L. Structure and Permeability in Human Placental Capillaries. Placenta 1997, 18, 205–213. [Google Scholar] [CrossRef]
- Needham, L.L.; Grandjean, P.; Heinzow, B.; Jørgensen, P.J.; Nielsen, F.; Sjödin, A.; Patterson, D.G.; Turner, W.E.; Weihe, P. Partition of Environmental Chemicals between Maternal and Fetal Blood and Tissues. Environ. Sci. Technol. 2011, 45, 1121–1126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitro, S.D.; Johnson, T.; Zota, A.R. Cumulative Chemical Exposures During Pregnancy and Early Development. Curr. Environ. Health Rep. 2015, 2, 367–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Animal Welfare Act|Animal Welfare Information Center|NAL|USDA. Available online: https://www.nal.usda.gov/awic/animal-welfare-act (accessed on 13 March 2021).
- Legislation for the Protection of Animals Used for Scientific Purposes—Environment—European Commission. Available online: https://ec.europa.eu/environment/chemicals/lab_animals/legislation_en.htm (accessed on 13 March 2021).
- Carter, A.M. Animal Models of Human Placentation—A Review. Placenta 2007, 28, S41–S47. [Google Scholar] [CrossRef]
- Orendi, K.; Kivity, V.; Sammar, M.; Grimpel, Y.; Gonen, R.; Meiri, H.; Lubzens, E.; Huppertz, B. Placental and Trophoblastic in Vitro Models to Study Preventive and Therapeutic Agents for Preeclampsia. Placenta 2011, 32, S49–S54. [Google Scholar] [CrossRef]
- Grigsby, P.L. Animal Models to Study Placental Development and Function throughout Normal and Dysfunctional Human Pregnancy. Semin. Reprod. Med. 2016, 34, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Barry, J.S.; Anthony, R.V. The Pregnant Sheep as a Model for Human Pregnancy. Theriogenology 2008, 69, 55–67. [Google Scholar] [CrossRef] [Green Version]
- Pemathilaka, R.L.; Reynolds, D.E.; Hashemi, N.N. Drug Transport across the Human Placenta: Review of Placenta-on-a-Chip and Previous Approaches. Interface Focus 2019, 9, 20190031. [Google Scholar] [CrossRef] [Green Version]
- Carter, A.M. Animal Models of Human Pregnancy and Placentation: Alternatives to the Mouse. Reproduction 2020, 160, R129–R143. [Google Scholar] [CrossRef]
- Mess, A. The Guinea Pig Placenta: Model of Placental Growth Dynamics. Placenta 2007, 28, 812–815. [Google Scholar] [CrossRef]
- Malassiné, A.; Frendo, J.L.; Evain-Brion, D. A Comparison of Placental Development and Endocrine Functions between the Human and Mouse Model. Hum. Reprod. Update 2003, 9, 531–539. [Google Scholar] [CrossRef] [Green Version]
- Binder, N.K.; Beard, S.A.; Kaitu’U-Lino, T.J.; Tong, S.; Hannan, N.J.; Gardner, D.K. Paternal Obesity in a Rodent Model Affects Placental Gene Expression in a Sex-Specific Manner. Reproduction 2015, 149, 435–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binder, N.K.; Hannan, N.J.; Gardner, D.K. Paternal Diet-Induced Obesity Retards Early Mouse Embryo Development, Mitochondrial Activity and Pregnancy Health. PLoS ONE 2012, 7, e52304. [Google Scholar] [CrossRef] [Green Version]
- Swanson, A.M.; David, A.L. Animal Models of Fetal Growth Restriction: Considerations for Translational Medicine. Placenta 2015, 36, 623–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghulmiyyah, L.; Sibai, B. Maternal Mortality From Preeclampsia/Eclampsia. Semin. Perinatol. 2012, 36, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Gatford, K.L.; Andraweera, P.H.; Roberts, C.T.; Care, A.S. Animal Models of Preeclampsia: Causes, Consequences, and Interventions. Hypertension 2020, 75, 1363–1381. [Google Scholar] [CrossRef] [PubMed]
- Susiarjo, M.; Sasson, I.; Mesaros, C.; Bartolomei, M.S. Bisphenol A Exposure Disrupts Genomic Imprinting in the Mouse. PLoS Genet. 2013, 9, e1003401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyu, N.; Hidetomo, I.; Risa, Y.; Nanako, K.; Hiroki, I.; Hiroshi, Y. Placental Transfer of Conjugated Bisphenol A and Subsequent Reactivation in the Rat Fetus. Environ. Health Perspect. 2010, 118, 1196–1203. [Google Scholar]
- Patterson, T.A.; Twaddle, N.C.; Roegge, C.S.; Callicott, R.J.; Fisher, J.W.; Doerge, D.R. Concurrent Determination of Bisphenol A Pharmacokinetics in Maternal and Fetal Rhesus Monkeys. Toxicol. Appl. Pharmacol. 2013, 267, 41–48. [Google Scholar] [CrossRef]
- Veiga-Lopez, A.; Luense, L.J.; Christenson, L.K.; Padmanabhan, V. Developmental Programming: Gestational Bisphenol-A Treatment Alters Trajectory of Fetal Ovarian Gene Expression. Endocrinology 2013, 154, 1873–1884. [Google Scholar] [CrossRef] [Green Version]
- Animals Used for Scientific Purposes—Environment—European Commission. Available online: https://ec.europa.eu/environment/archives/lab_animals/ewg_en.htm (accessed on 2 July 2021).
- DeGrazia, D. Nonhuman Primates, Human Need, and Ethical Constraints. Hastings Cent. Rep. 2016, 46, 27–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sooranna, S.R.; Oteng-Ntim, E.; Meah, R.; Ryder, T.A.; Bajoria, R. Characterization of Human Placental Explants: Morphological, Biochemical and Physiological Studies Using First and Third Trimester Placenta. Hum. Reprod. 1999, 14, 536–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, J.L.; Stone, P.R.; Chamley, L.W. The Effects of Oxygen Concentration and Gestational Age on Extravillous Trophoblast Outgrowth in a Human First Trimester Villous Explant Model. Hum. Reprod. 2006, 21, 2699–2705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karteris, E.; Vatish, M.; Hillhouse, E.W.; Grammatopoulos, D.K. Preeclampsia Is Associated with Impaired Regulation of the Placental Nitric Oxide-Cyclic Guanosine Monophosphate Pathway by Corticotropin-Releasing Hormone (CRH) and CRH-Related Peptides. J. Clin. Endocrinol. Metab. 2005, 90, 3680–3687. [Google Scholar] [CrossRef] [Green Version]
- Majewska, M.; Lipka, A.; Paukszto, L.; Jastrzebski, J.P.; Myszczynski, K.; Gowkielewicz, M.; Jozwik, M.; Majewski, M.K. Transcriptome Profile of the Human Placenta. Funct. Integr. Genom. 2017, 17, 551–563. [Google Scholar] [CrossRef] [Green Version]
- Pique-Regi, R.; Romero, R.; Tarca, A.L.; Sendler, E.D.; Xu, Y.; Garcia-Flores, V.; Leng, Y.; Luca, F.; Hassan, S.S.; Gomez-Lopez, N. Single Cell Transcriptional Signatures of the Human Placenta in Term and Preterm Parturition. Elife 2019, 8, e52004. [Google Scholar] [CrossRef]
- Sato, B.L.; Ward, M.A.; Astern, J.M.; Kendal-Wright, C.E.; Collier, A.C. Validation of Murine and Human Placental Explant Cultures for Use in Sex Steroid and Phase II Conjugation Toxicology Studies. Toxicol. In Vitro 2015, 29, 103–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Tan, B.; Karteris, E.; Zervou, S.; Digby, J.; Hillhouse, E.W.; Vatish, M.; Randeva, H.S. Secretion of Adiponectin by Human Placenta: Differential Modulation of Adiponectin and Its Receptors by Cytokines. Diabetologia 2006, 49, 1292–1302. [Google Scholar] [CrossRef] [Green Version]
- Cindrova-Davies, T.; Yung, H.W.; Johns, J.; Spasic-Boskovic, O.; Korolchuk, S.; Jauniaux, E.; Burton, G.J.; Charnock-Jones, D.S. Oxidative Stress, Gene Expression, and Protein Changes Induced in the Human Placenta during Labor. Am. J. Pathol. 2007, 171, 1168–1179. [Google Scholar] [CrossRef] [PubMed]
- Sitras, V.; Paulssen, R.H.; Grønaas, H.; Vårtun, Å.; Acharya, G. Gene Expression Profile in Labouring and Non-Labouring Human Placenta near Term. Mol. Hum. Reprod. 2008, 14, 61–65. [Google Scholar] [CrossRef] [Green Version]
- Panigel, M.; Pascaud, M.; Brun, J.L. Etude Radioangiographique de La Circulation Dans Les Villosités et l’espace Intervilleux Du Cotylédon Placentaire Humain Isolé Mantenu En Survie Paperfusion. J. Physiol. 1967, 59, 277. [Google Scholar]
- Schneider, H. IFPA Senior Award Lecture: Energy Metabolism of Human Placental Tissue Studied by Ex Vivo Perfusion of an Isolated Cotyledon. Placenta 2015, 36, S29–S34. [Google Scholar] [CrossRef]
- Schneider, H.; Panigel, M.; Dancis, J. Transfer across the Perfused Human Placenta of Antipyrine, Sodium, and Leucine. Am. J. Obstet. Gynecol. 1972, 114, 822–828. [Google Scholar] [CrossRef]
- Mose, T.; Knudsen, L.E.; Hedegaard, M.; Mortensen, G.K. Transplacental Transfer of Monomethyl Phthalate and Mono(2-Ethylhexyl) Phthalate in a Human Placenta Perfusion System. Int. J. Toxicol. 2007, 26, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Dallmann, A.; Liu, X.I.; Burckart, G.J.; van den Anker, J. Drug Transporters Expressed in the Human Placenta and Models for Studying Maternal-Fetal Drug Transfer. J. Clin. Pharmacol. 2019, 59, S70–S81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutson, J.R.; Garcia-Bournissen, F.; Davis, A.; Koren, G. The Human Placental Perfusion Model: A Systematic Review and Development of a Model to Predict in Vivo Transfer of Therapeutic Drugs. Clin. Pharmacol. Ther. 2011, 90, 67–76. [Google Scholar] [CrossRef]
- Lewis, R.M.; Cleal, J.K.; Sengers, B.G. Placental Perfusion and Mathematical Modelling. Placenta 2020, 93, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Madsen, K.E.; Mariager, C.Ø.; Duvald, C.S.; Hansen, E.S.S.; Bertelsen, L.B.; Pedersen, M.; Pedersen, L.H.; Uldbjerg, N.; Laustsen, C. Ex Vivo Human Placenta Perfusion, Metabolic and Functional Imaging for Obstetric Research—A Feasibility Study. Tomography 2019, 5, 333–338. [Google Scholar] [CrossRef]
- Huang, X.; Lüthi, M.; Ontsouka, E.C.; Kallol, S.; Baumann, M.U.; Surbek, D.V.; Albrecht, C. Establishment of a Confluent Monolayer Model with Human Primary Trophoblast Cells: Novel Insights into Placental Glucose Transport. Mol. Hum. Reprod. 2016, 22, 442–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melhem, H.; Kallol, S.; Huang, X.; Lüthi, M.; Ontsouka, C.E.; Keogh, A.; Stroka, D.; Thormann, W.; Schneider, H.; Albrecht, C. Placental Secretion of Apolipoprotein A1 and E: The Anti-Atherogenic Impact of the Placenta. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Fuenzalida, B.; Kallol, S.; Lüthi, M.; Albrecht, C.; Leiva, A. The Polarized Localization of Lipoprotein Receptors and Cholesterol Transporters in the Syncytiotrophoblast of the Placenta Is Reproducible in a Monolayer of Primary Human Trophoblasts. Placenta 2021, 105, 50–60. [Google Scholar] [CrossRef]
- Murrieta-Coxca, J.M.; Gutiérrez-Samudio, R.N.; El-Shorafa, H.M.; Groten, T.; Rodríguez-Martínez, S.; Cancino-Diaz, M.E.; Cancino-Diaz, J.C.; Favaro, R.R.; Markert, U.R.; Morales-Prieto, D.M. Role of IL-36 Cytokines in the Regulation of Angiogenesis Potential of Trophoblast Cells. Int. J. Mol. Sci. 2021, 22, 285. [Google Scholar] [CrossRef]
- Vega, M.; Mauro, M.; Williams, Z. Direct Toxicity of Insulin on the Human Placenta and Protection by Metformin. Fertil. Steril. 2019, 111, 489–496.e5. [Google Scholar] [CrossRef] [Green Version]
- King, A.; Thomas, L.; Bischof, P. Cell Culture Models of Trophoblast II: Trophoblast Cell Lines—A Workshop Report. Placenta 2000, 21, S113–S119. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, R.S.; Goldstein, D.P. Chorionic Tumors. N. Engl. J. Med. 1996, 335, 1740–1748. [Google Scholar] [CrossRef]
- Wolfe, M.W. Culture and Transfection of Human Choriocarcinoma Cells. Methods Mol. Med. 2006, 121, 229–239. [Google Scholar]
- Ntrivalas, E.; Kwak-Kim, J.; Beaman, K.; Mantouvalos, H.; Gilman-Sachs, A. An in Vitro Coculture Model to Study Cytokine Profiles of Natural Killer Cells during Maternal Immune Cell-Trophoblast Interactions. J. Soc. Gynecol. Investig. 2006, 13, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Mparmpakas, D.; Zachariades, E.; Foster, H.; Kara, A.; Harvey, A.; Goumenou, A.; Karteris, E. Expression of MTOR and Downstream Signalling Components in the JEG-3 and BeWo Human Placental Choriocarcinoma Cell Lines. Int. J. Mol. Med. 2010, 25, 65–69. [Google Scholar] [PubMed]
- de Aguiar Greca, S.-C.; Kyrou, I.; Pink, R.; Randeva, H.; Grammatopoulos, D.; Silva, E.; Karteris, E. Involvement of the Endocrine-Disrupting Chemical Bisphenol A (BPA) in Human Placentation. J. Clin. Med. 2020, 9, 405. [Google Scholar] [CrossRef] [Green Version]
- Horii, M.; Touma, O.; Bui, T.; Parast, M.M. Modeling Human Trophoblast, the Placental Epithelium at the Maternal Fetal Interface. Reproduction 2020, 160, R1–R11. [Google Scholar] [CrossRef]
- Thomson, J.A. Embryonic Stem Cell Lines Derived from Human Blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef] [Green Version]
- Xu, R.H.; Chen, X.; Li, D.S.; Li, R.; Addicks, G.C.; Glennon, C.; Zwaka, T.P.; Thomson, J.A. BMP4 Initiates Human Embryonic Stem Cell Differentiation to Trophoblast. Nat. Biotechnol. 2002, 20, 1261–1264. [Google Scholar] [CrossRef]
- Gerami-Naini, B.; Dovzhenko, O.V.; Durning, M.; Wegner, F.H.; Thomson, J.A.; Golos, T.G. Trophoblast Differentiation in Embryoid Bodies Derived from Human Embryonic Stem Cells. Endocrinology 2004, 145, 1517–1524. [Google Scholar] [CrossRef] [Green Version]
- Horii, M.; Bui, T.; Touma, O.; Cho, H.Y.; Parast, M.M. An Improved Two-Step Protocol for Trophoblast Differentiation of Human Pluripotent Stem Cells. Curr. Protoc. Stem Cell Biol. 2019, 50, e96. [Google Scholar] [CrossRef] [Green Version]
- Horii, M.; Morey, R.; Bui, T.; Touma, O.; Nelson, K.K.; Cho, H.Y.; Rishik, H.; Laurent, L.C.; Parast, M.M. Modeling Preeclampsia Using Human Induced Pluripotent Stem Cells. Sci. Rep. 2021, 11, 5877. [Google Scholar] [CrossRef]
- Bode, C.J.; Jin, H.; Rytting, E.; Silverstein, P.S.; Young, A.M.; Audus, K.L. In Vitro Models for Studying Trophoblast Transcellular Transport. Methods Mol. Med. 2006, 122, 225–239. [Google Scholar] [PubMed] [Green Version]
- Aengenheister, L.; Keevend, K.; Muoth, C.; Schönenberger, R.; Diener, L.; Wick, P.; Buerki-Thurnherr, T. An Advanced Human in Vitro Co-Culture Model for Translocation Studies across the Placental Barrier. Sci. Rep. 2018, 8, 5388. [Google Scholar] [CrossRef] [Green Version]
- Lelièvre, S.A.; Kwok, T.; Chittiboyina, S. Architecture in 3D Cell Culture: An Essential Feature for in Vitro Toxicology. Toxicol. Vitr. 2017, 45, 287–295. [Google Scholar] [CrossRef] [PubMed]
- White, T.E.; Saltzman, R.A.; Di Sant’Agnese, P.A.; Keng, P.C.; Sutherland, R.M.; Miller, R.K. Human Choriocarcinoma (JAr) Cells Grown as Multicellular Spheroids. Placenta 1988, 9, 583–598. [Google Scholar] [CrossRef]
- Wong, M.K.; Wahed, M.; Shawky, S.A.; Dvorkin-Gheva, A.; Raha, S. Transcriptomic and Functional Analyses of 3D Placental Extravillous Trophoblast Spheroids. Sci. Rep. 2019, 9, 12607. [Google Scholar] [CrossRef] [Green Version]
- Clevers, H. Modeling Development and Disease with Organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haider, S.; Meinhardt, G.; Saleh, L.; Kunihs, V.; Gamperl, M.; Kaindl, U.; Ellinger, A.; Burkard, T.R.; Fiala, C.; Pollheimer, J.; et al. Self-Renewing Trophoblast Organoids Recapitulate the Developmental Program of the Early Human Placenta. Stem. Cell Rep. 2018, 11, 537–551. [Google Scholar] [CrossRef] [Green Version]
- Sheridan, M.A.; Fernando, R.C.; Gardner, L.; Hollinshead, M.S.; Burton, G.J.; Moffett, A.; Turco, M.Y. Establishment and Differentiation of Long-Term Trophoblast Organoid Cultures from the Human Placenta. Nat. Protoc. 2020, 15, 3441–3463. [Google Scholar] [CrossRef]
- Kreuder, A.-E.; Bolaños-Rosales, A.; Palmer, C.; Thomas, A.; Geiger, M.-A.; Lam, T.; Amler, A.-K.; Markert, U.R.; Lauster, R.; Kloke, L. Inspired by the Human Placenta: A Novel 3D Bioprinted Membrane System to Create Barrier Models. Sci. Rep. 2020, 10, 15606. [Google Scholar] [CrossRef]
- Broguiere, N.; Isenmann, L.; Hirt, C.; Ringel, T.; Placzek, S.; Cavalli, E.; Ringnalda, F.; Villiger, L.; Züllig, R.; Lehmann, R.; et al. Growth of Epithelial Organoids in a Defined Hydrogel. Adv. Mater. 2018, 30, 1801621. [Google Scholar] [CrossRef]
- Nishiguchi, A.; Gilmore, C.; Sood, A.; Matsusaki, M.; Collett, G.; Tannetta, D.; Sargent, I.L.; McGarvey, J.; Halemani, N.D.; Hanley, J.; et al. In Vitro Placenta Barrier Model Using Primary Human Trophoblasts, Underlying Connective Tissue and Vascular Endothelium. Biomaterials 2019, 192, 140–148. [Google Scholar] [CrossRef]
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Hsin, H.Y.; Ingber, D.E. Reconstituting Organ-Level Lung Functions on a Chip. Science 2010, 328, 1662–1668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.S.; Romero, R.; Han, Y.M.; Kim, H.C.; Kim, C.J.; Hong, J.-S.; Huh, D. Placenta-on-a-Chip: A Novel Platform to Study the Biology of the Human Placenta. J. Matern. Neonatal Med. 2016, 29, 1046–1054. [Google Scholar] [CrossRef]
- Blundell, C.; Yi, Y.-S.; Ma, L.; Tess, E.R.; Farrell, M.J.; Georgescu, A.; Aleksunes, L.M.; Huh, D. Placental Drug Transport-on-a-Chip: A Microengineered In Vitro Model of Transporter-Mediated Drug Efflux in the Human Placental Barrier. Adv. Healthc. Mater. 2018, 7, 1700786. [Google Scholar] [CrossRef]
- Pemathilaka, R.L.; Caplin, J.D.; Aykar, S.S.; Montazami, R.; Hashemi, N.N. Placenta-on-a-Chip: In Vitro Study of Caffeine Transport across Placental Barrier Using Liquid Chromatography Mass Spectrometry. Glob. Chall. 2019, 3, 1800112. [Google Scholar] [CrossRef] [Green Version]
- Yin, F.; Zhu, Y.; Zhang, M.; Yu, H.; Chen, W.; Qin, J. A 3D Human Placenta-on-a-Chip Model to Probe Nanoparticle Exposure at the Placental Barrier. Toxicol. Vitr. 2019, 54, 105–113. [Google Scholar] [CrossRef]
- Jodat, Y.A.; Kang, M.G.; Kiaee, K.; Kim, G.J.; Martinez, A.F.H.; Rosenkranz, A.; Bae, H.; Shin, S.R. Human-Derived Organ-on-a-Chip for Personalized Drug Development. Curr. Pharm. Des. 2019, 24, 5471–5486. [Google Scholar] [CrossRef]
- Colquitt, R.B.; Colquhoun, D.A.; Thiele, R.H. In Silico Modelling of Physiologic Systems. Best Pract. Res. Clin. Anaesthesiol. 2011, 25, 499–510. [Google Scholar] [CrossRef]
- Roth, C.J.; Haeussner, E.; Ruebelmann, T.; Koch, F.V.; Schmitz, C.; Frank, H.G.; Wall, W.A. Dynamic Modeling of Uteroplacental Blood Flow in IUGR Indicates Vortices and Elevated Pressure in the Intervillous Space-a Pilot Study. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Codaccioni, M.; Bois, F.; Brochot, C. Placental Transfer of Xenobiotics in Pregnancy Physiologically-Based Pharmacokinetic Models: Structure and Data. Comput. Toxicol. 2019, 12, 100111. [Google Scholar] [CrossRef]
- Giaginis, C.; Zira, A.; Theocharis, S.; Tsantili-Kakoulidou, A. Application of Quantitative Structure-Activity Relationships for Modeling Drug and Chemical Transport across the Human Placenta Barrier: A Multivariate Data Analysis Approach. J. Appl. Toxicol. 2009, 29, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Cotter, S.L.; Klika, V.; Kimpton, L.; Collins, S.; Heazell, A.E.P. A Stochastic Model for Early Placental Development. J. R. Soc. Interface 2014, 11, 20140149. [Google Scholar] [CrossRef] [Green Version]
- Gill, J.S.; Salafia, C.M.; Grebenkov, D.; Vvedensky, D.D. Modeling Oxygen Transport in Human Placental Terminal Villi. J. Theor. Biol. 2011, 291, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Plitman Mayo, R.; Olsthoorn, J.; Charnock-Jones, D.S.; Burton, G.J.; Oyen, M.L. Computational Modeling of the Structure-Function Relationship in Human Placental Terminal Villi. J. Biomech. 2016, 49, 3780–3787. [Google Scholar] [CrossRef] [Green Version]
- Plitman Mayo, R.; Charnock-Jones, D.S.; Burton, G.J.; Oyen, M.L. Three-Dimensional Modeling of Human Placental Terminal Villi. Placenta 2016, 43, 54–60. [Google Scholar] [CrossRef] [Green Version]
- Pearce, P.; Brownbill, P.; Janáček, J.; Jirkovská, M.; Kubínová, L.; Chernyavsky, I.L.; Jensen, O.E. Image-Based Modeling of Blood Flow and Oxygen Transfer in Feto-Placental Capillaries. PLoS ONE 2016, 11, e0165369. [Google Scholar] [CrossRef] [Green Version]
- Erlich, A.; Pearce, P.; Mayo, R.P.; Jensen, O.E.; Chernyavsky, I.L. Physical and Geometric Determinants of Transport in Feto-Placental Microvascular Networks. Sci. Adv. 2019, 5, eaav6326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, M.; Mauroy, B.; James, J.L.; Tawhai, M.H.; Clark, A.R. A Multiscale Model of Placental Oxygen Exchange: The Effect of Villous Tree Structure on Exchange Efficiency. J. Theor. Biol. 2016, 408, 1–12. [Google Scholar] [CrossRef]
- Plitman Mayo, R. Advances in Human Placental Biomechanics. Comput. Struct. Biotechnol. J. 2018, 16, 298–306. [Google Scholar] [CrossRef]
- Clark, A.R.; Lin, M.; Tawhai, M.; Saghian, R.; James, J.L. Multiscale Modelling of the Feto-Placental Vasculature. Interface Focus 2015, 5, 20140078. [Google Scholar] [CrossRef] [Green Version]
- Saghian, R.; James, J.L.; Tawhai, M.H.; Collins, S.L.; Clark, A.R. Association of Placental Jets and Mega-Jets with Reduced Villous Density. J. Biomech. Eng. 2017, 139, 0510011–05100110. [Google Scholar] [CrossRef]
- Dallmann, A.; Pfister, M.; van den Anker, J.; Eissing, T. Physiologically Based Pharmacokinetic Modeling in Pregnancy: A Systematic Review of Published Models. Clin. Pharmacol. Ther. 2018, 104, 1110–1124. [Google Scholar] [CrossRef]
- De Sousa Mendes, M.; Lui, G.; Zheng, Y.; Pressiat, C.; Hirt, D.; Valade, E.; Bouazza, N.; Foissac, F.; Blanche, S.; Treluyer, J.M.; et al. A Physiologically-Based Pharmacokinetic Model to Predict Human Fetal Exposure for a Drug Metabolized by Several CYP450 Pathways. Clin. Pharmacokinet. 2017, 56, 537–550. [Google Scholar] [CrossRef]
- Zhang, Z.; Imperial, M.Z.; Patilea-Vrana, G.I.; Wedagedera, J.; Gaohua, L.; Unadkat, J.D. Development of a Novel Maternal-Fetal Physiologically Based Pharmacokinetic Model I: Insights into Factors That Determine Fetal Drug Exposure through Simulations and Sensitivity Analyses. Drug Metab. Dispos. 2017, 45, 920–938. [Google Scholar] [CrossRef] [Green Version]
- Schalkwijk, S.; Buaben, A.O.; Freriksen, J.J.M.; Colbers, A.P.; Burger, D.M.; Greupink, R.; Russel, F.G.M. Prediction of Fetal Darunavir Exposure by Integrating Human Ex-Vivo Placental Transfer and Physiologically Based Pharmacokinetic Modeling. Clin. Pharmacokinet. 2018, 57, 705–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abduljalil, K.; Johnson, T.N.; Jamei, M. Application of Feto-Maternal Physiologically-Based Pharmacokinetic Model To Predict Emtricitabine Concentration during Pregnancy. J. Pharmacokinet. Pharmacodyn. 2018, 45, S76. [Google Scholar]
- Tropsha, A.; Golbraikh, A. Predictive QSAR Modeling Workflow, Model Applicability Domains, and Virtual Screening. Curr. Pharm. Des. 2007, 13, 3494–3504. [Google Scholar] [CrossRef]
- Hewitt, M.; Madden, J.C.; Rowe, P.H.; Cronin, M.T.D. Structure-Based Modelling in Reproductive Toxicology: (Q)SARs for the Placental Barrier. SAR QSAR Environ. Res. 2007, 18, 57–76. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Xia, Z.N.; Yan, L.; Liu, S.S. Prediction of Placental Barrier Permeability: A Model Based on Partial Least Squares Variable Selection Procedure. Molecules 2015, 20, 8270–8286. [Google Scholar] [CrossRef] [Green Version]
- Lu, F.; He, Y.; Jiang, L.; Chen, J.; Cai, Y.; Zhang, Y. Prediction of Placenta Barrier Permeability and Reproductive Toxicity of Compounds in Tocolytic Chinese Herbs Using Support Vector Machine. In Proceedings of the 2015 International Conference on Materials Engineering and Information Technology Applications, Guilin, China, 30–31 August 2015; Chen, Y., Ed.; Spring Nature: Basingstoke, UK, 2015; Volume 28, pp. 650–655. [Google Scholar]
- Eguchi, A.; Hanazato, M.; Suzuki, N.; Matsuno, Y.; Todaka, E.; Mori, C. Maternal–Fetal Transfer Rates of PCBs, OCPs, PBDEs, and Dioxin-like Compounds Predicted through Quantitative Structure–Activity Relationship Modeling. Environ. Sci. Pollut. Res. 2018, 25, 7212–7222. [Google Scholar] [CrossRef] [PubMed]
- Takaku, T.; Nagahori, H.; Sogame, Y.; Takagi, T. Quantitative Structure-Activity Relationship Model for the Fetal-Maternal Blood Concentration Ratio of Chemicals in Humans. Biol. Pharm. Bull. 2015, 38, 930–934. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.C.; Lin, P.; Chou, C.Y.; Wang, S.S.; Tung, C.W. Prediction of Human Fetal-Maternal Blood Concentration Ratio of Chemicals. PeerJ 2020, 8, e9562. [Google Scholar]
- Slavov, S.; Beger, R.D. Quantitative Structure–Toxicity Relationships in Translational Toxicology. Curr. Opin. Toxicol. 2020, 23–24, 46–49. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, J.; Mackay, R.; de Aguiar Greca, S.-C.; Corti, A.; Silva, E.; Karteris, E.; Ahluwalia, A. The Role of the 3Rs for Understanding and Modeling the Human Placenta. J. Clin. Med. 2021, 10, 3444. https://doi.org/10.3390/jcm10153444
Costa J, Mackay R, de Aguiar Greca S-C, Corti A, Silva E, Karteris E, Ahluwalia A. The Role of the 3Rs for Understanding and Modeling the Human Placenta. Journal of Clinical Medicine. 2021; 10(15):3444. https://doi.org/10.3390/jcm10153444
Chicago/Turabian StyleCosta, Joana, Ruth Mackay, Sophie-Christine de Aguiar Greca, Alessandro Corti, Elisabete Silva, Emmanouil Karteris, and Arti Ahluwalia. 2021. "The Role of the 3Rs for Understanding and Modeling the Human Placenta" Journal of Clinical Medicine 10, no. 15: 3444. https://doi.org/10.3390/jcm10153444
APA StyleCosta, J., Mackay, R., de Aguiar Greca, S.-C., Corti, A., Silva, E., Karteris, E., & Ahluwalia, A. (2021). The Role of the 3Rs for Understanding and Modeling the Human Placenta. Journal of Clinical Medicine, 10(15), 3444. https://doi.org/10.3390/jcm10153444






