Decoding the Myeloid-Derived Suppressor Cells in Lymphoid Malignancies
Abstract
:1. Introduction
2. Phenotypic Characterization of MDSCs
3. Expansion and Activation
4. Recruitment
5. Mechanisms of Function
5.1. Deprivation of Essential Amino Acids
5.2. Reactive Oxygen Species (ROS) Production
5.3. Obstruction of Lymphocyte Trafficking
5.4. Induction of Immunosuppressive Cell Population and NK Cell Anergy
6. MDSCs in Hematological Malignancies
7. MDSCs in Mouse Models of Lymphoma
8. MDSCs in Lymphoid Malignancies
8.1. MDSCs in B-Cell Lymphomas
8.2. MDSCs in Hodgkin Lymphoma (HL)
8.3. B-Cell Chronic Lymphocytic Leukemia (B-CLL)
8.4. MDSCs in T/NK Lymphomas
9. Challenges and Open Questions
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bennett, J.A.; Srinivasa Rao, V.; Mitchell, M.S. Systemic Bacillus Calmette-Guerin (BCG) Activates Natural Suppressor Cells. Proc. Natl. Acad. Sci. USA 1978, 75, 5142–5144. [Google Scholar] [CrossRef] [Green Version]
- Talmadge, J.E.; Gabrilovich, D.I. History of Myeloid-Derived Suppressor Cells. Nat. Rev. Cancer 2013, 13, 739–752. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Bronte, V.; Chen, S.H.; Colombo, M.P.; Ochoa, A.; Ostrand-Rosenberg, S.; Schreiber, H. The Terminology Issue for Myeloid-Derived Suppressor Cells. Cancer Res. 2007, 67, 425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, R.; Roden, R.B. The Terminology Issue for Myeloid-Derived Suppressor Cells. Cancer Res. 2007, 67, 426. [Google Scholar] [CrossRef] [Green Version]
- Veglia, F.; Sanseviero, E.; Gabrilovich, D.I. Myeloid-Derived Suppressor Cells in the Era of Increasing Myeloid Cell Diversity. Nat. Rev. Immunol. 2021, 21, 485–498. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Bronte, V. Coordinated Regulation of Myeloid Cells by Tumours. Nat. Rev. Immunol. 2012, 12, 253–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ioannou, M.; Alissafi, T.; Lazaridis, I.; Deraos, G.; Matsoukas, J.; Gravanis, A.; Mastorodemos, V.; Plaitakis, A.; Sharpe, A.; Boumpas, D.; et al. Crucial Role of Granulocytic Myeloid-Derived Suppressor Cells in the Regulation of Central Nervous System Autoimmune Disease. J. Immunol. 2012, 188, 1136–1146. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, M.; Alissafi, T.; Boon, L.; Boumpas, D.; Verginis, P. In Vivo Ablation of Plasmacytoid Dendritic Cells Inhibits Autoimmunity through Expansion of Myeloid-Derived Suppressor Cells. J. Immunol. 2013, 190, 2631–2640. [Google Scholar] [CrossRef] [Green Version]
- Vlachou, K.; Mintzas, K.; Glymenaki, M.; Ioannou, M.; Papadaki, G.; Bertsias, G.K.; Sidiropoulos, P.; Boumpas, D.T.; Verginis, P. Elimination of Granulocytic Myeloid-Derived Suppressor Cells in Lupus-Prone Mice Linked to Reactive Oxygen Species-Dependent Extracellular Trap Formation. Arthritis Rheumatol. 2016, 68, 449–461. [Google Scholar] [CrossRef] [Green Version]
- Wood, K.J.; Bushell, A.; Hester, J. Regulatory Immune Cells in Transplantation. Nat. Rev. Immunol. 2012, 12, 417–430. [Google Scholar] [CrossRef]
- Bronte, V.; Brandau, S.; Chen, S.H.; Colombo, M.P.; Frey, A.B.; Greten, T.F.; Mandruzzato, S.; Murray, P.J.; Ochoa, A.; Ostrand-Rosenberg, S.; et al. Recommendations for Myeloid-Derived Suppressor Cell Nomenclature and Characterization Standards. Nat. Commun. 2016, 7, 12150. [Google Scholar] [CrossRef] [Green Version]
- Kusmartsev, S.A.; Li, Y.; Chen, S.H. Gr-1+ Myeloid Cells Derived from Tumor-Bearing Mice Inhibit Primary T Cell Activation Induced through CD3/CD28 Costimulation. J. Immunol. 2000, 165, 779–785. [Google Scholar] [CrossRef] [Green Version]
- Gabrilovich, D.; Ishida, T.; Oyama, T.; Ran, S.; Kravtsov, V.; Nadaf, S.; Carbone, D.P. Vascular Endothelial Growth Factor Inhibits the Development of Dendritic Cells and Dramatically Affects the Differentiation of Multiple Hematopoietic Lineages in Vivo. Blood 1998, 92, 4150–4166. [Google Scholar] [CrossRef]
- Watson, G.A.; Fu, Y.X.; Lopez, D.M. Splenic Macrophages from Tumor-Bearing Mice Co-Expressing MAC-1 and MAC-2 Antigens Exert Immunoregulatory Functions via Two Distinct Mechanisms. J. Leukoc. Biol. 1991, 49, 126–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youn, J.I.; Nagaraj, S.; Collazo, M.; Gabrilovich, D.I. Subsets of Myeloid-Derived Suppressor Cells in Tumor-Bearing Mice. J. Immunol. 2008, 181, 5791–5802. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, C.A.; Moses, K.; Trellakis, S.; Lang, S.; Brandau, S. Neutrophils and Granulocytic Myeloid-Derived Suppressor Cells: Immunophenotyping, Cell Biology and Clinical Relevance in Human Oncology. Cancer Immunol. Immunother. 2012, 61, 1155–1167. [Google Scholar] [CrossRef] [PubMed]
- Mandruzzato, S.; Brandau, S.; Britten, C.M.; Bronte, V.; Damuzzo, V.; Gouttefangeas, C.; Maurer, D.; Ottensmeier, C.; Van der Burg, S.H.; Welters, M.J.; et al. Toward Harmonized Phenotyping of Human Myeloid-Derived Suppressor Cells by Flow Cytometry: Results from an Interim Study. Cancer Immunol. Immunother. 2016, 65, 161–169. [Google Scholar] [CrossRef]
- Condamine, T.; Dominguez, G.A.; Youn, J.I.; Kossenkov, A.V.; Mony, S.; Alicea-Torres, K.; Tcyganov, E.; Hashimoto, A.; Nefedova, Y.; Lin, C.; et al. Lectin-Type Oxidized LDL Receptor-1 Distinguishes Population of Human Polymorphonuclear Myeloid-Derived Suppressor Cells in Cancer Patients. Sci. Immunol. 2016, 1, aaf8943. [Google Scholar] [CrossRef] [Green Version]
- Nan, J.; Xing, Y.F.; Hu, B.; Tang, J.X.; Dong, H.M.; He, Y.M.; Ruan, D.Y.; Ye, Q.J.; Cai, J.R.; Ma, X.K.; et al. Endoplasmic Reticulum Stress Induced LOX-1+CD15+ Polymorphonuclear Myeloid-Derived Suppressor Cells in Hepatocellular Carcinoma. Immunology 2018, 154, 144–155. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.R.; Park, S.M.; Seo, S.U.; Jung, I.; Yoon, H.I.; Gabrilovich, D.I.; Cho, B.C.; Seong, S.Y.; Ha, S.J.; Youn, J.I. The Ratio of Peripheral Regulatory T Cells to Lox-1+ Polymorphonuclear Myeloid-Derived Suppressor Cells Predicts the Early Response to Anti–PD-1 Therapy in Patients with Non–Small Cell Lung Cancer. Am. J. Respir. Crit. Care Med. 2018, 199, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Chai, E.; Zhang, L.; Li, C. LOX-1+ PMN-MDSC Enhances Immune Suppression Which Promotes Glioblastoma Multiforme Progression. Cancer Manag. Res. 2019, 11, 7307–7315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Si, Y.; Merz, S.F.; Jansen, P.; Wang, B.; Bruderek, K.; Altenhoff, P.; Mattheis, S.; Lang, S.; Gunzer, M.; Klode, J.; et al. Multidimensional Imaging Provides Evidence for Down-Regulation of T Cell Effector Function by MDSC in Human Cancer Tissue. Sci. Immunol. 2019, 4, eaaw9159. [Google Scholar] [CrossRef] [PubMed]
- Tavukcuoglu, E.; Horzum, U.; Yanik, H.; Uner, A.; Yoyen-Ermis, D.; Nural, S.K.; Aydin, B.; Sokmensuer, C.; Karakoc, D.; Yilmaz, K.B.; et al. Human Splenic Polymorphonuclear Myeloid-Derived Suppressor Cells (PMN-MDSC) Are Strategically Located Immune Regulatory Cells in Cancer. Eur. J. Immunol. 2020, 50, 2067–2074. [Google Scholar] [CrossRef]
- Bronte, V.; Chappell, D.B.; Apolloni, E.; Cabrelle, A.; Wang, M.; Hwu, P.; Restifo, N.P. Unopposed Production of Granulocyte-Macrophage Colony-Stimulating Factor by Tumors Inhibits CD8+ T Cell Responses by Dysregulating Antigen-Presenting Cell Maturation. J. Immunol. 1999, 162, 5728–5737. [Google Scholar]
- Waight, J.D.; Hu, Q.; Miller, A.; Liu, S.; Abrams, S.I. Tumor-Derived G-CSF Facilitates Neoplastic Growth through a Granulocytic Myeloid-Derived Suppressor Cell-Dependent Mechanism. PLoS ONE 2011, 6, e27690. [Google Scholar] [CrossRef]
- Sawanobori, Y.; Ueha, S.; Kurachi, M.; Shimaoka, T.; Talmadge, J.E.; Abe, J.; Shono, Y.; Kitabatake, M.; Kakimi, K.; Mukaida, N.; et al. Chemokine-Mediated Rapid Turnover of Myeloid-Derived Suppressor Cells in Tumor-Bearing Mice. Blood 2008, 111, 5457–5466. [Google Scholar] [CrossRef] [Green Version]
- Pan, P.Y.; Wang, G.X.; Yin, B.; Ozao, J.; Ku, T.; Divino, C.M.; Chen, S.H. Reversion of Immune Tolerance in Advanced Malignancy: Modulation of Myeloid-Derived Suppressor Cell Development by Blockade of Stem-Cell Factor Function. Blood 2008, 111, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Bunt, S.K.; Yang, L.; Sinha, P.; Clements, V.K.; Leips, J.; Ostrand-Rosenberg, S. Reduced Inflammation in the Tumor Microenvironment Delays the Accumulation of Myeloid-Derived Suppressor Cells and Limits Tumor Progression. Cancer Res. 2007, 67, 10019–10026. [Google Scholar] [CrossRef] [Green Version]
- Sinha, P.; Okoro, C.; Foell, D.; Freeze, H.H.; Ostrand-Rosenberg, S.; Srikrishna, G. Proinflammatory S100 Proteins Regulate the Accumulation of Myeloid-Derived Suppressor Cells. J. Immunol. 2008, 181, 4666–4675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinha, P.; Clements, V.K.; Fulton, A.M.; Ostrand-Rosenberg, S. Prostaglandin E2 Promotes Tumor Progression by Inducing Myeloid-Derived Suppressor Cells. Cancer Res. 2007, 67, 4507–4513. [Google Scholar] [CrossRef] [Green Version]
- Ochoa, A.C.; Zea, A.H.; Hernandez, C.; Rodriguez, P.C. Arginase, Prostaglandins, and Myeloid-Derived Suppressor Cells in Renal Cell Carcinoma. Clin. Cancer Res. 2007, 13, 721s–726s. [Google Scholar] [CrossRef] [Green Version]
- Condamine, T.; Gabrilovich, D.I. Molecular Mechanisms Regulating Myeloid-Derived Suppressor Cell Differentiation and Function. Trends Immunol. 2011, 32, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Condamine, T.; Mastio, J.; Gabrilovich, D.I. Transcriptional Regulation of Myeloid-Derived Suppressor Cells. J. Leukoc. Biol. 2015, 98, 913–922. [Google Scholar] [CrossRef]
- Wang, W.; Xia, X.; Mao, L.; Wang, S. The CCAAT/Enhancer-Binding Protein Family: Its Roles in MDSC Expansion and Function. Front. Immunol. 2019, 10, 1804. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-Derived Suppressor Cells as Regulators of the Immune System. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef]
- Hatziioannou, A.; Alissafi, T.; Verginis, P. Myeloid-Derived Suppressor Cells and T Regulatory Cells in Tumors: Unraveling the Dark Side of the Force. J. Leukoc. Biol. 2017, 102, 407–421. [Google Scholar] [CrossRef]
- Millrud, C.R.; Bergenfelz, C.; Leandersson, K. On the Origin of Myeloid-Derived Suppressor Cells. Oncotarget 2017, 8, 3649–3665. [Google Scholar] [CrossRef] [Green Version]
- Murdoch, C.; Giannoudis, A.; Lewis, C.E. Mechanisms Regulating the Recruitment of Macrophages into Hypoxic Areas of Tumors and Other Ischemic Tissues. Blood 2004, 104, 2224–2234. [Google Scholar] [CrossRef]
- Chun, E.; Lavoie, S.; Michaud, M.; Gallini, C.A.; Kim, J.; Soucy, G.; Odze, R.; Glickman, J.N.; Garrett, W.S. CCL2 Promotes Colorectal Carcinogenesis by Enhancing Polymorphonuclear Myeloid-Derived Suppressor Cell Population and Function. Cell Rep. 2015, 12, 244–257. [Google Scholar] [CrossRef] [Green Version]
- Huang, B.; Lei, Z.; Zhao, J.; Gong, W.; Liu, J.; Chen, Z.; Liu, Y.; Li, D.; Yuan, Y.; Zhang, G.M.; et al. CCL2/CCR2 Pathway Mediates Recruitment of Myeloid Suppressor Cells to Cancers. Cancer Lett. 2007, 252, 86–92. [Google Scholar] [CrossRef]
- Chang, A.L.; Miska, J.; Wainwright, D.A.; Dey, M.; Rivetta, C.V.; Yu, D.; Kanojia, D.; Pituch, K.C.; Qiao, J.; Pytel, P.; et al. CCL2 Produced by the Glioma Microenvironment Is Essential for the Recruitment of Regulatory T Cells and Myeloid-Derived Suppressor Cells. Cancer Res. 2016, 76, 5671–5682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luboshits, G.; Shina, S.; Kaplan, O.; Engelberg, S.; Nass, D.; Lifshitz-Mercer, B.; Chaitchik, S.; Keydar, I.; Ben-Baruch, A. Elevated Expression of the CC Chemokine Regulated on Activation, Normal T Cell Expressed and Secreted (RANTES) in Advanced Breast Carcinoma. Cancer Res. 1999, 59, 4681–4687. [Google Scholar] [PubMed]
- Zhang, Y.; Lv, D.; Kim, H.J.; Kurt, R.A.; Bu, W.; Li, Y.; Ma, X. A Novel Role of Hematopoietic CCL5 in Promoting Triple-Negative Mammary Tumor Progression by Regulating Generation of Myeloid-Derived Suppressor Cells. Cell Res. 2013, 23, 394–408. [Google Scholar] [CrossRef]
- Ichikawa, M.; Williams, R.; Wang, L.; Vogl, T.; Srikrishna, G. S100A8/A9 Activate Key Genes and Pathways in Colon Tumor Progression. Mol. Cancer Res. 2011, 9, 133–148. [Google Scholar] [CrossRef] [Green Version]
- Inamoto, S.; Itatani, Y.; Yamamoto, T.; Minamiguchi, S.; Hirai, H.; Iwamoto, M.; Hasegawa, S.; Taketo, M.M.; Sakai, Y.; Kawada, K. Loss of SMAD4 Promotes Colorectal Cancer Progression by Accumulation of Myeloid-Derived Suppressor Cells through the CCL15–CCR1 Chemokine Axis. Clin. Cancer Res. 2016, 22, 492–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, D.K.; Xu, I.M.; Lai, R.K.; Tse, A.P.; Wei, L.L.; Koh, H.Y.; Li, L.L.; Lee, D.; Lo, R.C.; Wong, C.M.; et al. Hypoxia Induces Myeloid-Derived Suppressor Cell Recruitment to Hepatocellular Carcinoma through Chemokine (C-C Motif) Ligand 26. Hepatology 2016, 64, 797–813. [Google Scholar] [CrossRef] [Green Version]
- Alfaro, C.; Teijeira, A.; Oñate, C.; Pérez, G.; Sanmamed, M.F.; Andueza, M.P.; Alignani, D.; Labiano, S.; Azpilikueta, A.; Rodriguez-Paulete, A.; et al. Tumor-Produced Interleukin-8 Attracts Human Myeloid-Derived Suppressor Cells and Elicits Extrusion of Neutrophil Extracellular Traps (NETs). Clin. Cancer Res. 2016, 22, 3924–3936. [Google Scholar] [CrossRef] [Green Version]
- Obermajer, N.; Muthuswamy, R.; Odunsi, K.; Edwards, R.P.; Kalinski, P. PGE(2)-Induced CXCL12 Production and CXCR4 Expression Controls the Accumulation of Human MDSCs in Ovarian Cancer Environment. Cancer Res. 2011, 71, 7463–7470. [Google Scholar] [CrossRef] [Green Version]
- Dolcetti, L.; Peranzoni, E.; Ugel, S.; Marigo, I.; Fernandez Gomez, A.; Mesa, C.; Geilich, M.; Winkels, G.; Traggiai, E.; Casati, A.; et al. Hierarchy of Immunosuppressive Strength among Myeloid-Derived Suppressor Cell Subsets Is Determined by GM-CSF. Eur. J. Immunol. 2010, 40, 22–35. [Google Scholar] [CrossRef]
- Movahedi, K.; Guilliams, M.; Van den Bossche, J.; Van den Bergh, R.; Gysemans, C.; Beschin, A.; De Baetselier, P.; Van Ginderachter, J.A. Identification of Discrete Tumor-Induced Myeloid-Derived Suppressor Cell Subpopulations with Distinct T Cell–Suppressive Activity. Blood 2008, 111, 4233–4244. [Google Scholar] [CrossRef]
- Bronte, V.; Zanovello, P. Regulation of Immune Responses by L-Arginine Metabolism. Nat. Rev. Immunol. 2005, 5, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, P.C.; Ochoa, A.C. Arginine Regulation by Myeloid Derived Suppressor Cells and Tolerance in Cancer: Mechanisms and Therapeutic Perspectives. Immunol. Rev. 2008, 222, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, P.C.; Zea, A.H.; Culotta, K.S.; Zabaleta, J.; Ochoa, J.B.; Ochoa, A.C. Regulation of t cell receptor cd3ζ chain expression byl-arginine. J. Biol. Chem. 2002, 277, 21123–21129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, P.C.; Quiceno, D.G.; Ochoa, A.C. L-Arginine Availability Regulates T-Lymphocyte Cell-Cycle Progression. Blood 2007, 109, 1568–1573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harari, O.; Liao, J.K. Inhibition of MHC II Gene Transcription by Nitric Oxide and Antioxidants. Curr. Pharm. Des. 2004, 10, 893–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzoni, A.; Bronte, V.; Visintin, A.; Spitzer, J.H.; Apolloni, E.; Serafini, P.; Zanovello, P.; Segal, D.M. Myeloid Suppressor Lines Inhibit T Cell Responses by an NO-Dependent Mechanism. J. Immunol. 2002, 168, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Apolloni, E.; Bronte, V.; Mazzoni, A.; Serafini, P.; Cabrelle, A.; Segal, D.M.; Young, H.A.; Zanovello, P. Immortalized Myeloid Suppressor Cells Trigger Apoptosis in Antigen-Activated T Lymphocytes. J. Immunol. 2000, 165, 6723–6730. [Google Scholar] [CrossRef] [PubMed]
- Koblish, H.K.; Hunter, C.A.; Wysocka, M.; Trinchieri, G.; Lee, W.M. Immune Suppression by Recombinant Interleukin (RIL)-12 Involves Interferon Gamma Induction of Nitric Oxide Synthase 2 (INOS) Activity: Inhibitors of NO Generation Reveal the Extent of RIL-12 Vaccine Adjuvant Effect. J. Exp. Med. 1998, 188, 1603–1610. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Li, Z.L.; Ye, S.B.; Ouyang, L.Y.; Chen, Y.S.; He, J.; Huang, H.Q.; Zeng, Y.X.; Zhang, X.S.; Li, J. Myeloid-Derived Suppressor Cells Inhibit T Cell Proliferation in Human Extranodal NK/T Cell Lymphoma: A Novel Prognostic Indicator. Cancer Immunol. Immunother. 2015, 64, 1587–1599. [Google Scholar] [CrossRef] [Green Version]
- Tadmor, T.; Fell, R.; Polliack, A.; Attias, D. Absolute Monocytosis at Diagnosis Correlates with Survival in Diffuse Large B-Cell Lymphoma—Possible Link with Monocytic Myeloid-Derived Suppressor Cells. Hematol. Oncol. 2013, 31, 65–71. [Google Scholar] [CrossRef]
- Lin, Y.; Gustafson, M.P.; Bulur, P.A.; Gastineau, D.A.; Witzig, T.E.; Dietz, A.B. Immunosuppressive CD14+HLA-DR(Low)/-Monocytes in B-Cell Non-Hodgkin Lymphoma. Blood 2011, 117, 872–881. [Google Scholar] [CrossRef] [Green Version]
- Khalifa, K.A.; Badawy, H.M.; Radwan, W.M.; Shehata, M.A.; Bassuoni, M.A. CD14+ HLA-DR Low/− Monocytes as Indicator of Disease Aggressiveness in B-Cell Non-Hodgkin Lymphoma. Int. J. Lab. Hematol. 2014, 36, 650–655. [Google Scholar] [CrossRef]
- Görgün, G.T.; Whitehill, G.; Anderson, J.L.; Hideshima, T.; Maguire, C.; Laubach, J.; Raje, N.; Munshi, N.C.; Richardson, P.G.; Anderson, K.C. Tumor-Promoting Immune-Suppressive Myeloid-Derived Suppressor Cells in the Multiple Myeloma Microenvironment in Humans. Blood 2013, 121, 2975–2987. [Google Scholar] [CrossRef] [Green Version]
- Giallongo, C.; Tibullo, D.; Parrinello, N.L.; La Cava, P.; Di Rosa, M.; Bramanti, V.; Di Raimondo, C.; Conticello, C.; Chiarenza, A.; Palumbo, G.A.; et al. Granulocyte-like Myeloid Derived Suppressor Cells (G-MDSC) Are Increased in Multiple Myeloma and Are Driven by Dysfunctional Mesenchymal Stem Cells (MSC). Oncotarget 2016, 7, 85764–85775. [Google Scholar] [CrossRef] [Green Version]
- Romano, A.; Parrinello, N.L.; La Cava, P.; Tibullo, D.; Giallongo, C.; Camiolo, G.; Puglisi, F.; Parisi, M.; Pirosa, M.C.; Martino, E.; et al. PMN-MDSC and Arginase Are Increased in Myeloma and May Contribute to Resistance to Therapy. Expert Rev. Mol. Diagn. 2018, 18, 675–683. [Google Scholar] [CrossRef]
- Srivastava, M.K.; Sinha, P.; Clements, V.K.; Rodriguez, P.; Ostrand-Rosenberg, S. Myeloid-Derived Suppressor Cells Inhibit T-Cell Activation by Depleting Cystine and Cysteine. Cancer Res. 2010, 70, 68–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Wang, X.; Wang, L.; Ma, X.; Gong, Z.; Zhang, S.; Li, Y. Targeting the IDO1 Pathway in Cancer: From Bench to Bedside. J. Hematol. Oncol. 2018, 11, 100. [Google Scholar] [CrossRef] [Green Version]
- Platten, M.; Nollen, E.A.A.; Röhrig, U.F.; Fallarino, F.; Opitz, C.A. Tryptophan Metabolism as a Common Therapeutic Target in Cancer, Neurodegeneration and Beyond. Nat. Rev. Drug Discov. 2019, 18, 379–401. [Google Scholar] [CrossRef]
- Corzo, C.A.; Cotter, M.J.; Cheng, P.; Cheng, F.; Kusmartsev, S.; Sotomayor, E.; Padhya, T.; McCaffrey, T.V.; McCaffrey, J.C.; Gabrilovich, D.I. Mechanism Regulating Reactive Oxygen Species in Tumor-Induced Myeloid-Derived Suppressor Cells. J. Immunol. 2009, 182, 5693–5701. [Google Scholar] [CrossRef]
- Kusmartsev, S.; Nefedova, Y.; Yoder, D.; Gabrilovich, D.I. Antigen-Specific Inhibition of CD8+ T Cell Response by Immature Myeloid Cells in Cancer Is Mediated by Reactive Oxygen Species. J. Immunol. 2004, 172, 989–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmielau, J.; Finn, O.J. Activated Granulocytes and Granulocyte-Derived Hydrogen Peroxide Are the Underlying Mechanism of Suppression of T-Cell Function in Advanced Cancer Patients. Cancer Res. 2001, 61, 4756–4760. [Google Scholar]
- Szuster-Ciesielska, A.; Hryciuk-Umer, E.; Stepulak, A.; Kupisz, K.; Kandefer-Szerszeń, M. Reactive Oxygen Species Production by Blood Neutrophils of Patients with Laryngeal Carcinoma and Antioxidative Enzyme Activity in Their Blood. Acta Oncol. 2004, 43, 252–258. [Google Scholar] [CrossRef] [Green Version]
- Geskin, L.J.; Akilov, O.E.; Kwon, S.; Schowalter, M.; Watkins, S.; Whiteside, T.L.; Butterfield, L.H.; Falo, L.D. Therapeutic Reduction of Cell-Mediated Immunosuppression in Mycosis Fungoides and Sézary Syndrome. Cancer Immunol. Immunother. 2018, 67, 423–434. [Google Scholar] [CrossRef]
- Ohl, K.; Tenbrock, K. Reactive Oxygen Species as Regulators of MDSC-Mediated Immune Suppression. Front. Immunol. 2018, 9, 2499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ku, A.W.; Muhitch, J.B.; Powers, C.A.; Diehl, M.; Kim, M.; Fisher, D.T.; Sharda, A.P.; Clements, V.K.; O’Loughlin, K.; Minderman, H.; et al. Tumor-Induced MDSC Act via Remote Control to Inhibit L-Selectin-Dependent Adaptive Immunity in Lymph Nodes. Elife 2016, 5, e17375. [Google Scholar] [CrossRef]
- Ostrand-Rosenberg, S.; Sinha, P. Myeloid-Derived Suppressor Cells: Linking Inflammation and Cancer. J. Immunol. 2009, 182, 4499–4506. [Google Scholar] [CrossRef]
- Hanson, E.M.; Clements, V.K.; Sinha, P.; Ilkovitch, D.; Ostrand-Rosenberg, S. Myeloid-Derived Suppressor Cells down-Regulate L-Selectin Expression on CD4+ and CD8+ T Cells. J. Immunol. 2009, 183, 937–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, B.; Pan, P.Y.; Li, Q.; Sato, A.I.; Levy, D.E.; Bromberg, J.; Divino, C.M.; Chen, S.H. Gr-1+CD115+ Immature Myeloid Suppressor Cells Mediate the Development of Tumor-Induced T Regulatory Cells and T-Cell Anergy in Tumor-Bearing Host. Cancer Res. 2006, 66, 1123–1131. [Google Scholar] [CrossRef] [Green Version]
- Jitschin, R.; Braun, M.; Büttner, M.; Dettmer-Wilde, K.; Bricks, J.; Berger, J.; Eckart, M.J.; Krause, S.W.; Oefner, P.J.; Le Blanc, K.; et al. CLL-Cells Induce IDOhi CD14+HLA-DRlo Myeloid-Derived Suppressor Cells That Inhibit T-Cell Responses and Promote TRegs. Blood 2014, 124, 750–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beury, D.W.; Parker, K.H.; Nyandjo, M.; Sinha, P.; Carter, K.A.; Ostrand-Rosenberg, S. Cross-Talk among Myeloid-Derived Suppressor Cells, Macrophages, and Tumor Cells Impacts the Inflammatory Milieu of Solid Tumors. J. Leukoc. Biol. 2014, 96, 1109–1118. [Google Scholar] [CrossRef]
- Li, H.; Han, Y.; Guo, Q.; Zhang, M.; Cao, X. Cancer-Expanded Myeloid-Derived Suppressor Cells Induce Anergy of NK Cells through Membrane-Bound TGF-Β1. J. Immunol. 2009, 182, 240–249. [Google Scholar] [CrossRef] [Green Version]
- Alissafi, T.; Hatzioannou, A.; Mintzas, K.; Barouni, R.M.; Banos, A.; Sormendi, S.; Polyzos, A.; Xilouri, M.; Wielockx, B.; Gogas, H.; et al. Autophagy Orchestrates the Regulatory Program of Tumor-Associated Myeloid-Derived Suppressor Cells. J. Clin. Investig. 2018, 128, 3840–3852. [Google Scholar] [CrossRef] [PubMed]
- Kalafati, L.; Kourtzelis, I.; Schulte-Schrepping, J.; Li, X.; Hatzioannou, A.; Grinenko, T.; Hagag, E.; Sinha, A.; Has, C.; Dietz, S.; et al. Innate Immune Training of Granulopoiesis Promotes Anti-Tumor Activity. Cell 2020, 183, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Bizymi, N.; Bjelica, S.; Kittang, A.O.; Mojsilovic, S.; Velegraki, M.; Pontikoglou, C.; Roussel, M.; Ersvær, E.; Santibañez, J.F.; Lipoldová, M.; et al. Myeloid-Derived Suppressor Cells in Hematologic Diseases: Promising Biomarkers and Treatment Targets. HemaSphere 2019, 3, e168. [Google Scholar] [CrossRef] [PubMed]
- Serafini, P.; Mgebroff, S.; Noonan, K.; Borrello, I. Myeloid-Derived Suppressor Cells Promote Cross-Tolerance in B-Cell Lymphoma by Expanding Regulatory T Cells. Cancer Res. 2008, 68, 5439–5449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; Ji, J.; Xu, J.; Li, D.; Shi, G.; Liu, F.; Ding, L.; Ren, J.; Dou, H.; Wang, T.; et al. MiR-30a Increases MDSC Differentiation and Immunosuppressive Function by Targeting SOCS3 in Mice with B-Cell Lymphoma. FEBS J. 2017, 284, 2410–2424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, S.; Jeong, A.L.; Lee, S.; Park, J.S.; Kim, K.D.; Choi, I.; Yoon, S.R.; Lee, M.S.; Lim, J.S.; Han, S.H.; et al. Adiponectin Deficiency Suppresses Lymphoma Growth in Mice by Modulating NK Cells, CD8 T Cells, and Myeloid-Derived Suppressor Cells. J. Immunol. 2013, 190, 4877–4886. [Google Scholar] [CrossRef] [Green Version]
- Abedi-Valugerdi, M.; Wolfsberger, J.; Pillai, P.R.; Zheng, W.; Sadeghi, B.; Zhao, Y.; Hassan, M. Suppressive Effects of Low-Dose 5-Fluorouracil, Busulfan or Treosulfan on the Expansion of Circulatory Neutrophils and Myeloid Derived Immunosuppressor Cells in Tumor-Bearing Mice. Int. Immunopharmacol. 2016, 40, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Abedi-Valugerdi, M.; Zheng, W.; Benkessou, F.; Zhao, Y.; Hassan, M. Differential Effects of Low-Dose Fludarabine or 5-Fluorouracil on the Tumor Growth and Myeloid Derived Immunosuppression Status of Tumor-Bearing Mice. Int. Immunopharmacol. 2017, 47, 173–181. [Google Scholar] [CrossRef]
- Pilot, T.; Fratti, A.; Thinselin, C.; Perrichet, A.; Demontoux, L.; Limagne, E.; Derangère, V.; Ilie, A.; Ndiaye, M.; Jacquin, E.; et al. Heat Shock and HSP70 Regulate 5-FU-Mediated Caspase-1 Activation in Myeloid-Derived Suppressor Cells and Tumor Growth in Mice. J. Immunother. Cancer 2020, 8, e000478. [Google Scholar] [CrossRef]
- Qin, H.; Lerman, B.; Sakamaki, I.; Wei, G.; Cha, S.C.; Rao, S.S.; Qian, J.; Hailemichael, Y.; Nurieva, R.; Dwyer, K.C.; et al. Generation of a New Therapeutic Peptide That Depletes Myeloid-Derived Suppressor Cells in Tumor-Bearing Mice. Nat. Med. 2014, 20, 676–681. [Google Scholar] [CrossRef]
- Sakamaki, I.; Kwak, L.W.; Cha, S.C.; Yi, Q.; Lerman, B.; Chen, J.; Surapaneni, S.; Bateman, S.; Qin, H. Lenalidomide Enhances the Protective Effect of a Therapeutic Vaccine and Reverses Immune Suppression in Mice Bearing Established Lymphomas. Leukemia 2014, 28, 329–337. [Google Scholar] [CrossRef] [Green Version]
- Lu, F.; Zhao, Y.; Pang, Y.; Ji, M.; Sun, Y.; Wang, H.; Zou, J.; Wang, Y.; Li, G.; Sun, T.; et al. NLRP3 Inflammasome Upregulates PD-L1 Expression and Contributes to Immune Suppression in Lymphoma. Cancer Lett. 2021, 497, 178–189. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, R.; Li, Q.; Wang, H.; Tao, Q.; Zhai, Z. Elevated M-MDSCs in Circulation Are Indicative of Poor Prognosis in Diffuse Large B-Cell Lymphoma Patients. J. Clin. Med. 2021, 10, 1768. [Google Scholar] [CrossRef]
- Wu, C.; Wu, X.; Zhang, X.; Chai, Y.; Guo, Q.; Li, L.; Yue, L.; Bai, J.; Wang, Z.; Zhang, L. Prognostic Significance of Peripheral Monocytic Myeloid-Derived Suppressor Cells and Monocytes in Patients Newly Diagnosed with Diffuse Large b-Cell Lymphoma. Int. J. Clin. Exp. Med. 2015, 8, 15173–15181. [Google Scholar]
- Wu, C.; Wu, X.; Liu, X.; Yang, P.; Xu, J.; Chai, Y.; Guo, Q.; Wang, Z.; Zhang, L. Prognostic Significance of Monocytes and Monocytic Myeloid-Derived Suppressor Cells in Diffuse Large B-Cell Lymphoma Treated with R-CHOP. Cell. Physiol. Biochem. 2016, 39, 521–530. [Google Scholar] [CrossRef]
- Azzaoui, I.; Uhel, F.; Rossille, D.; Pangault, C.; Dulong, J.; Le Priol, J.; Lamy, T.; Houot, R.; Le Gouill, S.; Cartron, G.; et al. T-Cell Defect in Diffuse Large B-Cell Lymphomas Involves Expansion of Myeloid-Derived Suppressor Cells. Blood 2016, 128, 1081–1092. [Google Scholar] [CrossRef]
- Xiu, B.; Lin, Y.; Grote, D.M.; Ziesmer, S.C.; Gustafson, M.P.; Maas, M.L.; Zhang, Z.; Dietz, A.B.; Porrata, L.F.; Novak, A.J.; et al. IL-10 Induces the Development of Immunosuppressive CD14+ HLA-DRlow/- Monocytes in B-Cell Non-Hodgkin Lymphoma. Blood Cancer J. 2015, 5, e328. [Google Scholar] [CrossRef] [Green Version]
- Romano, A.; Parrinello, N.L.; Vetro, C.; Forte, S.; Chiarenza, A.; Figuera, A.; Motta, G.; Palumbo, G.A.; Ippolito, M.; Consoli, U.; et al. Circulating Myeloid-Derived Suppressor Cells Correlate with Clinical Outcome in Hodgkin Lymphoma Patients Treated up-Front with a Risk-Adapted Strategy. Br. J. Haematol. 2015, 168, 689–700. [Google Scholar] [CrossRef]
- Marini, O.; Spina, C.; Mimiola, E.; Cassaro, A.; Malerba, G.; Todeschini, G.; Perbellini, O.; Scupoli, M.; Carli, G.; Facchinelli, D.; et al. Identification of Granulocytic Myeloid-Derived Suppressor Cells (G-MDSCs) in the Peripheral Blood of Hodgkin and Non-Hodgkin Lymphoma Patients. Oncotarget 2016, 7, 27676–27688. [Google Scholar] [CrossRef] [Green Version]
- Amini, R.M.; Enblad, G.; Hollander, P.; Laszlo, S.; Eriksson, E.; Ayoola Gustafsson, K.; Loskog, A.; Thörn, I. Altered Profile of Immune Regulatory Cells in the Peripheral Blood of Lymphoma Patients. BMC Cancer 2019, 19, 316. [Google Scholar] [CrossRef] [Green Version]
- Gustafson, M.P.; Abraham, R.S.; Lin, Y.; Wu, W.; Gastineau, D.A.; Zent, C.S.; Dietz, A.B. Association of an Increased Frequency of CD14 +HLA-DR Lo/Neg Monocytes with Decreased Time to Progression in Chronic Lymphocytic Leukaemia (CLL). Br. J. Haematol. 2012, 156, 674–676. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhou, Y.; Huang, Q.; Qiu, L. CD14+HLA-DRlow/- Expression: A Novel Prognostic Factor in Chronic Lymphocytic Leukemia. Oncol. Lett. 2015, 9, 1167–1172. [Google Scholar] [CrossRef] [Green Version]
- Zahran, A.M.; Moeen, S.M.; Thabet, A.F.; Rayan, A.; Abdel-Rahim, M.H.; Mohamed, W.M.; Hetta, H.F. Monocytic Myeloid-Derived Suppressor Cells in Chronic Lymphocytic Leukemia Patients: A Single Center Experience. Leuk. Lymphoma 2020, 61, 1645–1652. [Google Scholar] [CrossRef]
- Wilcox, R.A.; Feldman, A.L.; Wada, D.A.; Yang, Z.Z.; Comfere, N.I.; Dong, H.; Kwon, E.D.; Novak, A.J.; Markovic, S.N.; Pittelkow, M.R.; et al. B7-H1 (PD-L1, CD274) Suppresses Host Immunity in T-Cell Lymphoproliferative Disorders. Blood 2009, 114, 2149–2158. [Google Scholar] [CrossRef] [Green Version]
- Bontkes, H.J.; Jordanova, E.S.; Nijeboer, P.; Neefjes-Borst, E.A.; Cillessen, S.A.; Hayat, A.; Mulder, C.J.; Bouma, G.; Von Blomberg, B.M.; De Gruijl, T.D. High Myeloid-Derived Suppressor Cell Frequencies in the Duodenum Are Associated with Enteropathy Associated T-Cell Lymphoma and Its Precursor Lesions. Br. J. Haematol. 2017, 178, 988–991. [Google Scholar] [CrossRef] [PubMed]
- Enblad, G.; Karlsson, H.; Gammelgård, G.; Wenthe, J.; Lövgren, T.; Amini, R.M.; Wikstrom, K.I.; Essand, M.; Savoldo, B.; Hallböök, H.; et al. A Phase I/IIa Trial Using CD19-Targeted Third-Generation CAR T Cells for Lymphoma and Leukemia. Clin. Cancer Res. 2018, 24, 6185–6194. [Google Scholar] [CrossRef] [Green Version]
- Grosser, R.; Cherkassky, L.; Chintala, N.; Adusumilli, P.S. Combination Immunotherapy with CAR T Cells and Checkpoint Blockade for the Treatment of Solid Tumors. Cancer Cell 2019, 36, 471–482. [Google Scholar] [CrossRef]
- D’Aveni, M.; Notarantonio, A.B.; Bertrand, A.; Boulangé, L.; Pochon, C.; Rubio, M.T. Myeloid-Derived Suppressor Cells in the Context of Allogeneic Hematopoietic Stem Cell Transplantation. Front. Immunol. 2020, 11, 989. [Google Scholar] [CrossRef]
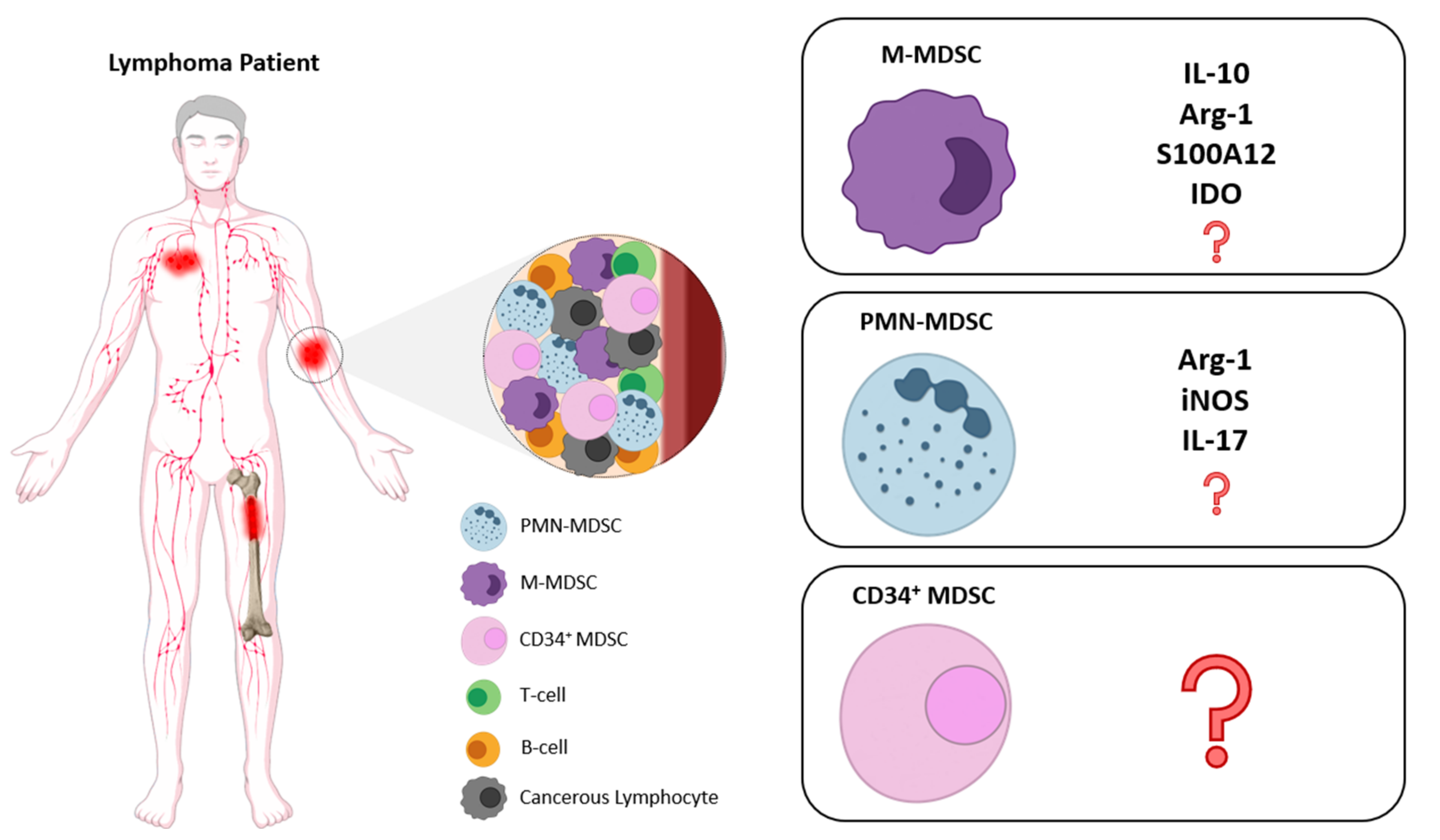
| Lymphoma Type | MDSCs Characterization | Mode of Action/Expansion | Clinical Implications | Reference |
|---|---|---|---|---|
| DLBCL Ν = 23 ND | CD14+HLADRlow/− (M-MDSCs) | - | Association with disease activity | 2011 [60] |
| DLBCL N = 144 ND | CD14+HLADRlow/− (M-MDSCs) | - | Significant difference of M-MDSC between GCB-DLBCL and non-GCB-DLBCL patients | 2015 [95] 2016 [96] |
| DLBCL N = 66 ND | LinnegHLA-DRlow/− CD123−CD33+CD11b+ (PMN-MDSCs) CD3−CD335−CD115+ CD14+ HLA-DR low (M-MDSCs) | IL-10, S100A12 and programmed death-ligand 1 (PD-L1) | Increased PMN- and M-MDSC compared to HC; Only M-MDSCs corelated with high-risk features and poor outcome | 2016 [97] |
| DLBCL TOTAL = 103 N = 65 ND 12 RELAPSED 26 REMISSION | CD14+HLA-DRlow/− (M-MDSCs) | IL-35 | MDSCs levels correlated with disease progression | 2021 [94] |
| NHLs, N = 40 R/R = 36, ND = 4 DLBCL = 18 FL = 14, MCL = 4, SLL = 2,MALT = 1, LPL = 1 | CD14+HLA-DRlow/− CD120blow (M-MDSCs) | Arginine metabolism. | increased M-MDSCs correlated with more aggressive disease | 2011 [61] |
| NHLs, N = 42 R/R = 26, ND = 16 DLBCL = 21, FL = 16, NHLi = 5 | CD14+HLA-DRlow/− (M-MDSCs) | arginase I | Increased in NHL vs. HC Correlated with: Advanced stage of disease (III/IV), aggressive histology and relapsed/refractory disease. | 2014 [62] |
| NHL, N = 22 (ND) DLBCL = 6, FL = 7, MCL = 4, MZL = 5 | CD14+HLA-DRlow/− (M-MDSCs) | IL-10 | elevated in NHL vs. HC Correlated with higher IPI. | 2015 [98] |
| HODGKIN ND N = 60 | CD14+HLA-DRlow/− (M-MDSCs) CD11b+CD33+CD14−HLA-DR− Lin− (PMN-MDSCs) CD11b+CD33+CD14–HLA-DR−CD34+ (CD34+MDSCs) | - | All MDSCs were increased in HL vs. HC CD34+MDSCs were strong predictors for PFS | 2015 [99] |
| NHL+HL, ND N = 124 HL = 31, DLBCL = 62 NHLi = 31 | CD66b+CD33dimHLADR− (PMN-MDSCs) | PMN-MDSCs were increased in all NHL+HL vs. HC | 2016 [100] | |
| NHL+HL, N = 43 ND DLBCL = 24 HL = 19 | CD11b+HLA-DRdimCD33dim CD14− (PMN-MDSCs) CD11b+HLADR−CD33+CD14+ (M-MDSCs) | - | Only PMN-MDSCs were increased in NHL+HL vs. HC Associated with better DFS in CHL | 2019 [101] |
| B-CLL, N = 29 untreated | CD14+HLADRlo/neg (M-MDSCs) | Diminished HLA-DR and CD86 expression | MDSCs correlated with disease progression MDSCs decreased to normal at remission | 2012 [102] |
| B-CLL, N = 79 Untreated | CD14+HLADRlo/neg (M-MDSCs) | Induction of Tregs and indoleamine 2,3-dioxygenase (IDO) activity | Increased in CLL vs. HC Associated with shorter LDT | 2014 [79] |
| B-CLL, N = 49 MBL, N = 23 Untreated | CD14+HLA-DR low/– (M-MDSCs) | - | Increased in CLL vs. MBL vs. HC MDSCs. Associated with advanced disease stage, IGHV unmutated CLL cases and worse OS | 2015 [103] |
| B-CLL, N = 50 | CD14+HLA-DR low/– (M-MDSCs) | - | Increased in CLL vs. HC correlated with disease stage, disease progression and OS | 2020 [104] |
| Cutaneous and Peripheral T-cell lymphomas | CD14+HLA-DR low/– (M-MDSCs) PBMCs | - | M-MDSCs expressing PD-L1 highly expand in CTCL patients | 2009 [105] |
| Extranodal NK, N = 32 | CD14+HLADR−CD33+CD11b+ (MDSCs) HLADR−CD33+CD11b+ CD15+ (PMN-MDSCs) | Arg-1, i-NOS and IL-17 | Higher levels in NHL vs. HC; M-MDSCs were independent predictors for DFS and OS. | 2015 [59] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papafragkos, I.; Markaki, E.; Kalpadakis, C.; Verginis, P. Decoding the Myeloid-Derived Suppressor Cells in Lymphoid Malignancies. J. Clin. Med. 2021, 10, 3462. https://doi.org/10.3390/jcm10163462
Papafragkos I, Markaki E, Kalpadakis C, Verginis P. Decoding the Myeloid-Derived Suppressor Cells in Lymphoid Malignancies. Journal of Clinical Medicine. 2021; 10(16):3462. https://doi.org/10.3390/jcm10163462
Chicago/Turabian StylePapafragkos, Iosif, Efrosyni Markaki, Christina Kalpadakis, and Panayotis Verginis. 2021. "Decoding the Myeloid-Derived Suppressor Cells in Lymphoid Malignancies" Journal of Clinical Medicine 10, no. 16: 3462. https://doi.org/10.3390/jcm10163462
APA StylePapafragkos, I., Markaki, E., Kalpadakis, C., & Verginis, P. (2021). Decoding the Myeloid-Derived Suppressor Cells in Lymphoid Malignancies. Journal of Clinical Medicine, 10(16), 3462. https://doi.org/10.3390/jcm10163462





