Female Gender Is Associated with Higher Susceptibility of Weight Induced Arterial Stiffening and Rise in Blood Pressure
Abstract
:1. Introduction
2. Methods:
2.1. Patient Cohort
2.2. Clinical Workup
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Association of the Female Gender with Obesity Mediated Vascular TOD
3.3. Gender Based Associations of Central Anthropometric Measures with Arterial Damage
4. Discussion
The Proposed Mechanism Predisposing the Female Gender to Obesity-Related Hypertension Compared to the Males
5. Conclusions
Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henry, S.L.; Barzel, B.; Wood-Bradley, R.J.; Burke, S.L.; Head, G.A.; Armitage, J.A. Developmental origins of obesity-related hypertension. Clin. Exp. Pharmacol. Physiol. 2012, 39, 799–806. [Google Scholar] [CrossRef]
- Kannel, W.B.; Zhang, T.; Garrison, R.J. Is obesity-related hypertension less of a cardiovascular risk? The Framingham study. Am. Heart J. 1990, 120, 1195–1201. [Google Scholar] [CrossRef]
- Payne, R.A.; Wilkinson, I.B.; Webb, D.J. Arterial stiffness and Hypertension-Emerging Concepts. Hypertension 2010, 55, 9–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ecobici, M.; Stoicescu, C. Arterial Stiffness and Hypertension—Which Comes First? Maedica 2017, 12, 184190. [Google Scholar]
- Kaess, B.M.; Rong, J.; Larson, M.G.; Hamburg, N.M.; Vita, J.A.; Levy, D.; Benjamin, E.J.; Vasan, R.S.; Mitchell, G.F. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA 2012, 308, 875–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Najjar, S.S.; Scuteri, A.; Shetty, V.; Wright, J.G.; Muller, D.C.; Fleg, J.L.; Spurgeon, H.P.; Ferrucci, L.; Lakatta, E.G. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the Baltimore Longitudinal Study of Aging. J. Am. Coll. Cardiol. 2008, 51, 1377–1383. [Google Scholar] [CrossRef] [Green Version]
- Yambe, M.; Tomiyama, H.; Yamada, J.; Koji, Y.; Motobe, K.; Shiina, K.; Yamamoto, Y.; Yamashina, A. Arterial stiffness and progression to hypertension in Japanese male subjects with high normal blood pressure. J. Hypertens. 2007, 25, 87–93. [Google Scholar] [CrossRef]
- Singhal, A.; Farooqi, I.S.; Cole, T.J.; O’Rahilly, S.; Fewtrell, M.; Kattenhorn, M.; Lucas, A.; Deanfield, J. Influence of leptin on arterial distensibility: A novel link between obesity and cardiovascular disease? Circulation 2002, 106, 1919–1924. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Martínez, E.; Miana, M.; Jurado-López, R.; Bartolomé, M.V.; Neto, F.S.; Salaices, M.; López-Andrés, N.; Cachofeiro, V. The potential role of leptin in the vascular remodeling associated with obesity. Int. J. Obes. 2014, 38, 1565–1572. [Google Scholar] [CrossRef]
- Du Toit, W.L.; Schutte, A.E.; Mels, C.M.C. The relationship of blood pressure with uric acid and bilirubin in young lean and overweight/obese men and women: The African-PREDICT study. J. Hum. Hypertens. 2020, 34, 648–656. [Google Scholar] [CrossRef]
- Oda, A.; Taniguchi, T.; Yokoyama, M. Leptin stimulates rat aortic smooth muscle cell proliferation and migration. Kobe J. Med. Sci. 2001, 47, 141–150. [Google Scholar] [CrossRef]
- Gong, M.; Wen, S.; Nguyen, T.; Wang, C.; Jin, J.; Zhou, L. Converging Relationships of Obesity and Hyperuricemia with Special Reference to Metabolic Disorders and Plausible Therapeutic Implications. Diabetes Metab. Syndr. Obes. 2020, 13, 943–962. [Google Scholar] [CrossRef] [Green Version]
- Carnagarin, R.; Gregory, C.; Azzam, O.; Hillis, G.S.; Schultz, C.; Watts, G.F.; Bell, D.; Matthews, V.; Schlaich, M.P. The Role of Sympatho-Inhibition in Combination Treatment of Obesity-Related Hypertension. Curr. Hypertens. Rep. 2017, 19, 99. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, G.E.; Beske, S.D.; Ballard, T.P.; Davy, K.P. Sympathetic neural activation in visceral obesity. Circulation 2002, 106, 2533–2536. [Google Scholar] [CrossRef] [Green Version]
- Abate, N.I.; Mansour, Y.H.; Tuncel, M.; Arbique, D.; Chavoshan, B.; Kizilbash, A.; Howell-Stampley, T.; Vongpatanasin, W.; Victor, R.G. Overweight and sympathetic overactivity in black Americans. Hypertension 2001, 38, 379–383. [Google Scholar] [CrossRef] [Green Version]
- Andersson, B.; Elam, M.; Wallin, B.G.; Bjorntorp, P.; Andersson, O.K. Effect of energy-restricted diet on sympathetic muscle nerve activity in obese women. Hypertension 1991, 18, 783–789. [Google Scholar] [CrossRef] [Green Version]
- Safar, M.E.; Czernichow, S.; Blacher, J. Obesity, arterial stiffness, and cardiovascular risk. J. Am. Soc. Nephrol. 2006, 17, S109–S111. [Google Scholar] [CrossRef]
- Van den Munckhof, I.C.L.; Holewijn, S.; de Graaf, J.; Rutten, J.H.W. Sex differences in fat distribution influence the association between BMI and arterial stiffness. J. Hypertens. 2017, 35, 1219–1225. [Google Scholar] [CrossRef]
- DuPont, J.J.; Kenney, R.M.; Patel, A.R.; Jaffe, I.Z. Sex differences in mechanisms of arterial stiffness. Br. J. Pharmacol. 2019, 176, 4208–4225. [Google Scholar] [CrossRef] [PubMed]
- Kramer, H.; Gutierrez, O.M.; Judd, S.E.; Muntner, P.; Warnock, D.G.; Tanner, R.M.; Panwar, B.; Shoham, D.A.; McClellan, W. Waist circumference, body mass index, and ESRD in the REGARDS (reasons for geographic and racial differences in stroke) study. Am. J. Kidney. Dis. 2016, 67, 62–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilsgaard, T.; Schirmer, H.; Arnesen, E. Impact of body weight on blood pressure with a focus on sex differences: The Tromsø study, 1986–1995. Arch. Intern. Med. 2000, 160, 2847–2853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujita, M.; Hata, A. Sex and age differences in the effect of obesity on incidence of hypertension in the Japanese population: A large historical cohort study. J. Am. Soc. Hypertens. 2014, 8, 64–70. [Google Scholar] [CrossRef]
- De Simone, G.; Devereux, R.B.; Chinali, M.; Roman, M.J.; Best, L.G.; Welty, T.K.; Lee, E.T.; Howard, B.V. Risk factors for arterial hypertension in adults with initial optimal blood pressure: The strong heart study. Hypertension 2006, 47, 162–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosca, L.; Barrett-Connor, E.; Wenger, N.K. Sex/gender differences in cardiovascular disease prevention: What a difference a decade makes. Circulation 2011, 124, 2145–2154. [Google Scholar] [CrossRef] [Green Version]
- Gudmundsdottir, H.; Hoieggen, A.; Stenehjem, A.; Waldum, B.; Os, I. Hypertension in women: Latest findings and clinical implications. Ther. Adv. Chronic Dis. 2012, 3, 137–146. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.K.; Alley, D.; Seeman, T.; Karlamangla, A.; Crimmins, E. Recent changes in cardiovascular risk factors among women and men. J. Womens Health 2006, 15, 734–746. [Google Scholar] [CrossRef] [Green Version]
- Malik, R.; Aneni, E.C.; Shahrayar, S.; Freitas, W.M.; Ali, S.S.; Veledar, E.; Latif, M.A.; Aziz, M.; Ahmed, R.; Khan, S.A.; et al. Elevated serum uric acid is associated with vascular inflammation but not coronary artery calcification in the healthy octogenarians: The Brazilian study on healthy aging. Aging Clin. Exp. Res. 2016, 28, 359–362. [Google Scholar] [CrossRef]
- Coutinho, T.; Goel, K.; Corrêa de Sá, D.; Carter, R.E.; Hodge, D.O.; Kragelund, C.; Kanaya, A.M.; Zeller, M.; Park, J.S.; Kober, L.; et al. Combining body mass index with measures of central obesity in the assessment of mortality in subjects with coronary disease: Role of “normal weight central obesity”. J. Am. Coll. Cardiol. 2013, 61, 553–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Recio-Rodriguez, J.I.; Gomez-Marcos, M.A.; Patino-Alonso, M.C.; Agudo-Conde, C.; Rodriguez-Sanchez, E.; Garcia-Ortiz, L. Abdominal obesity vs. general obesity for identifying arterial stiffness, subclinical atherosclerosis and wave reflection in healthy, diabetics and hypertensive. BMC Cardiovasc. Disord. 2012, 12, 3. [Google Scholar] [CrossRef] [Green Version]
- Nordstrand, N.; Gjevestad, E.; Dinh, K.N.; Hofsø, D.; Røislien, J.; Saltvedt, E.; Os, I.; Hjelmesæth, J. The relationship between various measures of obesity and arterial stiffness in morbidly obese patients. BMC Cardiovasc. Disord. 2011, 11, 7. [Google Scholar] [CrossRef] [Green Version]
- Rueda-Clausen, C.F.; Lahera, V.; Calderón, J.; Bolivar, I.C.; Castillo, V.R.; Gutiérrez, M.; Carreño, M.; del Pilar Oubiña, M.; Cachofeiro, V.; López-Jaramillo, P. The presence of abdominal obesity is associated with changes in vascular function independently of other cardiovascular risk factors. Int. J. Cardiol. 2010, 139, 32–41. [Google Scholar] [CrossRef]
- Chen, S.C.; Lo, T.C.; Chang, J.H.; Kuo, H.W. Variations in aging, gender, menopause, and obesity and their effects on hypertension in taiwan. Int. J. Hypertens. 2014, 2014, 515297. [Google Scholar] [CrossRef] [Green Version]
- Liao, Y.Y.; Chu, C.; Wang, Y.; Zheng, W.L.; Ma, Q.; Hu, J.W.; Yan, Y.; Wang, K.K.; Yuan, Y.; Chen, C.; et al. Sex differences in impact of long-term burden and trends of body mass index and blood pressure from childhood to adulthood on arterial stiffness in adults: A 30-year cohort study. Atherosclerosis 2020, 313, 118–125. [Google Scholar] [CrossRef]
- Zebekakis, P.E.; Nawrot, T.; Thijs, L.; Balkestein, E.J.; van der Heijden-Spek, J.; Van Bortel, L.M.; Struijker-Boudier, H.A.; Safar, M.E.; Staessen, J.A. Obesity is associated with increased arterial stiffness from adolescence until old age. J. Hypertens. 2005, 23, 1839–1846. [Google Scholar] [CrossRef]
- Sowers, J.R. Diabetes mellitus and cardiovascular disease in women. Arch. Intern. Med. 1998, 158, 617–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giltay, E.J.; Lambert, J.; Elbers, J.M.; Gooren, L.J.; Asscheman, H.; Stehouwer, C.D. Arterial compliance and distensibility are modulated by body composition in both men and women but by insulin sensitivity only in women. Diabetologia 1999, 42, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Rannelli, L.A.; MacRae, J.M.; Mann, M.C.; Ramesh, S.; Hemmelgarn, B.R.; Rabi, D.; Sola, D.Y.; Ahmed, S.B. Sex differences in associations between insulin resistance, heart rate variability, and arterial stiffness in healthy women and men: A physiology study. Can. J. Physiol. Pharmacol. 2016, 95, 349–355. [Google Scholar] [CrossRef]
- Gorzelniak, K.; Engeli, S.; Janke, J.; Luft, F.C.; Sharma, A.M. Hormonal regulation of the human adipose-tissue renin-angiotensin system: Relationship to obesity and hypertension. J. Hypertens. 2002, 20, 965–973. [Google Scholar] [CrossRef]
- Thethi, T.; Kamiyama, M.; Kobori, H. The link between the renin-angiotensin-aldosterone system and renal injury in obesity and the metabolic syndrome. Curr. Hypertens. Rep. 2012, 14, 160–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, M.C.; Fleeman, R.; Arnold, A.C. Sex differences in the metabolic effects of the renin-angiotensin system. Biol. Sex. Differ. 2019, 10, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Shoemaker, R.; Thatcher, S.E.; Batifoulier-Yiannikouris, F.; English, V.L.; Cassis, L.A. Administration of 17beta-estradiol to ovariectomized obese female mice reverses obesity-hypertension through an ace2-dependent mechanism. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E1066–E1075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huby, A.C.; Belin De Chantemele, E.J. Reviving the use of aldosterone inhibitors in treating hypertension in obesity. Am. J. Physiology. Regul. Integr. Comp. Physiol. 2015, 309, R1065–R1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinh Cat, A.N.; Friederich-Persson, M.; White, A.; Touyz, R.M. Adipocytes, aldosterone and obesity-related hypertension. J. Mol. Endocrinol. 2016, 57, F7–F21. [Google Scholar] [CrossRef] [Green Version]
- Goodfriend, T.L.; Kelley, D.E.; Goodpaster, B.H.; Winters, S.J. Visceral obesity and insulin resistance are associated with plasma aldosterone levels in women. Obes. Res. 1999, 7, 355–362. [Google Scholar] [CrossRef]
- Huby, A.C.; Antonova, G.; Groenendyk, J.; Gomez-Sanchez, C.E.; Bollag, W.B.; Filosa, J.A.; Belin de Chantemele, E.J. Adipocyte-derived hormone leptin is a direct regulator of aldosterone secretion, which promotes endothelial dysfunction and cardiac fibrosis. Circulation 2015, 132, 2134–2145. [Google Scholar] [CrossRef] [PubMed]
- Belin de Chantemele, E.J.; Ali, M.I.; Mintz, J.D.; Rainey, W.E.; Tremblay, M.L.; Fulton, D.J.; Stepp, D.W. Increasing peripheral insulin sensitivity by protein tyrosine phosphatase 1b deletion improves control of blood pressure in obesity. Hypertension 2012, 60, 1273–1279. [Google Scholar]
- Huby, A.C.; Otvos, L., Jr.; Belin de Chantemele, E.J. Leptin induces hypertension and endothelial dysfunction via aldosterone-dependent mechanisms in obese female mice. Hypertension 2016, 67, 1020–1028. [Google Scholar]
- Gruzdeva, O.; Borodkina, D.; Uchasova, E.; Dyleva, Y.; Barbarash, O. Leptin resistance: Underlying mechanisms and diagnosis. Diabetes Metab. Syndr. Obes. 2019, 12, 191–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanashiro-Takeuchi, R.M.; Heidecker, B.; Lamirault, G.; Dharamsi, J.W.; Hare, J.M. Sex-specific impact of aldosterone receptor antagonism on ventricular remodeling and gene expression after myocardial infarction. Clin. Transl. Sci. 2009, 2, 134–142. [Google Scholar] [CrossRef]
- Khosla, N.; Kalaitzidis, R.; Bakris, G.L. Predictors of hyperkalemia risk following hypertension control with aldosterone blockade. Am. J. Nephrol. 2009, 30, 418–424. [Google Scholar] [CrossRef]
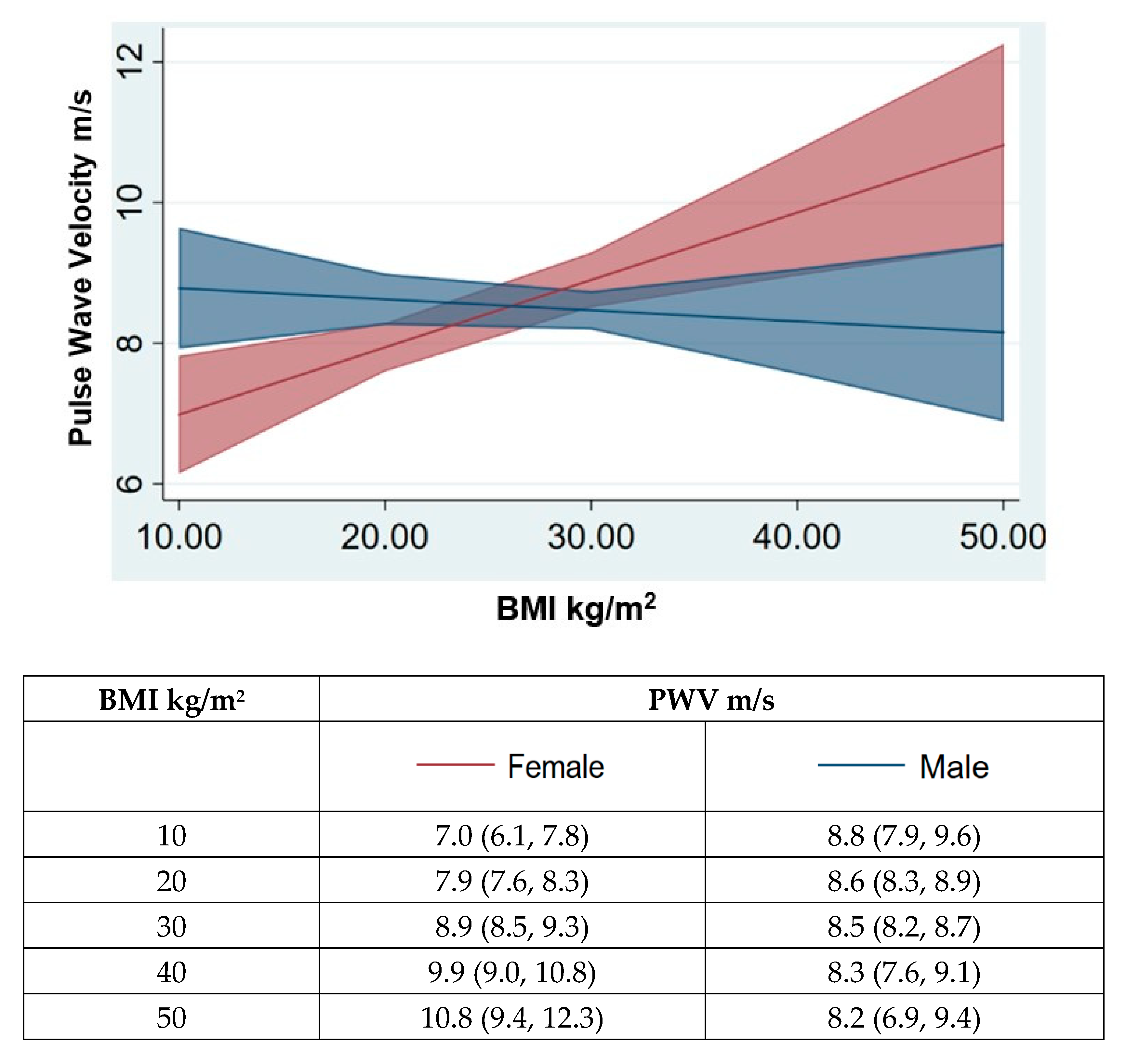
| Study Participants | BMI < 24 | BMI 24–28 | BMI > 28 | p Value between BMI Class | |
|---|---|---|---|---|---|
| n | 834 | 306 | 345 | 183 | |
| Age (years) | 54 (14) | 55 (16) | 55 (15) | 51 (14) | |
| Anthropometrics BMI | 25.5 (4) | 22 (2) | 26 (1) | 31 (3) | |
| WC (cm) | 91 (11) | 83 (8) | 92 (7) | 102 (10) | p < 0.001 |
| HC (cm) | 98 (7) | 93 (5) | 98 (5) | 105 (7) | p < 0.001 |
| WHR | 0.93 (0.08) | 0.90 (0.08) | 0.94 (0.07) | 0.97 (0.08) | p < 0.001 |
| WHtR | 0.55 (0.06) | 0.5 (0.05) | 0.55 (0.04) | 0.6 (0.06) | p < 0.001 |
| Arterial parameters | |||||
| CF PWV (m/s) | 8.5 (2.1) | 8.2 (2.2) | 8.6 (2.2) | 8.6 (2.2) | p < 0.001 |
| Brachial SP (mmHg) | 134 (19) | 131 (20) | 135 (19) | 135 (16) | p < 0.01 |
| Brachial DP (mmHg) | 77 (12) | 73(12) | 78 (12) | 79 (11) | p < 0.001 |
| Brachial PP (mmHg) | 57 (14) | 57 (15) | 57 (13) | 56 (13) | p < 0.001 |
| Central SP (mmHg) | 122 (19) | 120 (20) | 124 (20) | 122 (16) | p < 0.05 |
| Central DP (mmHg) | 78 (12) | 75 (12) | 79 (13) | 81 (11) | p < 0.001 |
| Central PP (mmHg) | 44 (13) | 45 (14) | 44 (13) | 41 (12) | p = 0.0643 |
| MAP (mmHg) | 97 (14) | 94 (15) | 99 (15) | 99 (13) | p < 0.001 |
| CAIx (%) | 139 (26) | 28 (12) | 26 (12) | 21 (13) | p < 0.001 |
| Systolic ejection duration | 315 (26) | 320 (25) | 315 (24) | 307 (27) | p < 0.001 |
| End systolic pressure | 110 (18) | 107 (18) | 111 (18) | 110 (15) | p < 0.05 |
| Biochemical Parameters | |||||
| Plasma glucose (mmol/L) | 5.86 (1.9) | 5.6 2 (1.7) | 5.95 (1.8) | 6.03 (2) | p < 0.05 |
| Liver profile | |||||
| Alanine transaminase (ALT) | 28.1 (27.8) | 20 (11) | 29 (19.7) | 36.9 (23.4) | p < 0.001 |
| Aspartate transaminase (AST) | 25.6 (22.8) | 22 (8.9) | 26.8 (25.6) | 29.5 (31) | p < 0.001 |
| Gamma-glutamyl transferase | 38.2 (36.9) | 26.1 (24) | 40 (33) | 54.7 (52) | p < 0.001 |
| Renal profile Blood urea nitrogen (BUN) | 6.1 (12.5) | 6.7 (20) | 5.8 (3.8) | 5.6 (1.5) | p = 0.458 |
| Creatinine | 79 (37.5) | 78 (55.6) | 79.33 (23) | 79 (16.6) | p = 0.814 |
| Lipid Profile | |||||
| Total Cholesterol (TC) | 4.8 (1.13) | 4.7 (1.1) | 4.8 (1.2) | 4.7 (1.2) | p = 0.644 |
| Triglycerides (TGL) | 2 (2.5) | 1.5 (0.9) | 2.23 (3.4) | 2.4 (2.4) | p < 0.05 |
| Low density lipoprotein (LDL) High density | 3.4 (6.3) | 3.1 (0.9) | 3.8 (9.6) | 3.3 (2.3) | p = 0.897 |
| lipoprotein (HDL) | 1.2 (0.5) | 1.2 (0.4) | 1.1 (0.7) | 1.1 (0.4) | p < 0.005 |
| Inflammatory marker: Uric acid (µmol/L) | 359.4 (100.1) | 321.9 (92.5) | 374 (98) | 394.39 (96.6) | p < 0.001 |
| Gender | Arterial Parameters | BMI Classes | Age in Years | p Value b/w BMI Classes | Post-hoc Analysis (Lean vs. Others BMI Classes) | p Value b/w Gender | |||
|---|---|---|---|---|---|---|---|---|---|
| 10–30 | 31–50 | 51–70 | >71 | ||||||
| n | 32 | 331 | 379 | 92 | |||||
| Male | CF PWV | lean | 6.6 (1.1) | 7.4 (1.4) | 9.0 (2.4) | 10.5 (0.0) | p = 0.21 | NS | p = 0.88 |
| overweight | 7.6 (0.2) | 7.7 (1.5) | 9.0 (2.0) | 10.4 (2.7) | |||||
| obese | 7.1 (2.2) | 8.3 (1.5) | 9.1 (2.0) | 9.8 (1.7) | |||||
| p = 0.82 | p < 0.005 | p = 0.90 | p = 0.76 | ||||||
| Female | lean | 5.5 (1.1) | 7.2 (1.7) | 8.2 (1.6) | 10.2 (1.9) | p < 0.005 | |||
| overweight | 6.4 (0.5) | 7.3 (0.8) | 9.2 (1.9) | 12.0 (3.5) | p < 0.005 | ||||
| obese | 6.3 (0.2) | 7.7 (1.4) | 9.1 (2.3) | 11.3 (1.9) | p < 0.005 | ||||
| p = 0.36 | p = 0.67 | p < 0.005 | p = 0.25 | ||||||
| Male | Brachial SP | lean | 133 (4.5) | 131 (17.5) | 136 (16.9) | 135 (17.8) | p = 0.78 | NS | p < 0.05 |
| overweight | 143 (10.9) | 131 (18) | 137 (20) | 135 (18.4) | |||||
| obese | 142 (20.2) | 133 (14.1) | 137 (17.0) | 125 (15.0) | |||||
| p = 0.70 | p = 0.56 | p = 0.93 | p = 0.32 | ||||||
| Female | lean | 116 (15.4) | 123 (20.1) | 129 (17.1) | 148 (30.1) | p < 0.005 | |||
| overweight | 125 (18.7) | 128 (19.5) | 143 (18.1) | 143 (26.1) | p < 0.005 | ||||
| obese | 114 (12.7) | 137 (16.8) | 139 (18.7) | 138 (7.9) | p < 0.05 | ||||
| p = 0.68 | p = 0.16 | p < 0.005 | p = 0.74 | ||||||
| Male | Brachial DP | lean | 77 (11.8) | 77 (13.5) | 79 (9.9) | 70 (8.5) | p = 0.12 | NS | p < 0.005 |
| overweight | 81 (7.0) | 79 (12.5) | 78 (11.7) | 73 (11.3) | |||||
| obese | 79 (14.8) | 82 (11.7) | 81 (10.0) | 64 (7.2) | |||||
| p = 0.94 | p = 0.16 | p = 0.50 | p = 0.62 | ||||||
| Female | lean | 70 (7.9) | 72 (14.3) | 71 (10.6) | 75 (14.4) | p < 0.001 | |||
| overweight | 74 (20.9) | 79 (13.0) | 79 (13.0) | 73 (10.5) | p < 0.005 | ||||
| obese | 68 (13.4) | 81 (9.4) | 79 (9.1) | 75 (6.0) | p < 0.005 | ||||
| p = 0.81 | p = 0.07 | p < 0.005 | p = 0.89 | ||||||
| Male | Central SP | lean | 118 (11.4) | 118 (19.2) | 126 (16.0) | 124 (19.1) | p = 0.94 | NS | p = 0.25 |
| overweight | 125 (6.0) | 119 (19.0) | 126 (19.2) | 123 (20.1) | |||||
| obese | 120 (16.9) | 120 (13.5) | 125 (16.5) | 110 (13.0) | |||||
| p = 0.82 | p = 0.77 | p = 0.94 | p = 0.14 | ||||||
| Female | lean | 104 (16.1) | 114 (21.4) | 119 (16.8) | 138 (29.8) | p < 0.05 | |||
| overweight | 112 (21.0) | 118 (20.0) | 132 (17.4) | 130 (25.0) | p < 0.005 | ||||
| obese | 99 (17.7) | 123 (18.6) | 128 (19.6) | 128 (8.4) | p < 0.05 | ||||
| p = 0.68 | p = 0.37 | p < 0.005 | p = 0.62 | ||||||
| Male | Central DP | lean | 78 (11.9) | 78 (13.7) | 80 (10.0) | 71 (8.6) | p = 0.13 | NS | p < 0.05 |
| overweight | 82 (7.1) | 80 (12.8) | 79 (12.0) | 74 (11.5) | |||||
| obese | 80 (15.0) | 83 (11.7) | 82 (10.2) | 65 (7.8) | |||||
| p = 0.95 | p = 0.08 | p = 0.44 | p = 0.07 | ||||||
| Female | lean | 71 (8.1) | 74 (14.6) | 72 (10.8) | 76 (14.8) | p < 0.001 | |||
| overweight | 76 (21.5) | 80 (13.1) | 80 (13.1) | 73 (11.0) | p < 0.005 | ||||
| obese | 69 (12.7) | 83 (9.5) | 80 (9.4) | 76 (5.8) | p < 0.005 | ||||
| p = 0.80 | p = 0.06 | p < 0.005 | p = 0.86 | ||||||
| Central Anthropometric Measures | WHR | WHtR | ||
|---|---|---|---|---|
| M | F | M | F | |
| CF PWV (m/s) | p = 0.004 | p = 0.000 | p = 0.003 | p = 0.000 |
| r = 0.145 | r = 0.386 | r = 0.145 | r = 0.412 | |
| Brachial SP (mmHg) | p = 0.372 | p = 0.001 | p = 0.063 | p = 0.000 |
| r = −0.044 | r = 0.202 | r = 0.201 | r = 0.291 | |
| Brachial PP (mmHg) | p = 0.651 | p = 0.008 | p = 0.55 | p = 0.002 |
| r = −0.022 | r = 0.166 | r = −0.029 | r = 0.198 | |
| Central SP (mmHg) | p = 0.146 | p = 0.002 | p = 0.54 | p = 0.000 |
| r = −0.072 | r = 0.196 | r = 0.03 | r = 0.271 | |
| Central PP (mmHg) | p = 0.239 | p = 0.00 | p = 0.109 | p = 0.003 |
| r = −0.058 | r = 0.174 | r = −0.079 | r = 0.185 | |
| Central MAP (mmHg) | p = 0.149 | p = 0.023 | p = 0.127 | p = 0.000 |
| r = −0.071 | r = 0.143 | r = 0.075 | r = 0.234 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, J.; Chao, H.; Tang, B.; Avolio, A.P.; Schlaich, M.P.; Nolde, J.M.; Adji, A.; Carnagarin, R. Female Gender Is Associated with Higher Susceptibility of Weight Induced Arterial Stiffening and Rise in Blood Pressure. J. Clin. Med. 2021, 10, 3479. https://doi.org/10.3390/jcm10163479
Zuo J, Chao H, Tang B, Avolio AP, Schlaich MP, Nolde JM, Adji A, Carnagarin R. Female Gender Is Associated with Higher Susceptibility of Weight Induced Arterial Stiffening and Rise in Blood Pressure. Journal of Clinical Medicine. 2021; 10(16):3479. https://doi.org/10.3390/jcm10163479
Chicago/Turabian StyleZuo, Junli, Huijuan Chao, Biwen Tang, Alberto P. Avolio, Markus P. Schlaich, Janis Marc Nolde, Audrey Adji, and Revathy Carnagarin. 2021. "Female Gender Is Associated with Higher Susceptibility of Weight Induced Arterial Stiffening and Rise in Blood Pressure" Journal of Clinical Medicine 10, no. 16: 3479. https://doi.org/10.3390/jcm10163479
APA StyleZuo, J., Chao, H., Tang, B., Avolio, A. P., Schlaich, M. P., Nolde, J. M., Adji, A., & Carnagarin, R. (2021). Female Gender Is Associated with Higher Susceptibility of Weight Induced Arterial Stiffening and Rise in Blood Pressure. Journal of Clinical Medicine, 10(16), 3479. https://doi.org/10.3390/jcm10163479






