Natural-Course Evaluation of Infants with Positional Severe Plagiocephaly Using a Three-Dimensional Scanner in Japan: Comparison with Those Who Received Cranial Helmet Therapy
Abstract
:1. Introduction
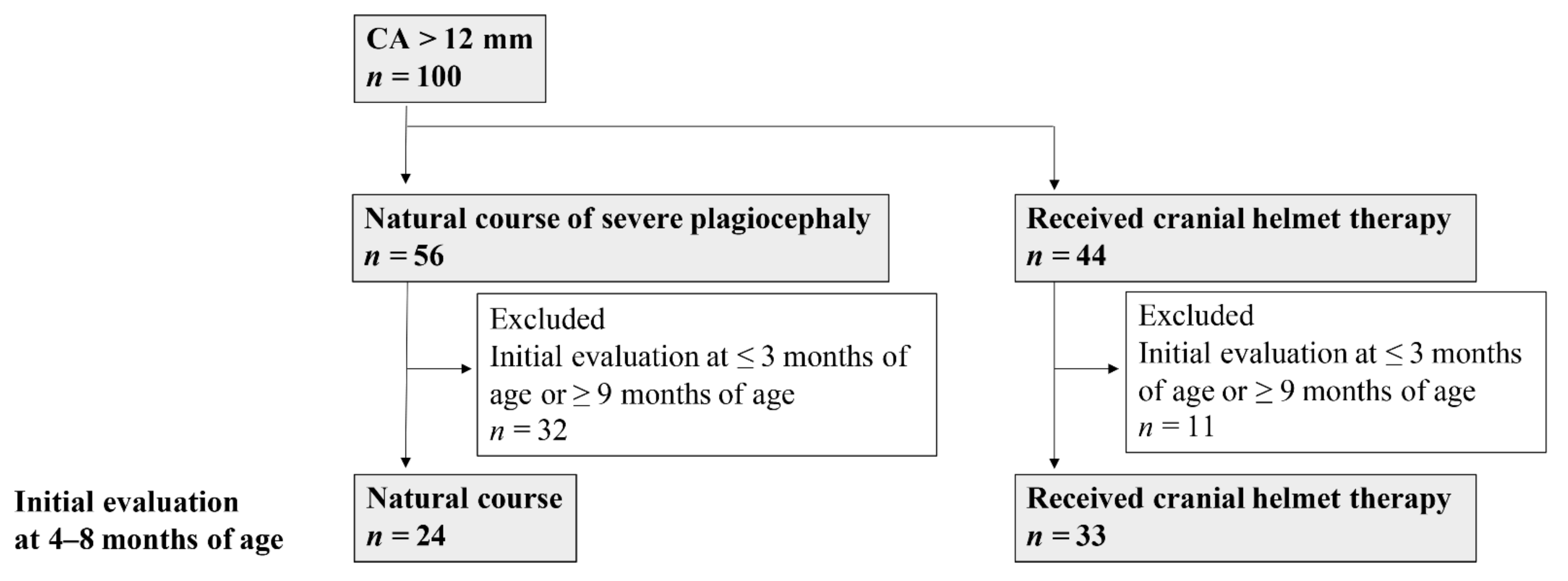
2. Materials and Methods
2.1. Subjects and Study Design
2.2. Study Methods
2.3. Primary Outcomes
2.4. Definition of Severity and Improvement
2.5. Data Acquisition Using the 3D Scanner
2.6. Data analysis Method
2.7. CHT
2.8. Statistical Analyses
3. Results
3.1. Study 1
3.1.1. Clinical Characteristics and Measured Values
3.1.2. Natural Course of Severe Plagiocephaly
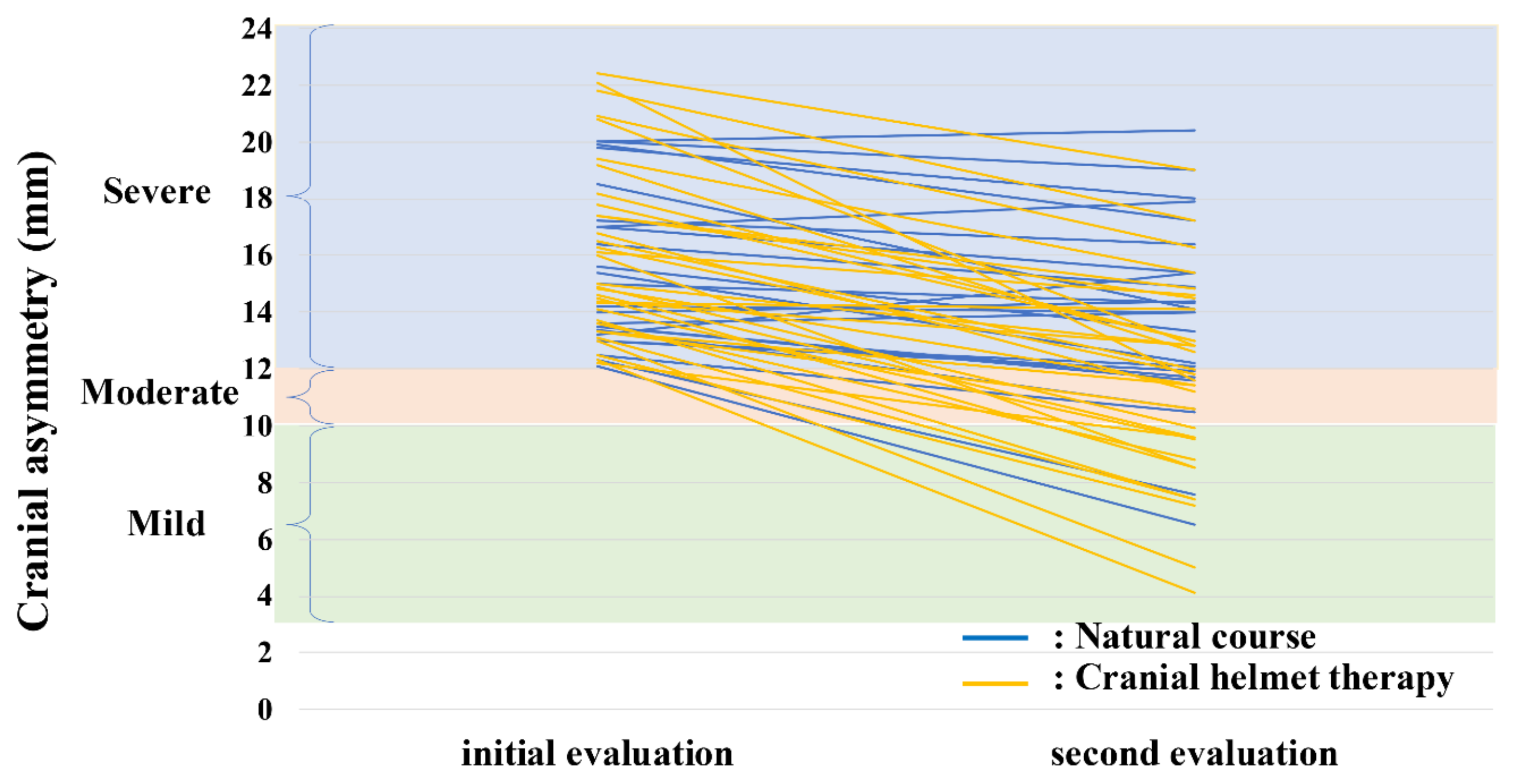
3.2. Study 2
3.2.1. Clinical Characteristics and Measured Values
3.2.2. Efficacy of CHT in Severe Plagiocephaly
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hutchison, B.L.; Hutchison, L.A.; Thompson, J.M.; Mitchell, E.A. Plagiocephaly and Brachycephaly in the First Two Years of Life: A Prospective Cohort Study. Pediatrics 2004, 114, 970–980. [Google Scholar] [CrossRef] [Green Version]
- Aihara, Y.; Komatsu, K.; Dairoku, H.; Kubo, O.; Hori, T.; Okada, Y. Cranial Molding Helmet Therapy and Establishment of Practical Criteria for Management in Asian Infant Positional Head Deformity. Childs Nerv. Syst. 2014, 30, 1499–1509. [Google Scholar] [CrossRef]
- Takamatsu, A.; Hikosaka, M.; Kaneko, T.; Mikami, M.; Kaneko, A. Evaluation of the Molding Helmet Therapy for Japanese Infants with Deformational Plagiocephaly. JMA J. 2021, 4, 50–60. [Google Scholar] [PubMed]
- Van Vlimmeren, L.A.; van der Graaf, Y.; Boere-Boonekamp, M.M.; L’Hoir, M.P.; Helders, P.J.; Engelbert, R.H. Risk Factors for Deformational Plagiocephaly at Birth and at 7 Weeks of Age: A Prospective Cohort Study. Pediatrics 2007, 119, e408–e418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutchison, B.L.; Hutchison, L.A.; Thompson, J.M.; Mitchell, E.A. Quantification of Plagiocephaly and Brachycephaly in Infants Using a Digital Photographic Technique. Cleft Palate Craniofac. J. 2005, 42, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Fahrenkopf, M.P.; Adams, N.S.; Mann, R.J.; Girotto, J.A. Deformational Plagiocephaly. In Nelson Textbook of Pediatrics, 21st ed.; Kliegman, R.M., St Geme, J.W., Blum, N.J., Shah, S.S., Tasker, R.C., Wilson, K.M., Eds.; Elsevier: Philadelphia, PA, USA, 2020; pp. 3082–3086. [Google Scholar]
- Looman, W.S.; Flannery, A.B. Evidence-Based Care of the Child with Deformational Plagiocephaly, Part I: Assessment and Diagnosis. J. Pediatr. Health Care 2012, 26, 242–250. [Google Scholar] [CrossRef]
- Moss, S.D. Nonsurgical, Nonorthotic Treatment of Occipital Plagiocephaly: What is the Natural History of the Misshapen Neonatal Head? J. Neurosurg. 1997, 87, 667–670. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.Y.; Park, M.S.; Yang, J.I.; Yim, S.Y. Comparison of Helmet Therapy and Counter Positioning for Deformational Plagiocephaly. Ann. Rehabil. Med. 2013, 37, 785–795. [Google Scholar] [CrossRef]
- Schweitzer, T.; Böhm, H.; Linz, C.; Jager, B.; Gerstl, L.; Kunz, F.; Stellzig-Eisenhauer, A.; Ernestus, R.I.; Krauß, J.; Meyer-Marcotty, P. Three-Dimensional Analysis of Positional Plagiocephaly Before and After Molding Helmet Therapy in Comparison to Normal Head Growth. Childs Nerv. Syst. 2013, 29, 1155–1161. [Google Scholar] [CrossRef]
- Kunz, F.; Schweitzer, T.; Große, S.; Waßmuth, N.; Stellzig-Eisenhauer, A.; Böhm, H.; Meyer-Marcotty, P.; Linz, C. Head Orthosis Therapy in Positional Plagiocephaly: Longitudinal 3D-Investigation of Long-Term Outcomes, Compared with Untreated Infants and with a Control Group. Eur. J. Orthod. 2019, 41, 29–37. [Google Scholar] [CrossRef]
- Kluba, S.; Kraut, W.; Calgeer, B.; Reinert, S.; Krimmel, M. Treatment of Positional Plagiocephaly—Helmet or No Helmet? J. Craniomaxillofac. Surg. 2014, 42, 683–688. [Google Scholar] [CrossRef]
- Mortenson, P.; Steinbok, P.; Smith, D. Deformational Plagiocephaly and Orthotic Treatment: Indications and Limitations. Childs Nerv. Syst. 2012, 28, 1407–1412. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.S.; Rah, D.K.; Kim, Y.O. Outcome Analysis of Cranial Molding Therapy in Nonsynostotic Plagiocephaly. Arch. Plast. Surg. 2012, 39, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Argenta, L.; David, L.; Thompson, J. Clinical Classification of Positional Plagiocephaly. J. Craniofac. Surg. 2004, 15, 368–372. [Google Scholar] [CrossRef]
- Wilbrand, J.F.; Lautenbacher, N.; Pons-Kühnemann, J.; Streckbein, P.; Kähling, C.; Reinges, M.H.; Howaldt, H.P.; Wilbrand, M. Treated Versus Untreated Positional Head Deformity. J. Craniofac. Surg. 2016, 27, 13–18. [Google Scholar] [CrossRef]
- Robinson, S.; Proctor, M. Diagnosis and Management of Deformational Plagiocephaly. J. Pediatr. Health Care 2009, 3, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Flannery, A.B.; Looman, W.S.; Kemper, K. Evidence-Based Care of the Child with Deformational Plagiocephaly, part II: Management. J. Pediatr. Health Care 2012, 26, 320–331. [Google Scholar] [CrossRef]
- Pastor-Pons, I.; Lucha-López, M.O.; Barrau-Lalmolda, M.; Rodes-Pastor, I.; Rodríguez-Fernández, Á.L.; Hidalgo-García, C.; Tricás-Moreno, J.M. Interrater and Intrarater Reliability of Cranial Anthropometric Measurements in Infants with Positional Plagiocephaly. Children 2020, 7, 306. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Pons, I.; Lucha-López, M.O.; Barrau-Lalmolda, M.; Rodes-Pastor, I.; Rodríguez-Fernández, Á.L.; Hidalgo-García, C.; Tricás-Moreno, J.M. Efficacy of Pediatric Integrative Manual Therapy in Positional Plagiocephaly: A Randomized Controlled Trial. Ital. J. Pediatr. 2021, 47, 132. [Google Scholar] [CrossRef]
- Van Wijk, R.M.; van Vlimmeren, L.A.; Groothuis-Oudshoorn, C.G.; Van der Ploeg, C.P.; Ijzerman, M.J.; Boere-Boonekamp, M.M. Helmet Therapy in Infants with Positional Skull Deformation: Randamised Controlled Trial. BMJ 2014, 348, g2741. [Google Scholar] [CrossRef] [Green Version]
- Van Wijk, R.M.; Boere-Boonekamp, M.M.; Groothuis-Oudshoorn, C.G.; van Vlimmeren, L.A.; IJzerman, M.J. Helmet Therapy Assessment in Infants with Deformed Skulls (HEAD): Protocol for a Randomised Controlled Trial. Trials 2012, 13, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]



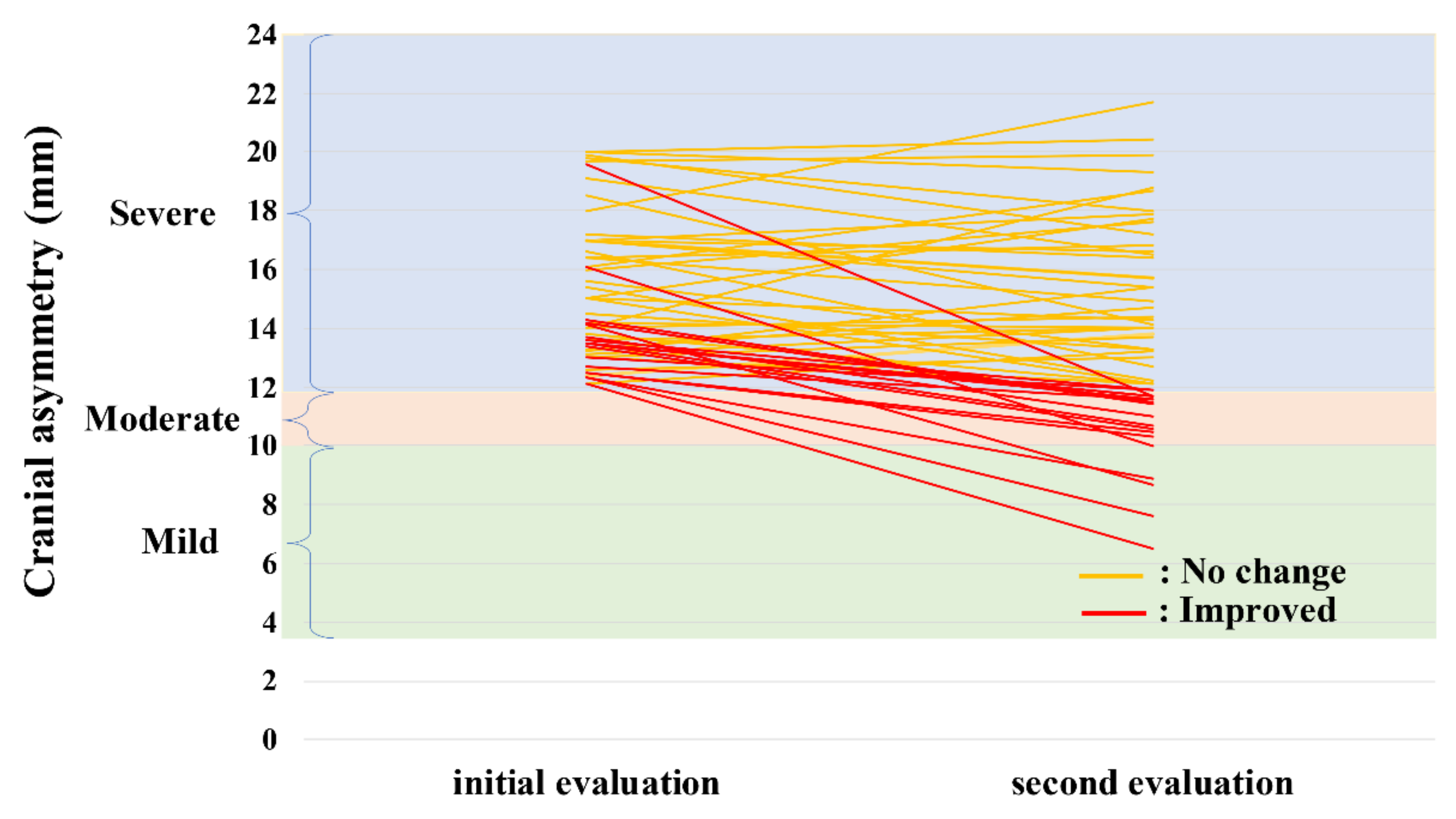
| No-Change Group n = 37 | Improved Group n = 19 | p-Value | |
|---|---|---|---|
| Birth weight, g | 3070 (1962–4144) | 3100 (1886–3820) | 0.729 |
| Birth order (first born) | 23 (62) | 11 (58) | 0.757 |
| Sex (male) | 23 (62) | 12 (63) | 0.942 |
| Gestational age at birth, weeks | 39 (35–41) | 38 (34–41) | 0.199 |
| Initial evaluation age, months | 3 (1–10) | 3 (1–10) | 0.634 |
| Second evaluation age, months | 6 (3–14) | 6 (3–12) | 0.328 |
| Evaluation interval, months | 2 (1–7) | 2 (1–4) | 0.221 |
| Initial evaluation CA, mm | 15.6 (12.1–20.0) | 13.5 (12.1–19.6) | 0.001 |
| Second evaluation CA, mm | 14.9 (12.1–21.7) | 11.0 (6.5–11.9) | <0.001 |
| CA-improvement value, mm | −0.2 (−4.4–4.8) | −2.8 (−7.9–−1.1) | <0.001 |
| Initial evaluation CVAI, % | 10.7 (8.0–14.4) | 9.4 (7.9–13.2) | 0.010 |
| Second evaluation CVAI, % | 9.9 (7.8–14.7) | 7.6 (4.3–8.5) | <0.001 |
| CVAI-mprovement value, % | −0.5 (−3.1–2.3) | −2.1 (−5.6–−0.9) | <0.001 |
| Initial head circumference, mm | 418.2 (351.3–472.3) | 410.1 (380.3–460.7) | 0.222 |
| Second head circumference, mm | 441.6 (393.4–496.7) | 432.6 (406.4–468.2) | 0.253 |
| Growth of head circumference, mm | 16.9 (6.5–42.1) | 17.7 (7.5–36.2) | 0.876 |
| Delivery mode | |||
| Vaginal delivery | 22 (60) | 11 (58) | 0.746 |
| Cesarean section | 11 (30) | 7 (37) | |
| Vacuum delivery | 2 (5) | 1 (5) | |
| Forceps delivery | 2 (5) | 0 (0) | |
| Fetal presentation | 0.696 | ||
| Cephalic presentation | 34 (92) | 18 (95) | |
| Breech presentation | 3 (8) | 1 (5) | |
| Nutrition | 0.067 | ||
| Breastfeeding | 4 (11) | 7 (37) | |
| Formula feeding | 6 (16) | 2 (10) | |
| Mixed feeding | 27 (73) | 10 (53) |
| Natural-Course Group n = 24 | CHT Group n = 33 | p-Value | |
|---|---|---|---|
| Birth weight, g | 3148 (1886–4144) | 3130 (2512–3642) | 0.651 |
| Birth order (first born) | 16 (67) | 18 (55) | 0.357 |
| Sex (male) | 20 (83) | 19 (57) | 0.039 |
| Gestational age at birth, weeks | 40 (34–41) | 39 (37–41) | 0.194 |
| Initial evaluation age, months | 4 (4–8) | 4 (4–8) | 0.874 |
| Second evaluation age, months | 6.5 (5–14) | 7 (6–10) | 0.822 |
| Evaluation interval, months | 2 (1–7) | 2 (1–4) | 0.564 |
| Delivery mode | 0.804 | ||
| Vaginal delivery | 17 (71) | 20 (60) | |
| Cesarean section | 5 (21) | 9 (27) | |
| Vacuum delivery | 1 (4) | 3 (10) | |
| Forceps delivery | 1 (4) | 1 (3) | |
| Fetal presentation | 0.316 | ||
| Cephalic presentation | 24 (100) | 30 (91) | |
| Breech presentation | 0 (0) | 2 (6) | |
| Transverse presentation | 0 (0) | 1 (3) | |
| Nutrition | 0.253 | ||
| Breast feeding | 6 (25) | 12 (36) | |
| Formula feeding | 7 (29) | 4 (12) | |
| Mixed feeding | 11 (46) | 17 (52) |
| Natural-Course Group n = 24 | CHT Group n = 33 | p-Value | |
|---|---|---|---|
| Initial evaluation CA, mm | 14.6 (12.1–20.0) | 15.0 (12.2–22.4) | 0.348 |
| Second evaluation CA, mm | 14.1 (6.5–20.4) | 11.6 (4.1–19) | 0.011 |
| CA-improvement value, mm | −1.6 (−5.6–2.2) | −4.6 (−10.5–0.2) | <0.001 |
| Initial evaluation CVAI, % | 10.0 (8.0–13.2) | 10.3 (8.1–14.9) | 0.316 |
| Second evaluation CVAI, % | 9.0 (4.3–12.5) | 7.8 (2.8–12.2) | 0.023 |
| CVAI-improvement value, % | −1.43 (−3.9–1.2) | −3.3 (−7.1– −0.3) | <0.001 |
| Initial head circumference, mm | 440.9 (395.0–466.2) | 432.6 (411.2–456.7) | 0.365 |
| Second head circumference, mm | 454.1 (412.2–496.7) | 446.2 (425.7–469.7) | 0.157 |
| Growth of head circumference, mm | 15.0 (6.5–33.0) | 12.9 (1.4–24.2) | 0.121 |
| Odds Ratio (95% Confidence Interval) | p-Value | |
|---|---|---|
| Sex (female/male) | 3.52 (0.80–18.6) | 0.0981 |
| CA-improvement value | 0.45 (0.28–0.65) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noto, T.; Nagano, N.; Kato, R.; Hashimoto, S.; Saito, K.; Miyabayashi, H.; Sasano, M.; Sumi, K.; Yoshino, A.; Morioka, I. Natural-Course Evaluation of Infants with Positional Severe Plagiocephaly Using a Three-Dimensional Scanner in Japan: Comparison with Those Who Received Cranial Helmet Therapy. J. Clin. Med. 2021, 10, 3531. https://doi.org/10.3390/jcm10163531
Noto T, Nagano N, Kato R, Hashimoto S, Saito K, Miyabayashi H, Sasano M, Sumi K, Yoshino A, Morioka I. Natural-Course Evaluation of Infants with Positional Severe Plagiocephaly Using a Three-Dimensional Scanner in Japan: Comparison with Those Who Received Cranial Helmet Therapy. Journal of Clinical Medicine. 2021; 10(16):3531. https://doi.org/10.3390/jcm10163531
Chicago/Turabian StyleNoto, Takanori, Nobuhiko Nagano, Risa Kato, Shin Hashimoto, Katsuya Saito, Hiroshi Miyabayashi, Mari Sasano, Koichiro Sumi, Atsuo Yoshino, and Ichiro Morioka. 2021. "Natural-Course Evaluation of Infants with Positional Severe Plagiocephaly Using a Three-Dimensional Scanner in Japan: Comparison with Those Who Received Cranial Helmet Therapy" Journal of Clinical Medicine 10, no. 16: 3531. https://doi.org/10.3390/jcm10163531
APA StyleNoto, T., Nagano, N., Kato, R., Hashimoto, S., Saito, K., Miyabayashi, H., Sasano, M., Sumi, K., Yoshino, A., & Morioka, I. (2021). Natural-Course Evaluation of Infants with Positional Severe Plagiocephaly Using a Three-Dimensional Scanner in Japan: Comparison with Those Who Received Cranial Helmet Therapy. Journal of Clinical Medicine, 10(16), 3531. https://doi.org/10.3390/jcm10163531






