Apolipoprotein and LRP1-Based Peptides as New Therapeutic Tools in Atherosclerosis
Abstract
:1. Introduction
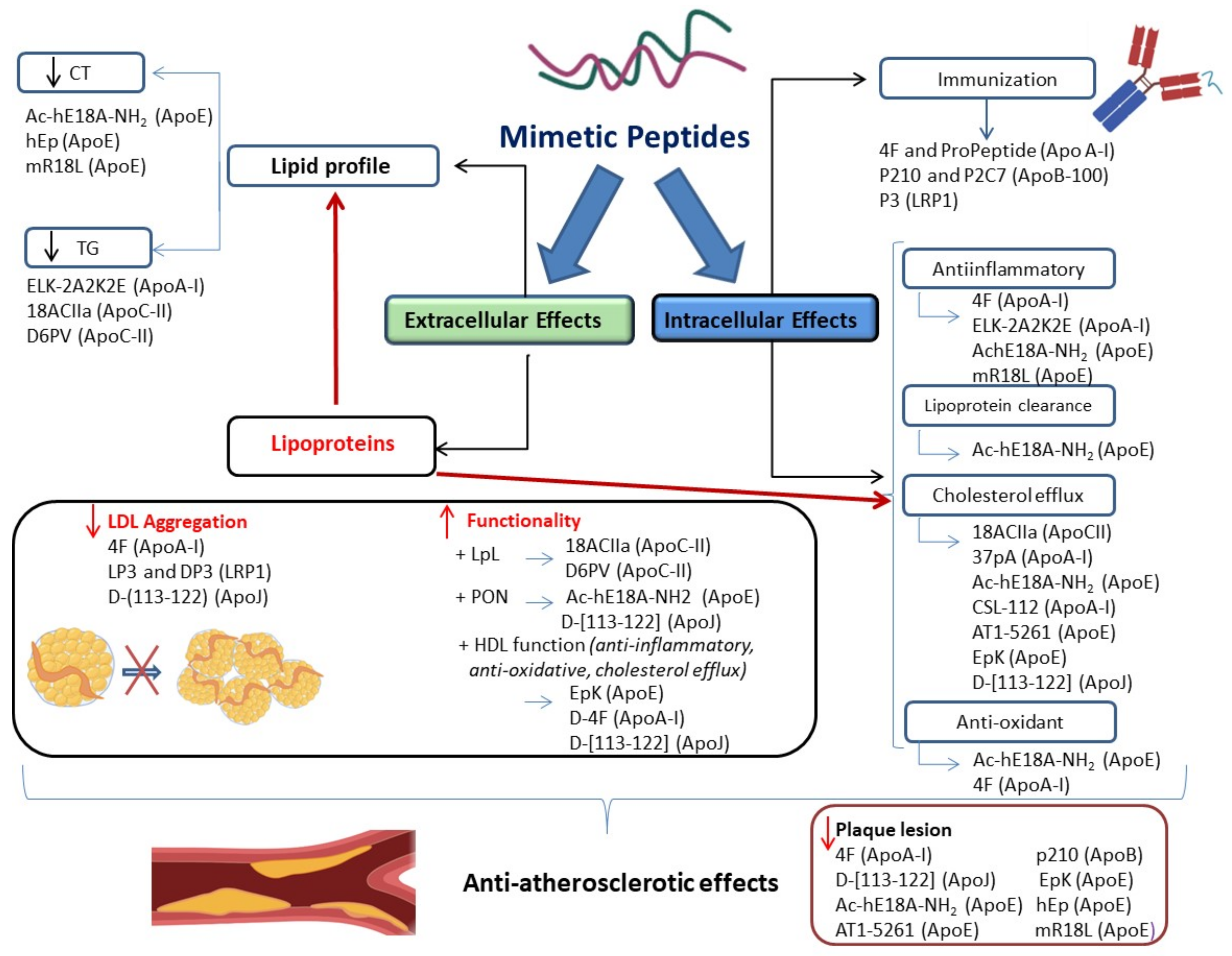
2. Apolipoprotein and LRP1-Based Peptides. Structure and Protective Effects against LDL Modification
2.1. ApoA-I-Based Peptides
2.2. ApoC-II-Based Peptides
2.3. ApoE-Based Peptides
2.4. ApoJ-Based Peptides
2.5. LRP1-Based Peptides
3. Impact of Peptides on Atherosclerosis
3.1. ApoA-I-Based Peptides
3.2. ApoC-II-Based Peptides
3.3. ApoE-Based Peptides
3.4. ApoJ-Based Peptides
3.5. LRP1-Based Peptides
4. Peptide-Based Immunization against Atherosclerosis
4.1. ApoA-I-Based Peptides
4.2. ApoB-100-Based Peptides
4.3. LRP1-Based Peptides
5. Peptides in Clinical Phases
5.1. ApoA-I-Based Peptides
5.2. ApoE-Based Peptides
6. Opportunities and Risk of Peptide-Based Treatments
7. Conclusions and Further Directions
8. Review Methodology, Search Strategy and Selection Criteria
Funding
Conflicts of Interest
Abbreviations
| AA | amino acid |
| ABCA1 ATP | binding cassette subfamily A member 1 |
| ACS | acute coronary syndrome |
| agLDL | aggregated low-density lipoprotein |
| Apo | apoprotein |
| CGRP | calcitonin gene-related peptide |
| FA | fatty acid |
| GLP-1 | glucagon-like peptide-1 |
| hcVSMC | human coronary vascular smooth muscle cells |
| HDL | high-density lipoprotein |
| HII HDL | inflammation index |
| IDL | intermediate density lipoprotein |
| IL-1β | Interleukin 1 β |
| IRAK-1 | interleukin-1 receptor associated kinase-1 |
| LDL | low density lipoproteins |
| LDL(-) | electronegative low-density lipoprotein |
| LDLR | low density lipoprotein receptor |
| LPL | lipoprotein lipase |
| LPS | lipopolysaccharides |
| LRP1 | low density lipoprotein receptor-related protein 1 |
| LXR | liver X receptor |
| MTP1 | microsomal triglyceride transfer protein |
| PCI | percutaneous coronary intervention |
| PCSK9 | proprotein convertase subtilisin/kexin type 9 |
| PLA2 | phospholipase A2 |
| PON | paraoxonase |
| QCA | quantitative coronary angiography |
| RCT | reverse cholesterol transport |
| SMase | sphingomyelinase |
| TG | triglyceride |
| TMR | transparency market research |
| TNF-α | tumor necrosis factor α |
| VLDL | very-low-density lipoprotein |
References
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and challenges in translating the biology of atherosclerosis. Nature 2011, 473, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Borén, J.; Williams, K.J. The central role of arterial retention of cholesterol-rich apolipoprotein-B-containing lipoproteins in the pathogenesis of atherosclerosis: A triumph of simplicity. Curr. Opin. Lipidol. 2016, 27, 473–483. [Google Scholar] [CrossRef]
- Orekhov, A.N. LDL and foam cell formation as the basis of atherogenesis. Curr. Opin. Lipidol. 2018, 29, 279–284. [Google Scholar] [CrossRef]
- Mahmood, S.S.; Levy, D.; Vasan, R.S.; Wang, T.J. The Framingham heart study and the epidemiology of cardiovascular disease: A historical perspective. Lancet 2014, 383, 999–1008. [Google Scholar] [CrossRef] [Green Version]
- Aiman, U.; Najmi, A.; Khan, R.A. Statin induced diabetes and its clinical implications. J. Pharmacol. Pharmacother. 2014, 5, 181–185. [Google Scholar] [CrossRef] [Green Version]
- Barylski, M.; Nikolic, D.; Banach, M.; Toth, P.; Montalto, G.; Rizzo, M. Statins and new-onset diabetes. Curr. Pharm. Des. 2014, 20, 3657–3664. [Google Scholar] [CrossRef]
- Ray, K.K.; Seshasai, S.R.K.; Erqou, S.; Sever, P.; Jukema, J.W.; Ford, I.; Sattar, N. Statins and all-cause mortality in high-risk primary prevention: A meta-analysis of 11 randomized controlled trials involving 65,229 participants. Arch. Intern. Med. 2010, 170, 1024–1031. [Google Scholar] [CrossRef] [Green Version]
- Ascaso, J.F.; Civeira, F.; Guijarro, C.; López Miranda, J.; Masana, L.; Mostaza, J.M.; Pedro-Botet, J.; Pintó, X.; Valdivielso, P. Indications of PCSK9 inhibitors in clinical practice. Recommendations of the Spanish Sociey of Arteriosclerosis (SEA), 2019. Clin. Investig. Arterioscler. 2019, 31, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, K.N.; Breslow, J.L. Antibodies to PCSK9: A superior way to lower LDL cholesterol? Circ. Res. 2012, 111, 274–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, J.C.; Boerwinkle, E.; Mosley, T.H.J.; Hobbs, H.H. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N. Engl. J. Med. 2006, 354, 1264–1272. [Google Scholar] [CrossRef]
- Amput, P.; McSweeney, C.; Palee, S.; Phrommintikul, A.; Chattipakorn, S.C.; Chattipakorn, N. The effects of proprotein convertase subtilisin/kexin type 9 inhibitors on lipid metabolism and cardiovascular function. Biomed. Pharmacother. 2019, 109, 1171–1180. [Google Scholar] [CrossRef]
- Recio, C.; Maione, F.; Iqbal, A.J.; Mascolo, N.; De Feo, V. The potential therapeutic application of peptides and peptidomimetics in cardiovascular disease. Front. Pharmacol. 2017, 7, 526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riggs, A.D. Making, cloning, and the expression of human insulin genes in bacteria: The path to humulin. Endocr. Rev. 2021, 42, 374–380. [Google Scholar] [CrossRef]
- Puttagunta, A.L.; Toth, E.L. Insulin lispro (Humalog), the first marketed insulin analogue: Indications, contraindications and need for further study. Can. Med. Assoc. J. 1998, 158, 506–511. [Google Scholar]
- Russell, F.A.; King, R.; Smillie, S.J.; Kodji, X.; Brain, S.D. Calcitonin gene-related peptide: Physiology and pathophysiology. Physiol. Rev. 2014, 94, 1099–1142. [Google Scholar] [CrossRef] [Green Version]
- Cardiovascular Drugs Market Size, Share & Trend|Industry Analysis Report 2017–2025. Available online: https://www.transparencymarketresearch.com/cardiovascular-drugs-market.html (accessed on 22 March 2021).
- Osei-Hwedieh, D.O.; Amar, M.; Sviridov, D.; Remaley, A.T. Apolipoprotein mimetic peptides: Mechanisms of action as anti-atherogenic agents. Pharmacol. Ther. 2011, 130, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Salnikov, E.S.; Aisenbrey, C.; Anantharamaiah, G.M.; Bechinger, B. Solid-state NMR structural investigations of peptide-based nanodiscs and of transmembrane helices in bicellar arrangements. Chem. Phys. Lipids 2019, 219, 58–71. [Google Scholar] [CrossRef]
- Remaley, A.T.; Thomas, F.; Stonik, J.A.; Demosky, S.J.; Bark, S.E.; Neufeld, E.B.; Bocharov, A.V.; Vishnyakova, T.G.; Patterson, A.P.; Eggerman, T.L.; et al. Synthetic amphipathic helical peptides promote lipid efflux from cells by an ABCA1-dependent and an ABCA1-independent pathway. J. Lipid Res. 2003, 44, 828–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, C.R.; Datta, G.; Wilson, L.; Palgunachari, M.N.; Anantharamaiah, G.M. The apoA-I mimetic peptide 4F protects apolipoprotein A-I from oxidative damage. Chem. Phys. Lipids 2019, 219, 28–35. [Google Scholar] [CrossRef]
- Ditiatkovski, M.; D’Souza, W.; Kesani, R.; Chin-Dusting, J.; de Haan, J.B.; Remaley, A.; Sviridov, D. An apolipoprotein A-I mimetic peptide designed with a reductionist approach stimulates reverse cholesterol transport and reduces atherosclerosis in mice. PLoS ONE 2013, 8, e68802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amar, M.J.A.; Sakurai, T.; Sakurai-Ikuta, A.; Sviridov, D.; Freeman, L.; Ahsan, L.; Remaley, A.T. A novel apolipoprotein C-II mimetic peptide that activates lipoprotein lipase and decreases serum triglycerides in apolipoprotein E-knockout mice. J. Pharmacol. Exp. Ther. 2015, 352, 227–235. [Google Scholar] [CrossRef] [Green Version]
- Wolska, A.; Lo, L.; Sviridov, D.O.; Pourmousa, M.; Pryor, M.; Ghosh, S.S.; Kakkar, R.; Davidson, M.; Wilson, S.; Pastor, R.W.; et al. A dual apolipoprotein C-II mimetic-apolipoprotein C-III antagonist peptide lowers plasma triglycerides. Sci. Transl. Med. 2020, 12, eaaw7905. [Google Scholar] [CrossRef]
- Xie, Q.; Li, F.; Zhao, S.-P. Ac-hE-18A-NH2, a novel dual-domain apolipoprotein mimetic peptide, inhibits apoptosis in macrophages by promoting cholesterol efflux. Mol. Med. Rep. 2014, 9, 1851–1856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bielicki, J.K.; Zhang, H.; Cortez, Y.; Zheng, Y.; Narayanaswami, V.; Patel, A.; Johansson, J.; Azhar, S. A new HDL mimetic peptide that stimulates cellular cholesterol efflux with high efficiency greatly reduces atherosclerosis in mice. J. Lipid Res. 2010, 51, 1496–1503. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Du, F.; Zhang, M.; Sun, S.; Yu, H.; Fan, D. A new recombinant human apolipoprotein E mimetic peptide with high-density lipoprotein binding and function enhancing activity. Exp. Biol. Med. 2011, 236, 1468–1476. [Google Scholar] [CrossRef]
- Liu, S.; McCormick, K.D.; Zhao, W.; Zhao, T.; Fan, D.; Wang, T. Human apolipoprotein E peptides inhibit hepatitis C virus entry by blocking virus binding. Hepatology 2012, 56, 484–491. [Google Scholar] [CrossRef] [Green Version]
- Handattu, S.P.; Nayyar, G.; Garber, D.W.; Palgunachari, M.N.; Monroe, C.E.; Keenum, T.D.; Mishra, V.K.; Datta, G.; Anantharamaiah, G.M. Two apolipoprotein E mimetic peptides with similar cholesterol reducing properties exhibit differential atheroprotective effects in LDL-R null mice. Atherosclerosis 2013, 227, 58–64. [Google Scholar] [CrossRef] [Green Version]
- Nikoulin, I.R.; Curtiss, L.K. An apolipoprotein E synthetic peptide targets to lipoproteins in plasma and mediates both cellular lipoprotein interactions in vitro and acute clearance of cholesterol-rich lipoproteins in vivo. J. Clin. Investig. 1998, 101, 223–234. [Google Scholar] [CrossRef] [Green Version]
- Hossain, A.; Tauhid, L.; Davenport, I.; Huckaba, T.; Graves, R.; Mandal, T.; Muniruzzaman, S.; Ahmed, S.A.; Bhattacharjee, P.S. LRP-1 Pathway Targeted Inhibition of Vascular Abnormalities in the Retina of Diabetic Mice. Curr. Eye Res. 2017, 42, 640–647. [Google Scholar] [CrossRef] [Green Version]
- Navab, M.; Anantharamaiah, G.M.; Reddy, S.T.; Van Lenten, B.J.; Wagner, A.C.; Hama, S.; Hough, G.; Bachini, E.; Garber, D.W.; Mishra, V.K.; et al. An oral apoJ peptide renders HDL antiinflammatory in mice and monkeys and dramatically reduces atherosclerosis in apolipoprotein E-null mice. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1932–1937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benitez-Amaro, A.; Pallara, C.; Nasarre, L.; Rivas-Urbina, A.; Benitez, S.; Vea, A.; Bornachea, O.; de Gonzalo-Calvo, D.; Serra-Mir, G.; Villegas, S.; et al. Molecular basis for the protective effects of low-density lipoprotein receptor-related protein 1 (LRP1)-derived peptides against LDL aggregation. Biochim. Biophys. Acta BBA Biomembr. 2019, 1861, 1302–1316. [Google Scholar] [CrossRef]
- Gordon, S.M.; Davidson, W.S. Apolipoprotein A-I mimetics and high-density lipoprotein function. Curr. Opin. Endocrinol. Diabetes Obes. 2012, 19, 109–114. [Google Scholar] [CrossRef]
- Liu, H.; Scraba, D.G.; Ryan, R.O. Prevention of phospholipase-C induced aggregation of low density lipoprotein by amphipathic apolipoproteins. FEBS Lett. 1993, 316, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Khoo, J.C.; Miller, E.; McLoughlin, P.; Steinberg, D. Prevention of low density lipoprotein aggregation by high density lipoprotein or apolipoprotein A-I. J. Lipid Res. 1990, 31, 645–652. [Google Scholar] [CrossRef]
- Nguyen, S.D.; Javanainen, M.; Rissanen, S.; Zhao, H.; Huusko, J.; Kivelä, A.M.; Ylä-Herttuala, S.; Navab, M.; Fogelman, A.M.; Vattulainen, I.; et al. Apolipoprotein A-I mimetic peptide 4F blocks sphingomyelinase-induced LDL aggregation. J. Lipid Res. 2015, 56, 1206–1221. [Google Scholar] [CrossRef] [Green Version]
- Sneck, M.; Nguyen, S.D.; Pihlajamaa, T.; Yohannes, G.; Riekkola, M.-L.; Milne, R.; Kovanen, P.T.; Öörni, K. Conformational changes of apoB-100 in SMase-modified LDL mediate formation of large aggregates at acidic pH. J. Lipid Res. 2012, 53, 1832–1839. [Google Scholar] [CrossRef] [Green Version]
- Kolodgie, F.D.; Burke, A.P.; Skorija, K.S.; Ladich, E.; Kutys, R.; Makuria, A.T.; Virmani, R. Lipoprotein-associated phospholipase A2 protein expression in the natural progression of human coronary atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2523–2529. [Google Scholar] [CrossRef] [Green Version]
- Hovingh, G.K.; Bochem, A.E.; Kastelein, J.J.P. Apolipoprotein A-I mimetic peptides. Curr. Opin. Lipidol. 2010, 21, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Björkegren, J.; Karpe, F.; Milne, R.W.; Hamsten, A. Differences in apolipoprotein and lipid composition between human chylomicron remnants and very low density lipoproteins isolated from fasting and postprandial plasma. J. Lipid Res. 1998, 39, 1412–1420. [Google Scholar] [CrossRef]
- Zdunek, J.; Martinez, G.V.; Schleucher, J.; Lycksell, P.O.; Yin, Y.; Nilsson, S.; Shen, Y.; Olivecrona, G.; Wijmenga, S. Global structure and dynamics of human apolipoprotein CII in complex with micelles: Evidence for increased mobility of the helix involved in the activation of lipoprotein lipase. Biochemistry 2003, 42, 1872–1889. [Google Scholar] [CrossRef]
- Reimund, M.; Wolska, A.; Risti, R.; Wilson, S.; Sviridov, D.; Remaley, A.T.; Lookene, A. Apolipoprotein C-II mimetic peptide is an efficient activator of lipoprotein lipase in human plasma as studied by a calorimetric approach. Biochem. Biophys. Res. Commun. 2019, 519, 67–72. [Google Scholar] [CrossRef]
- Fisher, C.A.; Ryan, R.O. Lipid binding-induced conformational changes in the N-terminal domain of human apolipoprotein E. J. Lipid Res. 1999, 40, 93–99. [Google Scholar] [CrossRef]
- Weisgraber, K.H. Apolipoprotein E distribution among human plasma lipoproteins: Role of the cysteine-arginine interchange at residue 112. J. Lipid Res. 1990, 31, 1503–1511. [Google Scholar] [CrossRef]
- Nguyen, D.; Dhanasekaran, P.; Nickel, M.; Nakatani, R.; Saito, H.; Phillips, M.C.; Lund-Katz, S. Molecular basis for the differences in lipid and lipoprotein binding properties of human apolipoproteins E3 and E4. Biochemistry 2010, 49, 10881–10889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasmussen, K.L. Plasma levels of apolipoprotein E, APOE genotype and risk of dementia and ischemic heart disease: A review. Atherosclerosis 2016, 255, 145–155. [Google Scholar] [CrossRef] [Green Version]
- Yamazaki, Y.; Zhao, N.; Caulfield, T.R.; Liu, C.-C.; Bu, G. Apolipoprotein E and Alzheimer disease: Pathobiology and targeting strategies. Nat. Rev. Neurol. 2019, 15, 501–518. [Google Scholar] [CrossRef] [PubMed]
- Rull, A.; Martínez-Bujidos, M.; Pérez-Cuellar, M.; Pérez, A.; Ordóñez-Llanos, J.; Sánchez-Quesada, J.L. Increased concentration of clusterin/apolipoprotein J (apoJ) in hyperlipemic serum is paradoxically associated with decreased apoJ content in lipoproteins. Atherosclerosis 2015, 241, 463–470. [Google Scholar] [CrossRef] [PubMed]
- De Silva, H.V.; Stuart, W.D.; Duvic, C.R.; Wetterau, J.R.; Ray, M.J.; Ferguson, D.G.; Albers, H.W.; Smith, W.R.; Harmony, J.A. A 70-kDa apolipoprotein designated ApoJ is a marker for subclasses of human plasma high density lipoproteins. J. Biol. Chem. 1990, 265, 13240–13247. [Google Scholar] [CrossRef]
- Bancells, C.; Canals, F.; Benítez, S.; Colomé, N.; Julve, J.; Ordóñez-Llanos, J.; Sánchez-Quesada, J.L. Proteomic analysis of electronegative low-density lipoprotein. J. Lipid Res. 2010, 51, 3508–3515. [Google Scholar] [CrossRef] [Green Version]
- Estruch, M.; Sánchez-Quesada, J.L.; Ordóñez Llanos, J.; Benítez, S. Electronegative LDL: A circulating modified LDL with a role in inflammation. Mediat. Inflamm. 2013, 2013, 181324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Bujidos, M.; Rull, A.; González-Cura, B.; Pérez-Cuéllar, M.; Montoliu-Gaya, L.; Villegas, S.; Ordoñez-Llaños, J.; Sánchez-Quesada, J.L. Clusterin/apolipoprotein J binds to aggregated LDL in human plasma and plays a protective role against LDL aggregation. FASEB J. 2015, 29, 1688–1700. [Google Scholar] [CrossRef]
- Rivas-Urbina, A.; Rull, A.; Montoliu-Gaya, L.; Pérez-Cuellar, M.; Ordóñez-Llanos, J.; Villegas, S.; Sánchez-Quesada, J.L. Low-density lipoprotein aggregation is inhibited by apolipoprotein J-derived mimetic peptide D-[113–122] apoJ. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2020, 1865, 158541. [Google Scholar] [CrossRef]
- Llorente-Cortés, V.; Martínez-González, J.; Badimon, L. LDL receptor-related protein mediates uptake of aggregated LDL in human vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1572–1579. [Google Scholar] [CrossRef] [Green Version]
- Llorente-Cortés, V.; Otero-Viñas, M.; Sánchez, S.; Rodríguez, C.; Badimon, L. Low-Density Lipoprotein Upregulates Low-Density Lipoprotein Receptor-Related Protein Expression in Vascular Smooth Muscle Cells. Circulation 2002, 106, 3104. [Google Scholar] [CrossRef]
- Costales, P.; Aledo, R.; Vérnia, S.; Das, A.; Shah, V.H.; Casado, M.; Badimon, L.; Llorente-Cortés, V. Selective role of sterol regulatory element binding protein isoforms in aggregated LDL-induced vascular low density lipoprotein receptor-related protein-1 expression. Atherosclerosis 2010, 213, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Llorente-Cortés, V.; Costales, P.; Bernués, J.; Camino-Lopez, S.; Badimon, L. Sterol Regulatory Element-binding Protein-2 Negatively Regulates Low Density Lipoprotein Receptor-related Protein Transcription. J. Mol. Biol. 2006, 359, 950–960. [Google Scholar] [CrossRef] [PubMed]
- Costales, P.; Fuentes-Prior, P.; Castellano, J.; Revuelta-Lopez, E.; Corral-Rodríguez, M.Á.; Nasarre, L.; Badimon, L.; Llorente-Cortes, V. K domain CR9 of low density lipoprotein (LDL) receptor-related protein 1 (LRP1) is critical for aggregated LDL-induced foam cell formation from human vascular smooth muscle cells. J. Biol. Chem. 2015, 290, 14852–14865. [Google Scholar] [CrossRef] [Green Version]
- Benitez-Amaro, A.; Pallara, C.; Nasarre, L.; Ferreira, R.; de Gonzalo-Calvo, D.; Prades, R.; Tarragó, T.; Llorente-Cortés, V. Development of Innovative Antiatherosclerotic Peptides through the Combination of Molecular Modeling and a Dual (Biochemical-Cellular) Screening System. Adv. Ther. 2020, 3, 2000037. [Google Scholar] [CrossRef]
- Navab, M.; Anantharamaiah, G.M.; Reddy, S.T.; Hama, S.; Hough, G.; Grijalva, V.R.; Wagner, A.C.; Frank, J.S.; Datta, G.; Garber, D.; et al. Oral D-4F causes formation of pre-beta high-density lipoprotein and improves high-density lipoprotein-mediated cholesterol efflux and reverse cholesterol transport from macrophages in apolipoprotein E-null mice. Circulation 2004, 109, 3215–3220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Souza, W.; Stonik, J.A.; Murphy, A.; Demosky, S.J.; Sethi, A.A.; Moore, X.L.; Chin-Dusting, J.; Remaley, A.T.; Sviridov, D. Structure/function relationships of apolipoprotein a-I mimetic peptides: Implications for antiatherogenic activities of high-density lipoprotein. Circ. Res. 2010, 107, 217–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.-L.; Gong, D.; Hu, X.-Y.; Wu, S.; Zheng, X.-L.; Wu, J.; Tang, X.-E.; Zhang, D.-W.; Tang, C.-K. ApoA-1 Mimetic Peptide ELK-2A2K2E Decreases Inflammatory Factor Levels Through the ABCA1-JAK2-STAT3-TTP Axis in THP-1-Derived Macrophages. J. Cardiovasc. Pharmacol. 2018, 72, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Dunbar, R.L.; Wolska, A.; Sikora, T.U.; Escobar, M.D.R.; Seliktar, N.; deGoma, E.; DerOhannessian, S.; Morrell, L.; McIntyre, A.D.; et al. A Novel APOC2 Missense Mutation Causing Apolipoprotein C-II Deficiency with Severe Triglyceridemia and Pancreatitis. J. Clin. Endocrinol. Metab. 2017, 102, 1454–1457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Gates, K.P.; Fang, L.; Amar, M.J.; Schneider, D.A.; Geng, H.; Huang, W.; Kim, J.; Pattison, J.; Zhang, J.; et al. Apoc2 loss-of-function zebrafish mutant as a genetic model of hyperlipidemia. Dis. Model. Mech. 2015, 8, 989–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakurai, T.; Sakurai, A.; Vaisman, B.L.; Amar, M.J.; Liu, C.; Gordon, S.M.; Drake, S.K.; Pryor, M.; Sampson, M.L.; Yang, L.; et al. Creation of Apolipoprotein C-II (ApoC-II) Mutant Mice and Correction of Their Hypertriglyceridemia with an ApoC-II Mimetic Peptide. J. Pharmacol. Exp. Ther. 2016, 356, 341–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komatsu, T.; Sakurai, T.; Wolska, A.; Amar, M.J.; Sakurai, A.; Vaisman, B.L.; Sviridov, D.; Demosky, S.; Pryor, M.; Ikewaki, K.; et al. Apolipoprotein C-II Mimetic Peptide Promotes the Plasma Clearance of Triglyceride-Rich Lipid Emulsion and the Incorporation of Fatty Acids into Peripheral Tissues of Mice. J. Nutr. Metab. 2019, 2019, 7078241. [Google Scholar] [CrossRef] [PubMed]
- Linton, M.F.; Yancey, P.G.; Davies, S.S.; Jerome, W.G.; Linton, E.F.; Song, W.L.; Doran, A.C.; Vickers, K.C. The Role of Lipids and Lipoproteins in Atherosclerosis. In Endotext (Internet); NBK393489; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Grossman, A., Hershman, J.M., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Yu, H.; Zhang, W.; Yancey, P.G.; Koury, M.J.; Zhang, Y.; Fazio, S.; Linton, M.F. Macrophage apolipoprotein E reduces atherosclerosis and prevents premature death in apolipoprotein E and scavenger receptor-class BI double-knockout mice. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 150–156. [Google Scholar] [CrossRef] [Green Version]
- Nayyar, G.; Garber, D.W.; Palgunachari, M.N.; Monroe, C.E.; Keenum, T.D.; Handattu, S.P.; Mishra, V.K.; Anantharamaiah, G.M. Apolipoprotein E mimetic is more effective than apolipoprotein A-I mimetic in reducing lesion formation in older female apo E null mice. Atherosclerosis 2012, 224, 326–331. [Google Scholar] [CrossRef] [Green Version]
- Valanti, E.K.; Chroni, A.; Sanoudou, D. The future of apolipoprotein E mimetic peptides in the prevention of cardiovascular disease. Curr. Opin. Lipidol. 2019, 30, 326–341. [Google Scholar] [CrossRef]
- Gupta, H.; White, C.R.; Handattu, S.; Garber, D.W.; Datta, G.; Chaddha, M.; Dai, L.; Gianturco, S.H.; Bradley, W.A.; Anantharamaiah, G.M. Apolipoprotein E mimetic Peptide dramatically lowers plasma cholesterol and restores endothelial function in watanabe heritable hyperlipidemic rabbits. Circulation 2005, 111, 3112–3118. [Google Scholar] [CrossRef] [Green Version]
- Handattu, S.P.; Datta, G.; Epand, R.M.; Epand, R.F.; Palgunachari, M.N.; Mishra, V.K.; Monroe, C.E.; Keenum, T.D.; Chaddha, M.; Anantharamaiah, G.M.; et al. Oral administration of L-mR18L, a single domain cationic amphipathic helical peptide, inhibits lesion formation in ApoE null mice. J. Lipid Res. 2010, 51, 3491–3499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharifov, O.F.; Nayyar, G.; Ternovoy, V.V.; Mishra, V.K.; Litovsky, S.H.; Palgunachari, M.N.; Garber, D.W.; Anantharamaiah, G.M.; Gupta, H. Cationic peptide mR18L with lipid lowering properties inhibits LPS-induced systemic and liver inflammation in rats. Biochem. Biophys. Res. Commun. 2013, 436, 705–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, J.; Xu, Y.; Shang, L.; Liu, H.-M.; Du, F.; Yu, H. Effect of The Apolipoprotein E Mimetic Peptide EpK on Atherosclerosis in apoE (-/-) Mice. Prog. Biochem. Biophys. 2015, 42, 833–842. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, H.; Liu, M.; Li, F.; Liu, L.; Du, F.; Fan, D.; Yu, H. A human apolipoprotein E mimetic peptide reduces atherosclerosis in aged apolipoprotein E null mice. Am. J. Transl. Res. 2016, 8, 3482–3492. [Google Scholar] [PubMed]
- Yang, N.; Qin, Q. Apolipoprotein J: A New Predictor and Therapeutic Target in Cardiovascular Disease? Chin. Med. J. 2015, 128, 2530–2534. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Akasaka, Y.; Ishii, T.; Komiyama, K.; Masuda, S.; Asuwa, N.; Choi-Miura, N.H.; Tomita, M. Distribution and synthesis of apolipoprotein J in the atherosclerotic aorta. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 665–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navab, M.; Hama-Levy, S.; Van Lenten, B.J.; Fonarow, G.C.; Cardinez, C.J.; Castellani, L.W.; Brennan, M.L.; Lusis, A.J.; Fogelman, A.M.; La Du, B.N. Mildly oxidized LDL induces an increased apolipoprotein J/paraoxonase ratio. J. Clin. Investig. 1997, 99, 2005–2019. [Google Scholar] [CrossRef] [Green Version]
- Gelissen, I.C.; Hochgrebe, T.; Wilson, M.R.; Easterbrook-Smith, S.B.; Jessup, W.; Dean, R.T.; Brown, A.J. Apolipoprotein J (clusterin) induces cholesterol export from macrophage-foam cells: A potential anti-atherogenic function? Biochem. J. 1998, 331, 231–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, R.M.; Mekary, R.A.; da Cruz Rodrigues, K.C.; Anaruma, C.P.; Ropelle, E.R.; da Silva, A.S.R.; Cintra, D.E.; Pauli, J.R.; de Moura, L.P. Protective molecular mechanisms of clusterin against apoptosis in cardiomyocytes. Heart Fail. Rev. 2018, 23, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Urbina, A.; Rull, A.; Aldana-Ramos, J.; Santos, D.; Puig, N.; Farre-Cabrerizo, N.; Benitez, S.; Perez, A.; de Gonzalo-Calvo, D.; Escola-Gil, J.C.; et al. Subcutaneous Administration of Apolipoprotein J-Derived Mimetic Peptide d-[113–122] apoJ Improves LDL and HDL Function and Prevents Atherosclerosis in LDLR-KO Mice. Biomolecules 2020, 10, 829. [Google Scholar] [CrossRef]
- Allahverdian, S.; Chehroudi, A.C.; McManus, B.M.; Abraham, T.; Francis, G.A. Contribution of intimal smooth muscle cells to cholesterol accumulation and macrophage-like cells in human atherosclerosis. Circulation 2014, 129, 1551–1559. [Google Scholar] [CrossRef] [Green Version]
- Llorente-Cortés, V.; Royo, T.; Juan-Babot, O.; Badimon, L. Adipocyte differentiation-related protein is induced by LRP1-mediated aggregated LDL internalization in human vascular smooth muscle cells and macrophages. J. Lipid Res. 2007, 48, 2133–2140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruuth, M.; Nguyen, S.D.; Vihervaara, T.; Hilvo, M.; Laajala, T.D.; Kondadi, P.K.; Gisterå, A.; Lähteenmäki, H.; Kittilä, T.; Huusko, J.; et al. Susceptibility of low-density lipoprotein particles to aggregate depends on particle lipidome, is modifiable, and associates with future cardiovascular deaths. Eur. Heart J. 2018, 39, 2562–2573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Gonzalo-Calvo, D.; Elosua, R.; Vea, A.; Subirana, I.; Sayols-Baixeras, S.; Marrugat, J.; Llorente-Cortés, V. Soluble low-density lipoprotein receptor-related protein 1 as a biomarker of coronary risk: Predictive capacity and association with clinical events. Atherosclerosis 2019, 287, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Castellano, J.; Aledo, R.; Sendra, J.; Costales, P.; Juan-Babot, O.; Badimon, L.; Llorente-Cortés, V. Hypoxia stimulates low-density lipoprotein receptor-related protein-1 expression through hypoxia-inducible factor-1α in human vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1411–1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCarthy, J.J.; Parker, A.; Salem, R.; Moliterno, D.J.; Wang, Q.; Plow, E.F.; Rao, S.; Shen, G.; Rogers, W.J.; Newby, L.K.; et al. Large scale association analysis for identification of genes underlying premature coronary heart disease: Cumulative perspective from analysis of 111 candidate genes. J. Med. Genet. 2004, 41, 334–341. [Google Scholar] [CrossRef] [Green Version]
- Schulz, S.; Schagdarsurengin, U.; Greiser, P.; Birkenmeier, G.; Müller-Werdan, U.; Hagemann, M.; Riemann, D.; Werdan, K.; Gläser, C. The LDL receptor-related protein (LRP1/A2MR) and coronary atherosclerosis–novel genomic variants and functional consequences. Hum. Mutat. 2002, 20, 404. [Google Scholar] [CrossRef]
- Wool, G.D.; Cabana, V.G.; Lukens, J.; Shaw, P.X.; Binder, C.J.; Witztum, J.L.; Reardon, C.A.; Getz, G.S. 4F Peptide reduces nascent atherosclerosis and induces natural antibody production in apolipoprotein E-null mice. FASEB J. 2011, 25, 290–300. [Google Scholar] [CrossRef] [Green Version]
- Klingenberg, R.; Lebens, M.; Hermansson, A.; Fredrikson, G.N.; Strodthoff, D.; Rudling, M.; Ketelhuth, D.F.J.; Gerdes, N.; Holmgren, J.; Nilsson, J.; et al. Intranasal immunization with an apolipoprotein B-100 fusion protein induces antigen-specific regulatory T cells and reduces atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 946–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, Z.; Cao, B.; Guo, X.; Li, W.; Li, S.; Chen, J.; Zhou, W.; Zheng, C.; Wei, Y. Apolipoprotein B-100 peptide 210 antibody inhibits atherosclerosis by regulation of macrophages that phagocytize oxidized lipid. Am. J. Transl. Res. 2018, 10, 1817–1828. [Google Scholar]
- Chyu, K.-Y.; Zhao, X.; Dimayuga, P.C.; Zhou, J.; Li, X.; Yano, J.; Lio, W.M.; Chan, L.F.; Kirzner, J.; Trinidad, P.; et al. CD8+ T cells mediate the athero-protective effect of immunization with an ApoB-100 peptide. PLoS ONE 2012, 7, e30780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fredrikson, G.N.; Björkbacka, H.; Söderberg, I.; Ljungcrantz, I.; Nilsson, J. Treatment with apo B peptide vaccines inhibits atherosclerosis in human apo B-100 transgenic mice without inducing an increase in peptide-specific antibodies. J. Intern. Med. 2008, 264, 563–570. [Google Scholar] [CrossRef]
- Kazuma, S.M.; Cavalcante, M.F.; Telles, A.E.R.; Maranhão, A.Q.; Abdalla, D.S.P. Cloning and expression of an anti-LDL (-) single-chain variable fragment, and its inhibitory effect on experimental atherosclerosis. MAbs 2013, 5, 763–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bornachea, O.; Benitez-Amaro, A.; Vea, A.; Nasarre, L.; de Gonzalo-Calvo, D.; Escola-Gil, J.C.; Cedo, L.; Iborra, A.; Martínez-Martínez, L.; Juarez, C.; et al. Immunization with the Gly1127-Cys1140 amino acid sequence of the LRP1 receptor reduces atherosclerosis in rabbits. Molecular, immunohistochemical and nuclear imaging studies. Theranostics 2020, 10, 3263. [Google Scholar] [CrossRef]
- Nilsson, J.; Nordin Fredrikson, G.; Schiopu, A.; Shah, P.K.; Jansson, B.; Carlsson, R. Oxidized LDL antibodies in treatment and risk assessment of atherosclerosis and associated cardiovascular disease. Curr. Pharm. Des. 2007, 13, 1021–1030. [Google Scholar] [CrossRef]
- Caligiuri, G.; Khallou-Laschet, J.; Vandaele, M.; Gaston, A.-T.; Delignat, S.; Mandet, C.; Kohler, H.V.; Kaveri, S.V.; Nicoletti, A. Phosphorylcholine-targeting immunization reduces atherosclerosis. J. Am. Coll. Cardiol. 2007, 50, 540–546. [Google Scholar] [CrossRef] [Green Version]
- Palinski, W.; Miller, E.; Witztum, J.L. Immunization of low density lipoprotein (LDL) receptor-deficient rabbits with homologous malondialdehyde-modified LDL reduces atherogenesis. Proc. Natl. Acad. Sci. USA 1995, 92, 821–825. [Google Scholar] [CrossRef] [Green Version]
- Teixeira Damasceno, N.R.; Apolinário, E.; Dias Flauzino, F.; Fernandes, I.; Abdalla, D.S.P. Soy isoflavones reduce electronegative low-density lipoprotein (LDL (-)) and anti-LDL (-) autoantibodies in experimental atherosclerosis. Eur. J. Nutr. 2007, 46, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Damasceno, N.R.T.; Sevanian, A.; Apolinário, E.; Oliveira, J.M.A.; Fernandes, I.; Abdalla, D.S.P. Detection of electronegative low density lipoprotein (LDL-) in plasma and atherosclerotic lesions by monoclonal antibody-based immunoassays. Clin. Biochem. 2006, 39, 28–38. [Google Scholar] [CrossRef]
- Grosso, D.M.; Ferderbar, S.; Wanschel, A.C.B.A.; Krieger, M.H.; Higushi, M.L.; Abdalla, D.S.P. Antibodies against electronegative LDL inhibit atherosclerosis in LDLr-/- mice. Braz. J. Med. Biol. Res. 2008, 41, 1086–1092. [Google Scholar] [CrossRef] [Green Version]
- Faulin, T.d.E.S.; Kazuma, S.M.; Tripodi, G.L.; Cavalcante, M.F.; Wakasuqui, F.; Oliveira, C.L.P.; Degenhardt, M.F.d.S.; Michaloski, J.; Giordano, R.J.; Ketelhuth, D.F.J.; et al. Proinflammatory Action of a New Electronegative Low-Density Lipoprotein Epitope. Biomolecules 2019, 9, 386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawamura, A.; Baitsch, D.; Telgmann, R.; Feuerborn, R.; Weissen-Plenz, G.; Hagedorn, C.; Saku, K.; Brand-Herrmann, S.-M.; von Eckardstein, A.; Assmann, G.; et al. Apolipoprotein E interrupts interleukin-1beta signaling in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1610–1617. [Google Scholar] [CrossRef] [Green Version]
- El Asmar, Z.; Terrand, J.; Jenty, M.; Host, L.; Mlih, M.; Zerr, A.; Justiniano, H.; Matz, R.L.; Boudier, C.; Scholler, E.; et al. Convergent signaling pathways controlled by LRP1 (Receptor-related Protein 1) cytoplasmic and extracellular domains limit cellular cholesterol accumulation. J. Biol. Chem. 2016, 291, 5116–5127. [Google Scholar] [CrossRef] [Green Version]
- Potere, N.; Del Buono, M.G.; Mauro, A.G.; Abbate, A.; Toldo, S. Low Density Lipoprotein Receptor-Related Protein-1 in Cardiac Inflammation and Infarct Healing. Front. Cardiovasc. Med. 2019, 6, 51. [Google Scholar] [CrossRef]
- Gaultier, A.; Arandjelovic, S.; Li, X.; Janes, J.; Dragojlovic, N.; Zhou, G.P.; Dolkas, J.; Myers, R.R.; Gonias, S.L.; Campana, W.M. A shed form of LDL receptor-related protein-1 regulates peripheral nerve injury and neuropathic pain in rodents. J. Clin. Investig. 2008, 118, 161–172. [Google Scholar] [CrossRef] [Green Version]
- Toldo, S.; Austin, D.; Mauro, A.G.; Mezzaroma, E.; Van Tassell, B.W.; Marchetti, C.; Carbone, S.; Mogelsvang, S.; Gelber, C.; Abbate, A. Low-Density Lipoprotein Receptor–Related Protein-1 Is a Therapeutic Target in Acute Myocardial Infarction. JACC Basic Transl. Sci. 2017, 2, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.E.; Weissbach, N.; Kjems, L.; Ayalasomayajula, S.; Zhang, Y.; Chang, I.; Navab, M.; Hama, S.; Hough, G.; Reddy, S.T.; et al. Treatment of patients with cardiovascular disease with L-4F, an apo-A1 mimetic, did not improve select biomarkers of HDL function. J. Lipid Res. 2011, 52, 361–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bloedon, L.T.; Dunbar, R.; Duffy, D.; Pinell-Salles, P.; Norris, R.; DeGroot, B.J.; Movva, R.; Navab, M.; Fogelman, A.M.; Rader, D.J. Safety, pharmacokinetics, and pharmacodynamics of oral apoA-I mimetic peptide D-4F in high-risk cardiovascular patients. J. Lipid Res. 2008, 49, 1344–1352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of Fx-5A in Healthy Volunteers-Full Text View-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04216342?cond=Safety%2C+Tolerability%2C+Pharmacokinetics+and+Pharmacodynamics+of+Fx-5A+in+Healthy+Volunteers&draw=2&rank=1 (accessed on 22 March 2021).
- Nicholls, S.J.; Puri, R.; Ballantyne, C.M.; Jukema, J.W.; Kastelein, J.J.P.; Koenig, W.; Wright, R.S.; Kallend, D.; Wijngaard, P.; Borgman, M.; et al. Effect of Infusion of High-Density Lipoprotein Mimetic Containing Recombinant Apolipoprotein A-I Milano on Coronary Disease in Patients with an Acute Coronary Syndrome in the MILANO-PILOT Trial: A Randomized Clinical Trial. JAMA Cardiol. 2018, 3, 806–814. [Google Scholar] [CrossRef] [Green Version]
- Study to Investigate CSL112 in Subjects with Acute Coronary Syndrome-Full Text View-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03473223?cond=Study+to+Investigate+CSL112+in+Subjects+With+Acute+Coronary+Syndrome&draw=2&rank=1 (accessed on 22 March 2021).
- Tardif, J.-C.; Ballantyne, C.M.; Barter, P.; Dasseux, J.-L.; Fayad, Z.A.; Guertin, M.-C.; Kastelein, J.J.P.; Keyserling, C.; Klepp, H.; Koenig, W.; et al. Effects of the high-density lipoprotein mimetic agent CER-001 on coronary atherosclerosis in patients with acute coronary syndromes: A randomized trial. Eur. Heart J. 2014, 35, 3277–3286. [Google Scholar] [CrossRef] [PubMed]
- White, C.R.; Goldberg, D.I.; Anantharamaiah, G.M. Recent developments in modulating atherogenic lipoproteins. Curr. Opin. Lipidol. 2015, 26, 369–375. [Google Scholar] [CrossRef]
- Amar, M.J.A.; D’Souza, W.; Turner, S.; Demosky, S.; Sviridov, D.; Stonik, J.; Luchoomun, J.; Voogt, J.; Hellerstein, M.; Sviridov, D.; et al. 5A apolipoprotein mimetic peptide promotes cholesterol efflux and reduces atherosclerosis in mice. J. Pharmacol. Exp. Ther. 2010, 334, 634–641. [Google Scholar] [CrossRef] [Green Version]
- Nissen, S.E.; Tsunoda, T.; Tuzcu, E.M.; Schoenhagen, P.; Cooper, C.J.; Yasin, M.; Eaton, G.M.; Lauer, M.A.; Sheldon, W.S.; Grines, C.L.; et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: A randomized controlled trial. JAMA 2003, 290, 2292–2300. [Google Scholar] [CrossRef] [PubMed]
- Easton, R.; Gille, A.; D’Andrea, D.; Davis, R.; Wright, S.D.; Shear, C. A multiple ascending dose study of CSL112, an infused formulation of ApoA-I. J. Clin. Pharmacol. 2014, 54, 301–310. [Google Scholar] [CrossRef]
- Michael Gibson, C.; Korjian, S.; Tricoci, P.; Daaboul, Y.; Yee, M.; Jain, P.; Alexander, J.H.; Steg, P.G.; Lincoff, A.M.; Kastelein, J.J.P.; et al. Safety and Tolerability of CSL112, a Reconstituted, Infusible, Plasma-Derived Apolipoprotein A-I, After Acute Myocardial Infarction: The AEGIS-I Trial (ApoA-I Event Reducing in Ischemic Syndromes I). Circulation 2016, 134, 1918–1930. [Google Scholar] [CrossRef] [PubMed]
- Abstract 15525: CER-001, a Synthetic HDL-Mimetic, Safely Mobilizes Cholesterol in Healthy Dyslipidemic Volunteers|Circulation. Available online: https://www.ahajournals.org/doi/10.1161/circ.124.suppl_21.A15525 (accessed on 22 March 2021).
- Collins, J.L.; Fivush, A.M.; Watson, M.A.; Galardi, C.M.; Lewis, M.C.; Moore, L.B.; Parks, D.J.; Wilson, J.G.; Tippin, T.K.; Binz, J.G.; et al. Identification of a nonsteroidal liver X receptor agonist through parallel array synthesis of tertiary amines. J. Med. Chem. 2002, 45, 1963–1966. [Google Scholar] [CrossRef] [PubMed]
- Van der Hoorn, J.; Lindén, D.; Lindahl, U.; Bekkers, M.; Voskuilen, M.; Nilsson, R.; Oscarsson, J.; Lindstedt, E.; Princen, H. Low dose of the liver X receptor agonist, AZ876, reduces atherosclerosis in APOE*3Leiden mice without affecting liver or plasma triglyceride levels. Br. J. Pharmacol. 2011, 162, 1553–1563. [Google Scholar] [CrossRef] [Green Version]
- Blom, D.J.; Raal, F.J.; Santos, R.D.; Marais, A.D. Lomitapide and Mipomersen-Inhibiting Microsomal Triglyceride Transfer Protein (MTP) and apoB100 Synthesis. Curr. Atheroscler. Rep. 2019, 21, 48. [Google Scholar] [CrossRef]
- Ma, Z.; Deng, C.; Hu, W.; Zhou, J.; Fan, C.; Di, S.; Liu, D.; Yang, Y.; Wang, D. Liver X Receptors and their Agonists: Targeting for Cholesterol Homeostasis and Cardiovascular Diseases. Curr. Issues. Mol. Biol. 2017, 22, 41–64. [Google Scholar] [CrossRef] [Green Version]
- Brahm, A.J.; Hegele, R.A. Lomitapide for the treatment of hypertriglyceridemia. Expert. Opin. Investig. Drugs 2016, 25, 1457–1463. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.K. An Overview of Drug Delivery Systems. Methods Mol. Biol. 2020, 2059, 1–54. [Google Scholar] [CrossRef]
- Jain, A.; Jain, A.; Gulbake, A.; Shilpi, S.; Hurkat, P.; Jain, S.K. Peptide and protein delivery using new drug delivery systems. Crit. Rev. Ther. Drug Carrier Syst. 2013, 30, 293–329. [Google Scholar] [CrossRef] [PubMed]
| Derived | Peptide | Sequences | Ref |
|---|---|---|---|
| ApoA-I | 18A | Ac-DWLKAFYDKVAEKLKEAF- NH2 | [18] |
| 37pA | 18A-P-18A | [19] | |
| 4F (L-4F and D-4F) | Ac-DWFKAFYDKVAEKFKEAF-NH2 | [20] | |
| ELK-2A2K2E | EKLKAKLEELKAKLEELL-P-EKLKAKLEELKAKLEELL | [21] | |
| ApoC-II | 18A-C-II-a | 18A-P-AMSTYTGIFTDQVLSVLKGEE | [22] |
| D6PV | DYLKEVFEKLRDLYEKFTPAVSTYTGIFTDQVLSVLKGEE | [23] | |
| ApoE | Ac-hE18A-NH2 | Ac-LRKLRKRLLR-18A-NH2 | [24] |
| AT1-5261 | EVRSKLEEWFAAFREFAEEFLARLKS | [25] | |
| EpK | NH2-CRRKLRKRLLRKKKKKKQVAEVRAKLEEQAQQIRLQAE-COOH | [26] | |
| hEp | EELRVRLASHLRKLRKRLLRDADDLQKRLAVYEEQAQQIRLQAEAFQARLKSWFEPLVEDM | [27] | |
| mR18L | AcGFRRFLGSWARIYRAFVGNH2 | [28] | |
| ApoE (141–155) dimer | Ac-LRKLRKRLLRDADDLLRKLRKRLLRDADDL | [29] | |
| ApoEdp | Ac-LRKLRKRLLLRKLRKRLL-NH2 | [30] | |
| ApoJ | D-(113–122) ApoJ | Ac-LVGRQLEEFL-NH2 | [31] |
| LRP1 | LP3 | H-GDNDSEDNSDEENC-NH2 | [32] |
| DP3 | H-NEEDSNDESDNDG-NH2 | [32] |
| Original | Peptide | Sequences | Animal Model | Adjuvant/ Carrier | Administration Route | Ref |
|---|---|---|---|---|---|---|
| ApoA-I | 4F | DWFKAFYDKVAEKFKEAF | apoE −/− mice | sterile PBS | Intraperitoneal injections | [89] |
| Pro peptide | 4F-P-4F | apoE −/− mice | sterile PBS | Intraperitoneal injections | [89] | |
| ApoB-100 | p210 | KTTKQSFDLSVKAQYKKNKH | apoE −/− mice | Cholera toxin B or Alum + cationized BSA | Intranasal or subcutaneous injection | [90,91,92] |
| LDLR −/−/hapoB-100 mice | Alum + cationized BSA | Subcutaneous injection | [93] | |||
| p2C7 | CMPSVILPSC | LDLR −/− mice | none | Passive immunization | [94] | |
| LRP1 | P3 | GDNDSEDNSDEENC | NZW Rabbit | KLH (Keyhole limpet haemocyanin) | Subcutaneous injection | [95] |
| Original | Peptide | Company | Administration Route | Conclusions | Stage | Ref and NCT |
|---|---|---|---|---|---|---|
| ApoA-I | D-4F | Novartis | Oral | Poor bioavailability HII improve | Phase 2 | [108] |
| L-4F | Novartis | Intravenous or subcutaneous | Reach plasma levels HII not improve | Phase 2 | [109] | |
| 5A | NHLBI | Intravenous | Not yet | Phase 1 | [110] NCT04216342 | |
| HDL mimetic | ApoA-I milano | Esperion Therapeutics Pfizer’s | Intravenous | No plaque regression | Phase 1 | [111] NCT02678923 |
| CSL-112 | CSL Behring Inc | Intravenous | Not yet | Phase 3 | [112] NCT03473223 | |
| CER-001 | Cerenis Therapeutics | Intravenous | No plaque reduction | Phase 2 | [113] NCT01201837 | |
| ApoE | Ac-hE18A-NH2 | LipimetiX Development | Intravenous | Safety profile Reduce TG and VLDL-C | Phase 1 | [114] NCT02100839 |
| Opportunities | Risks |
|---|---|
| Improvement of lipid and lipoprotein profile and LDL protection | Interactions derived from the multiple functions of apolipoproteins |
| Wide versatility to treat CVDs due to low product costs | Conformational peptide alterations depending on pH |
| New knowledge about non-classical roles of apolipoproteins and their implications in multiple events | Requirements of controlled release delivery device or frequent dosing |
| Extracellular and focalized actions | Ability to overcome cell membrane permeability |
| Wide variety of routes for peptide delivery | Low oral bioavailability |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benitez Amaro, A.; Solanelles Curco, A.; Garcia, E.; Julve, J.; Rives, J.; Benitez, S.; Llorente Cortes, V. Apolipoprotein and LRP1-Based Peptides as New Therapeutic Tools in Atherosclerosis. J. Clin. Med. 2021, 10, 3571. https://doi.org/10.3390/jcm10163571
Benitez Amaro A, Solanelles Curco A, Garcia E, Julve J, Rives J, Benitez S, Llorente Cortes V. Apolipoprotein and LRP1-Based Peptides as New Therapeutic Tools in Atherosclerosis. Journal of Clinical Medicine. 2021; 10(16):3571. https://doi.org/10.3390/jcm10163571
Chicago/Turabian StyleBenitez Amaro, Aleyda, Angels Solanelles Curco, Eduardo Garcia, Josep Julve, Jose Rives, Sonia Benitez, and Vicenta Llorente Cortes. 2021. "Apolipoprotein and LRP1-Based Peptides as New Therapeutic Tools in Atherosclerosis" Journal of Clinical Medicine 10, no. 16: 3571. https://doi.org/10.3390/jcm10163571
APA StyleBenitez Amaro, A., Solanelles Curco, A., Garcia, E., Julve, J., Rives, J., Benitez, S., & Llorente Cortes, V. (2021). Apolipoprotein and LRP1-Based Peptides as New Therapeutic Tools in Atherosclerosis. Journal of Clinical Medicine, 10(16), 3571. https://doi.org/10.3390/jcm10163571








