Clinical Management of Moyamoya Patients
Abstract
:1. Introduction
2. Pathophysiology
3. Clinical Features
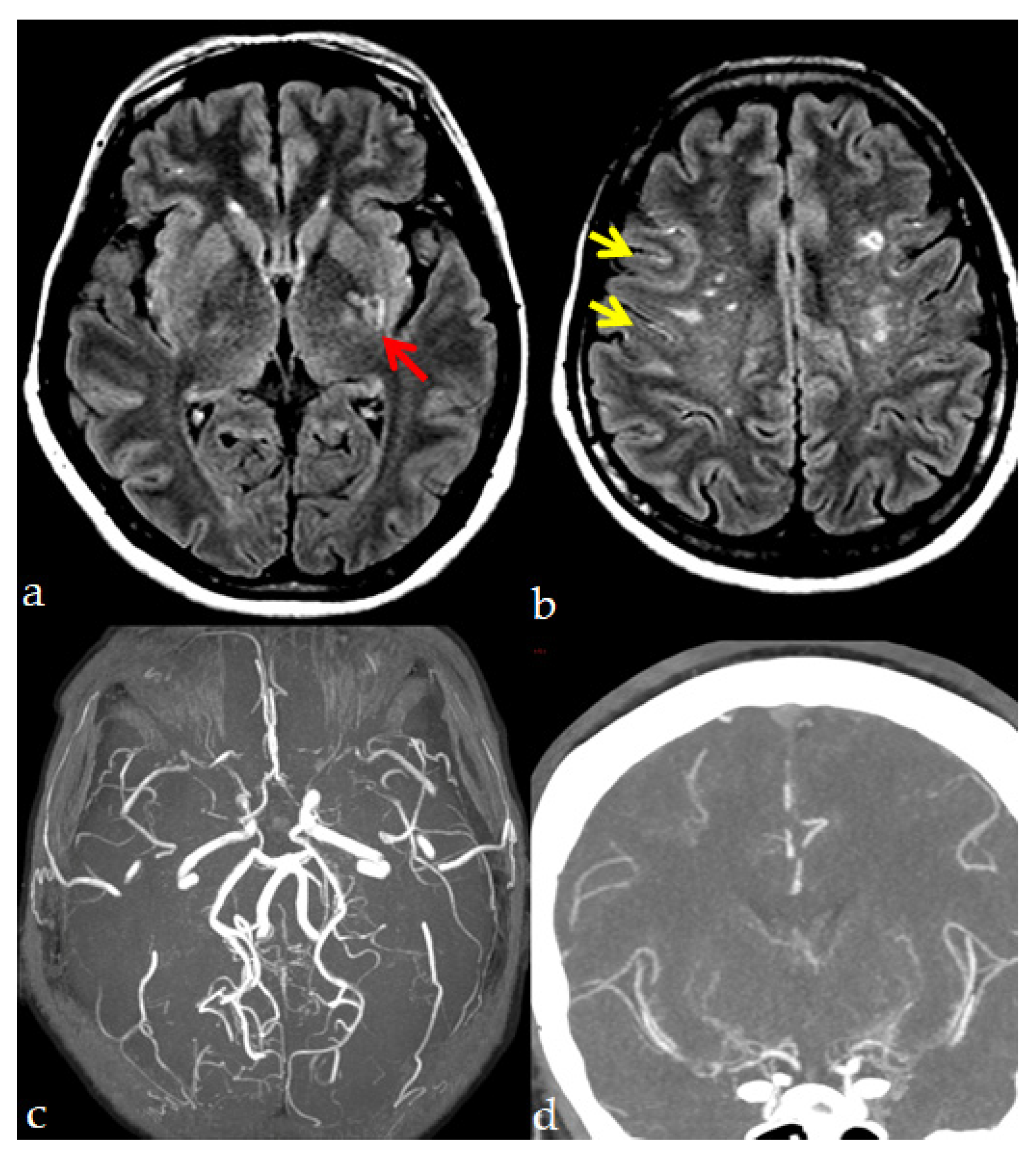
4. Neuroradiological Diagnosis
- -
- MRA showing stenosis or occlusion of the terminal portion of the intracranial ICA or proximal portions of the ACA and/or the MCA.
- -
- Presence of the abnormal vascular networks near the occlusive or stenotic lesions by MRA or MRI demonstrating two or more flow voids in the basal ganglia on each hemisphere.
5. Medical Treatment
5.1. Headache Management
5.2. Epilepsy Management
- before stroke: the typical epileptogenic focus of MMA is located in the territory of the ICA, probably being an expression of ischemic damage;
- after stroke: epilepsy in MMA is a recognized type of poststroke epilepsy;
- post-surgery: the surgical procedure itself, by causing a breach and reorganizing vascular dynamics, might be epileptogenic.
6. Surgical Treatment
- recurrent symptoms related to cerebral ischemic mechanisms;
- cerebral hemodynamic impairment with decreased regional CBF, vascular response, and perfusion reserve seen in hemodynamic neuro-radiological studies;
7. Prognosis
8. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fukui, M. Guidelines for the diagnosis and treatment of spontaneous occlusion of the circle of Willis (“moyamoya” disease). Research Committee on Spontaneous Occlusion of the Circle of Willis (Moyamoya Disease) of the Ministry of Health and Welfare, Japan. Clin. Neurol. Neurosurg. 1997, 99 (Suppl. 2), S238–S240. [Google Scholar] [CrossRef]
- Scott, R.M.; Smith, E.R. Moyamoya Disease and Moyamoya Syndrome. N. Engl. J. Med. 2009, 360, 1226–1237. [Google Scholar] [CrossRef] [Green Version]
- Guey, S.; Tournier-Lasserve, E.; Hervé, D.; Kossorotoff, M. Moyamoya disease and syndromes: From genetics to clinical management. Appl. Clin. Genet. 2015, 8, 49–68. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, E.; Saeki, N.; Oishi, H.; Hirai, S.; Yamaura, A. Long-term natural history of hemorrhagic moyamoya disease in 42 patients. J. Neurosurg. 2000, 93, 976–980. [Google Scholar] [CrossRef]
- Bersano, A.; Guey, S.; Bedini, G.; Nava, S.; Hervé, D.; Vajkoczy, P.; Tatlisumak, T.; Sareela, M.; Van Der Zwan, A.; Klijn, C.J.M.; et al. Research progresses in understanding the pathophysiology of moyamoya disease. Cerebrovasc. Dis. 2016, 41, 105–118. [Google Scholar] [CrossRef] [Green Version]
- Czabanka, M.; Boschi, A.; Acker, G.; Peña-Tapia, P.; Schubert, G.A.; Schmiedek, P.; Vajkoczy, P. Grading of moyamoya disease allows stratification for postoperative ischemia in bilateral revascularization surgery. Acta Neurochir. 2016, 158, 1895–1900. [Google Scholar] [CrossRef]
- Graf, J.; Schwitalla, J.C.; Albrecht, P.; Veltkamp, R.; Berlit, P.; Hartung, H.-P.; Aktas, O.; Kraemer, M. Misdiagnoses and delay of diagnoses in Moyamoya angiopathy-a large Caucasian case series. J. Neurol. 2019, 266, 1153–1159. [Google Scholar] [CrossRef]
- Fang, Y.-C.; Wei, L.-F.; Hu, C.-J.; Tu, Y.-K. Pathological Circulating Factors in Moyamoya Disease. Int. J. Mol. Sci. 2021, 22, 1696. [Google Scholar] [CrossRef]
- Reid, A.J.; Bhattacharjee, M.B.; Regalado, E.S.; Milewicz, A.L.; El-Hakam, L.M.; Dauser, R.C.; Milewicz, D.M. Diffuse and uncontrolled vascular smooth muscle cell proliferation in rapidly progressing pediatric moyamoya disease. J. Neurosurg. Pediatr. 2010, 6, 244–249. [Google Scholar] [CrossRef]
- Kang, H.-S.; Moon, Y.-J.; Kim, Y.-Y.; Park, W.-Y.; Park, A.K.; Wang, K.-C.; Kim, J.E.; Phi, J.H.; Lee, J.Y.; Kim, S.-K. Smooth-muscle progenitor cells isolated from patients with moyamoya disease: Novel experimental cell model. J. Neurosurg. 2014, 120, 415–425. [Google Scholar] [CrossRef]
- Yoshihara, T.; Taguchi, A.; Matsuyama, T.; Shimizu, Y.; Kikuchi-Taura, A.; Soma, T.; Stern, D.M.; Yoshikawa, H.; Kasahara, Y.; Moriwaki, H.; et al. Increase in circulating CD34-positive cells in patients with angiographic evidence of moyamoya-like vessels. J. Cereb. Blood Flow Metab. 2008, 28, 1086–1089. [Google Scholar] [CrossRef] [Green Version]
- Rafat, N.; Beck, G.C.; Peña-Tapia, P.G.; Schmiedek, P.; Vajkoczy, P. Increased levels of circulating endothelial progenitor cells in patients with Moyamoya disease. Stroke 2009, 40, 432–438. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Jung, J.-H.; Phi, J.H.; Kang, H.-S.; Kim, J.E.; Chae, J.H.; Kim, S.-J.; Kim, Y.-H.; Kim, Y.Y.; Cho, B.-K.; et al. Decreased level and defective function of circulating endothelial progenitor cells in children with moyamoya disease. J. Neurosci. Res. 2010, 88, 510–518. [Google Scholar] [CrossRef]
- Takekawa, Y.; Umezawa, T.; Ueno, Y.; Sawada, T.; Kobayashi, M. Pathological and immunohistochemical findings of an autopsy case of adult moyamoya disease. Neuropathology 2004, 24, 236–242. [Google Scholar] [CrossRef]
- Bedini, G.; Blecharz, K.; Nava, S.; Vajkoczy, P.; Alessandri, G.; Ranieri, M.; Acerbi, F.; Ferroli, P.; Riva, D.; Esposito, S.; et al. Vasculogenic and Angiogenic Pathways in Moyamoya Disease. Curr. Med. Chem. 2016, 23, 315–345. [Google Scholar] [CrossRef]
- Liu, W.; Morito, D.; Takashima, S.; Mineharu, Y.; Kobayashi, H.; Hitomi, T.; Hashikata, H.; Matsuura, N.; Yamazaki, S.; Toyoda, A.; et al. Identification of RNF213 as a susceptibility gene for moyamoya disease and its possible role in vascular development. PLoS ONE 2011, 6, e22542. [Google Scholar] [CrossRef] [Green Version]
- Raso, A.; Biassoni, R.; Mascelli, S.; Nozza, P.; Ugolotti, E.; Di Marco, E.; De Marco, P.; Merello, E.; Cama, A.; Pavanello, M.; et al. Moyamoya vasculopathy shows a genetic mutational gradient decreasing from East to West. J. Neurosurg. Sci. 2020, 64, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-G.; Zhang, Q.; Yu, L.-B.; Zhao, J.-Z. Role of Ring Finger Protein 213 in Moyamoya Disease. Chin. Med. J. 2016, 129, 2497–2501. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Deguchi, K.; Tanigawara, T.; Takenaka, K.; Nishimura, Y.; Shinoda, J.; Hattori, T.; Andoh, T.; Sakai, N. The relationship between moyamoya disease and bacterial infection. Clin. Neurol. Neurosurg. 1997, 99 (Suppl. 2), S221–S224. [Google Scholar] [CrossRef]
- Matsuo, M.; Nadanaka, S.; Soga, M.; Sugiyama, T.; Serigano, S.; Shimano, K.; Ichinose, F.; Nakamura, T.; Maeda, T.; Houkin, K.; et al. Vulnerability to shear stress caused by altered peri-endothelial matrix is a key feature of Moyamoya disease. Sci. Rep. 2021, 11, 1552. [Google Scholar] [CrossRef]
- Matsushige, T.; Kraemer, M.; Schlamann, M.; Berlit, P.; Forsting, M.; Ladd, M.E.; Sure, U.; Wrede, K.H. Ventricular Microaneurysms in Moyamoya Angiopathy Visualized with 7T MR Angiography. AJNR Am. J. Neuroradiol. 2016, 37, 1669–1672. [Google Scholar] [CrossRef] [Green Version]
- Mauro, A.J.; Johnson, E.S.; Chikos, P.M.; Alvord, E.C.J. Lipohyalinosis and miliary microaneurysms causing cerebral hemorrhage in a patient with moyamoya. A clinicopathological study. Stroke 1980, 11, 405–412. [Google Scholar] [CrossRef] [Green Version]
- Kawaguchi, S.; Sakaki, T.; Morimoto, T.; Kakizaki, T.; Kamada, K. Characteristics of intracranial aneurysms associated with moyamoya disease. Acta Neurochir. 1996, 138, 1287–1294. [Google Scholar] [CrossRef]
- Hamauchi, S.; Shichinohe, H.; Houkin, K. Review of past and present research on experimental models of moyamoya disease. Brain Circ. 2015, 1, 88–96. [Google Scholar] [CrossRef]
- Roberts, J.M.; Maniskas, M.E.; Fraser, J.F.; Bix, G.J. Internal carotid artery stenosis: A novel surgical model for moyamoya syndrome. PLoS ONE 2018, 13, e0191312. [Google Scholar] [CrossRef] [Green Version]
- Sugiyama, T.; Kuroda, S.; Nakayama, N.; Tanaka, S.; Houkin, K. Bone marrow-derived endothelial progenitor cells participate in the initiation of moyamoya disease. Neurol. Med. Chir. 2011, 51, 767–773. [Google Scholar] [CrossRef] [Green Version]
- Kuroda, S.; Kashiwazaki, D.; Akioka, N.; Koh, M.; Hori, E.; Nishikata, M.; Umemura, K.; Horie, Y.; Noguchi, K.; Kuwayama, N. Specific Shrinkage of Carotid Forks in Moyamoya Disease: A Novel Key Finding for Diagnosis. Neurol. Med. Chir. 2015, 55, 796–804. [Google Scholar] [CrossRef] [Green Version]
- Ryoo, S.; Cha, J.; Kim, S.J.; Choi, J.W.; Ki, C.-S.; Kim, K.H.; Jeon, P.; Kim, J.-S.; Hong, S.-C.; Bang, O.Y. High-resolution magnetic resonance wall imaging findings of Moyamoya disease. Stroke 2014, 45, 2457–2460. [Google Scholar] [CrossRef] [Green Version]
- Jeon, C.; Yeon, J.Y.; Jo, K.I.; Hong, S.-C.; Kim, J.-S. Clinical Role of Microembolic Signals in Adult Moyamoya Disease with Ischemic Stroke. Stroke 2019, 50, 1130–1135. [Google Scholar] [CrossRef]
- Goto, Y.; Yonekawa, Y. Worldwide distribution of moyamoya disease. Neurol. Med. Chir. 1992, 32, 883–886. [Google Scholar] [CrossRef] [Green Version]
- Yonekawa, Y.; Ogata, N.; Kaku, Y.; Taub, E.; Imhof, H.G. Moyamoya disease in Europe, past and present status. Clin. Neurol. Neurosurg. 1997, 99 (Suppl. 2), S58–S60. [Google Scholar] [CrossRef]
- Kraemer, M.; Heienbrok, W.; Berlit, P. Moyamoya disease in Europeans. Stroke 2008, 39, 3193–3200. [Google Scholar] [CrossRef] [Green Version]
- Kainth, D.; Chaudhry, S.A.; Kainth, H.; Suri, F.K.; Qureshi, A.I. Epidemiological and clinical features of moyamoya disease in the USA. Neuroepidemiology 2013, 40, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Kossorotoff, M.; Hervé, D.; Toulgoat, F.; Renaud, C.; Presles, E.; Chabriat, H.; Chabrier, S. Paediatric moyamoya in mainland France: A comprehensive survey of academic neuropaediatric centres. Cerebrovasc. Dis. 2012, 33, 76–79. [Google Scholar] [CrossRef]
- Vetrano, I.G.; Bersano, A.; Canavero, I.; Restelli, F.; Raccuia, G.; Ciceri, E.F.; Faragò, G.; Gioppo, A.; Broggi, M.; Schiariti, M. Characteristics of Moyamoya Disease in the Older Population: Is It Possible to Define a Typical Presentation and Optimal Therapeutical Management? J. Clin. Med. 2021, 10, 2287. [Google Scholar] [CrossRef]
- Kazumata, K.; Tha, K.K.; Narita, H.; Kusumi, I.; Shichinohe, H.; Ito, M.; Nakayama, N.; Houkin, K. Chronic ischemia alters brain microstructural integrity and cognitive performance in adult moyamoya disease. Stroke 2015, 46, 354–360. [Google Scholar] [CrossRef] [Green Version]
- Weinberg, D.G.; Rahme, R.J.; Aoun, S.G.; Batjer, H.H.; Bendok, B.R. Moyamoya disease: Functional and neurocognitive outcomes in the pediatric and adult populations. Neurosurg. Focus 2011, 30, E21. [Google Scholar] [CrossRef]
- Hara, S.; Hori, M.; Murata, S.; Ueda, R.; Tanaka, Y.; Inaji, M.; Maehara, T.; Aoki, S.; Nariai, T. Microstructural Damage in Normal-Appearing Brain Parenchyma and Neurocognitive Dysfunction in Adult Moyamoya Disease. Stroke 2018, 49, 2504–2507. [Google Scholar] [CrossRef]
- Karzmark, P.; Zeifert, P.D.; Bell-Stephens, T.E.; Steinberg, G.K.; Dorfman, L.J. Neurocognitive impairment in adults with moyamoya disease without stroke. Neurosurgery 2012, 70, 634–638. [Google Scholar] [CrossRef]
- Kraemer, M.; Lee, S.-I.; Ayzenberg, I.; Schwitalla, J.C.; Diehl, R.R.; Berlit, P.; Bosche, B.; Katsarava, Z.; Obermann, M. Headache in Caucasian patients with Moyamoya angiopathy—A systematic cohort study. Cephalalgia 2017, 37, 496–500. [Google Scholar] [CrossRef]
- Mikami, T.; Ochi, S.; Houkin, K.; Akiyama, Y.; Wanibuchi, M.; Mikuni, N. Predictive factors for epilepsy in moyamoya disease. J. Stroke Cerebrovasc. Dis. 2015, 24, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Ahn, E.S.; Scott, R.M.; Robertson, R.L.J.; Smith, E.R. Chorea in the clinical presentation of moyamoya disease: Results of surgical revascularization and a proposed clinicopathological correlation. J. Neurosurg. Pediatr. 2013, 11, 313–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavallieri, F.; Zedde, M.; Assenza, F.; Valzania, F. Steroid-Responsive Acute Left-Arm Chorea as a Presenting Symptom of Moyamoya Disease. Can. J. Neurol. Sci. 2021, 48, 287–289. [Google Scholar] [CrossRef] [PubMed]
- Research Committee on the Pathology and Treatment of Spontaneous Occlusion of the Circle of Willis. Guidelines for diagnosis and treatment of moyamoya disease (spontaneous occlusion of the circle of Willis). Neurol. Med. Chir. 2012, 52, 245–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleinloog, R.; Regli, L.; Rinkel, G.J.E.; Klijn, C.J.M. Regional differences in incidence and patient characteristics of moyamoya disease: A systematic review. J. Neurol. Neurosurg. Psychiatry 2012, 83, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Jeon, J.S. An update on the diagnosis and treatment of adult Moyamoya disease taking into consideration controversial issues. Neurol. Res. 2014, 36, 407–416. [Google Scholar] [CrossRef]
- Suzuki, J.; Takaku, A. Cerebrovascular “moyamoya” disease. Disease showing abnormal net-like vessels in base of brain. Arch. Neurol. 1969, 20, 288–299. [Google Scholar] [CrossRef]
- Fujimura, M.; Tominaga, T. Diagnosis of moyamoya disease: International standard and regional differences. Neurol. Med. Chir. 2015, 55, 189–193. [Google Scholar] [CrossRef] [Green Version]
- Fujimura, M.; Tominaga, T. Lessons learned from moyamoya disease: Outcome of direct/indirect revascularization surgery for 150 affected hemispheres. Neurol. Med. Chir. 2012, 52, 327–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houkin, K.; Nakayama, N.; Kuroda, S.; Nonaka, T.; Shonai, T.; Yoshimoto, T. Novel magnetic resonance angiography stage grading for moyamoya disease. Cerebrovasc. Dis. 2005, 20, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Funaki, T.; Takahashi, J.C.; Yoshida, K.; Takagi, Y.; Fushimi, Y.; Kikuchi, T.; Mineharu, Y.; Okada, T.; Morimoto, T.; Miyamoto, S. Periventricular anastomosis in moyamoya disease: Detecting fragile collateral vessels with MR angiography. J. Neurosurg. 2016, 124, 1766–1772. [Google Scholar] [CrossRef] [Green Version]
- Ryu, J.; Hamano, E.; Nishimura, M.; Satow, T.; Takahashi, J.C. Difference in periventricular anastomosis in child and adult moyamoya disease: A vascular morphology study. Acta Neurochir. 2020, 162, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Liu, Z.; Wang, Z.; Li, B.; Xu, L.; Xiao, X. High-resolution MR imaging of the arterial wall in moyamoya disease. Neurosci. Lett. 2015, 584, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Son, J.P.; Yeon, J.Y.; Kim, G.-M.; Kim, J.-S.; Hong, S.-C.; Bang, O.Y. Infarct Pattern and Collateral Status in Adult Moyamoya Disease: A Multimodal Magnetic Resonance Imaging Study. Stroke 2017, 48, 111–116. [Google Scholar] [CrossRef]
- Yoon, H.-K.; Shin, H.-J.; Chang, Y.W. “Ivy sign” in childhood moyamoya disease: Depiction on FLAIR and contrast-enhanced T1-weighted MR images. Radiology 2002, 223, 384–389. [Google Scholar] [CrossRef]
- Wolf, R.L. Intraarterial signal on fluid-attenuated inversion recovery images: A measure of hemodynamic stress? AJNR Am. J. Neuroradiol. 2001, 22, 1015–1016. [Google Scholar]
- Toyoda, K.; Ida, M.; Fukuda, K. Fluid-attenuated inversion recovery intraarterial signal: An early sign of hyperacute cerebral ischemia. AJNR Am. J. Neuroradiol. 2001, 22, 1021–1029. [Google Scholar]
- Iancu-Gontard, D.; Oppenheim, C.; Touzé, E.; Méary, E.; Zuber, M.; Mas, J.-L.; Frédy, D.; Meder, J.-F. Evaluation of hyperintense vessels on FLAIR MRI for the diagnosis of multiple intracerebral arterial stenoses. Stroke 2003, 34, 1886–1891. [Google Scholar] [CrossRef] [Green Version]
- Maeda, M.; Yagishita, A.; Yamamoto, T.; Sakuma, H.; Takeda, K. Abnormal hyperintensity within the subarachnoid space evaluated by fluid-attenuated inversion-recovery MR imaging: A spectrum of central nervous system diseases. Eur. Radiol. 2003, 13 (Suppl. 4), L192–L201. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhao, Y.; Li, M.; Ma, L.; Chen, Y.; Wang, R.; Ye, X.; Wang, H.; Chen, X.; Zhao, Y. Clinical Implications of the “Brush Sign” in Susceptibility-Weighted Imaging for Moyamoya Disease. Cerebrovasc. Dis. 2021, 50, 147–155. [Google Scholar] [CrossRef]
- Fan, A.P.; Jahanian, H.; Holdsworth, S.J.; Zaharchuk, G. Comparison of cerebral blood flow measurement with [15O]-water positron emission tomography and arterial spin labeling magnetic resonance imaging: A systematic review. J. Cereb. Blood Flow Metab. 2016, 36, 842–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nariai, T.; Matsushima, Y.; Imae, S.; Tanaka, Y.; Ishii, K.; Senda, M.; Ohno, K. Severe haemodynamic stress in selected subtypes of patients with moyamoya disease: A positron emission tomography study. J. Neurol. Neurosurg. Psychiatry 2005, 76, 663–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, Y.; Nariai, T.; Nagaoka, T.; Akimoto, H.; Ishiwata, K.; Ishii, K.; Matsushima, Y.; Ohno, K. Quantitative evaluation of cerebral hemodynamics in patients with moyamoya disease by dynamic susceptibility contrast magnetic resonance imaging—Comparison with positron emission tomography. J. Cereb. Blood Flow Metab. 2006, 26, 291–300. [Google Scholar] [CrossRef] [Green Version]
- Huang, A.; Lee, C.-W.; Liu, H.-M. Time to peak and full width at half maximum in MR perfusion: Valuable indicators for monitoring moyamoya patients after revascularization. Sci. Rep. 2021, 11, 479. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Ma, N.; Li, J.; Shen, Y.; Gu, W.; Ma, G.; Zhang, D.; Zhao, X. Cerebral Hemodynamic Changes After Revascularization in Patients With Hemorrhagic Moyamoya Disease. Front. Neurol. 2020, 11, 72. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, W.; Guo, X.; Shi, Z.; Sun, Z.; Gao, L.; Jin, F.; Wang, J.; Chen, W.; Yang, Y. CT perfusion assessment of Moyamoya syndrome before and after direct revascularization (superficial temporal artery to middle cerebral artery bypass). Eur. Radiol. 2016, 26, 254–261. [Google Scholar] [CrossRef]
- Togao, O.; Mihara, F.; Yoshiura, T.; Tanaka, A.; Noguchi, T.; Kuwabara, Y.; Kaneko, K.; Matsushima, T.; Honda, H. Cerebral hemodynamics in Moyamoya disease: Correlation between perfusion-weighted MR imaging and cerebral angiography. AJNR Am. J. Neuroradiol. 2006, 27, 391–397. [Google Scholar]
- Zhang, J.; Wang, J.; Geng, D.; Li, Y.; Song, D.; Gu, Y. Whole-brain CT perfusion and CT angiography assessment of Moyamoya disease before and after surgical revascularization: Preliminary study with 256-slice CT. PLoS ONE 2013, 8, e57595. [Google Scholar] [CrossRef] [Green Version]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2018, 49, e46–e110. [Google Scholar] [CrossRef]
- Kraemer, M.; Berlit, P.; Diesner, F.; Khan, N. What is the expert’s option on antiplatelet therapy in moyamoya disease? Results of a worldwide Survey. Eur. J. Neurol. 2012, 19, 163–167. [Google Scholar] [CrossRef]
- Yamada, S.; Oki, K.; Itoh, Y.; Kuroda, S.; Houkin, K.; Tominaga, T.; Miyamoto, S.; Hashimoto, N.; Suzuki, N. Effects of Surgery and Antiplatelet Therapy in Ten-Year Follow-Up from the Registry Study of Research Committee on Moyamoya Disease in Japan. J. Stroke Cerebrovasc. Dis. 2016, 25, 340–349. [Google Scholar] [CrossRef]
- Ye, F.; Li, J.; Wang, T.; Lan, K.; Li, H.; Yin, H.; Guo, T.; Zhang, X.; Yang, T.; Liang, J.; et al. Efficacy and Safety of Antiplatelet Agents for Adult Patients With Ischemic Moyamoya Disease. Front. Neurol. 2020, 11, 608000. [Google Scholar] [CrossRef]
- Chiu, D.; Shedden, P.; Bratina, P.; Grotta, J.C. Clinical features of moyamoya disease in the United States. Stroke 1998, 29, 1347–1351. [Google Scholar] [CrossRef] [Green Version]
- Kuriyama, S.; Kusaka, Y.; Fujimura, M.; Wakai, K.; Tamakoshi, A.; Hashimoto, S.; Tsuji, I.; Inaba, Y.; Yoshimoto, T. Prevalence and clinicoepidemiological features of moyamoya disease in Japan: Findings from a nationwide epidemiological survey. Stroke 2008, 39, 42–47. [Google Scholar] [CrossRef] [Green Version]
- Duan, L.; Bao, X.-Y.; Yang, W.-Z.; Shi, W.-C.; Li, D.-S.; Zhang, Z.-S.; Zong, R.; Han, C.; Zhao, F.; Feng, J. Moyamoya disease in China: Its clinical features and outcomes. Stroke 2012, 43, 56–60. [Google Scholar] [CrossRef] [Green Version]
- Ahn, I.M.; Park, D.-H.; Hann, H.J.; Kim, K.H.; Kim, H.J.; Ahn, H.S. Incidence, prevalence, and survival of moyamoya disease in Korea: A nationwide, population-based study. Stroke 2014, 45, 1090–1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P.-C.; Yang, S.-H.; Chien, K.-L.; Tsai, I.-J.; Kuo, M.-F. Epidemiology of moyamoya disease in Taiwan: A nationwide population-based study. Stroke 2014, 45, 1258–1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rashad, S.; Fujimura, M.; Niizuma, K.; Endo, H.; Tominaga, T. Long-term follow-up of pediatric moyamoya disease treated by combined direct-indirect revascularization surgery: Single institute experience with surgical and perioperative management. Neurosurg. Rev. 2016, 39, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, Q.; Zhang, D.; Zhao, Y. Effect of Aspirin in Postoperative Management of Adult Ischemic Moyamoya Disease. World Neurosurg. 2017, 105, 728–731. [Google Scholar] [CrossRef]
- Chiba, T.; Setta, K.; Shimada, Y.; Yoshida, J.; Fujimoto, K.; Tsutsui, S.; Yoshida, K.; Kobayashi, M.; Kubo, Y.; Fujiwara, S.; et al. Comparison of Effects between Clopidogrel and Cilostazol on Cerebral Perfusion in Nonsurgical Adult Patients with Symptomatically Ischemic Moyamoya Disease: Subanalysis of a Prospective Cohort. J. Stroke Cerebrovasc. Dis. 2018, 27, 3373–3379. [Google Scholar] [CrossRef]
- Miyoshi, K.; Chida, K.; Kobayashi, M.; Kubo, Y.; Yoshida, K.; Terasaki, K.; Ogasawara, K. Two-Year Clinical, Cerebral Hemodynamic, and Cognitive Outcomes of Adult Patients Undergoing Medication Alone for Symptomatically Ischemic Moyamoya Disease Without Cerebral Misery Perfusion: A Prospective Cohort Study. Neurosurgery 2019, 84, 1233–1241. [Google Scholar] [CrossRef]
- Ando, S.; Tsutsui, S.; Miyoshi, K.; Sato, S.; Yanagihara, W.; Setta, K.; Chiba, T.; Fujiwara, S.; Kobayashi, M.; Yoshida, K.; et al. Cilostazol may improve cognition better than clopidogrel in non-surgical adult patients with ischemic moyamoya disease: Subanalysis of a prospective cohort. Neurol. Res. 2019, 41, 480–487. [Google Scholar] [CrossRef]
- Seo, W.-K.; Kim, J.-Y.; Choi, E.-H.; Kim, Y.-S.; Chung, J.-W.; Saver, J.L.; Bang, O.Y.; Kim, G.-M. Association of Antiplatelet Therapy, Including Cilostazol, With Improved Survival in Patients With Moyamoya Disease in a Nationwide Study. J. Am. Heart Assoc. 2021, 10, e017701. [Google Scholar] [CrossRef]
- Savolainen, M.; Mustanoja, S.; Pekkola, J.; Tyni, T.; Uusitalo, A.-M.; Ruotsalainen, S.; Poutiainen, E.; Hernesniemi, J.; Kivipelto, L.; Tatlisumak, T. Moyamoya angiopathy: Long-term follow-up study in a Finnish population. J. Neurol. 2019, 266, 574–581. [Google Scholar] [CrossRef] [Green Version]
- Savolainen, M.; Pekkola, J.; Mustanoja, S.; Tyni, T.; Hernesniemi, J.; Kivipelto, L.; Tatlisumak, T. Moyamoya angiopathy: Radiological follow-up findings in Finnish patients. J. Neurol. 2020, 267, 2301–2306. [Google Scholar] [CrossRef] [PubMed]
- Argetsinger, D.S.; Miller, J.W.; Fletcher, J.J. Intravenous thrombolysis, mechanical embolectomy, and intracranial stenting for hyperacute ischemic stroke in a patient with moyamoya disease. J. Clin. Neurosci. 2016, 29, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.M.; Eun, M.-Y.; Seo, W.-K.; Suh, S. il Thrombolysis for acute ischemic stroke in a patient with Moyamoya disease. Can. J. Neurol. Sci. 2012, 39, 687–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokoyama, S.; Manabe, Y.; Fujii, D.; Ikeda-Sakai, Y.; Narai, H.; Omori, N.; Abe, K. Intravenous tissue plasminogen activator therapy for an acute ischemic stroke patient with later diagnosed unilateral moyamoya syndrome. J. Stroke Cerebrovasc. Dis. 2013, 22, 1190–1192. [Google Scholar] [CrossRef] [PubMed]
- Iwama, T.; Morimoto, M.; Hashimoto, N.; Goto, Y.; Todaka, T.; Sawada, M. Mechanism of intracranial rebleeding in moyamoya disease. Clin. Neurol. Neurosurg. 1997, 99 (Suppl. 2), S187–S190. [Google Scholar] [CrossRef]
- Okada, Y.; Kawamata, T.; Kawashima, A.; Yamaguchi, K.; Ono, Y.; Hori, T. The efficacy of superficial temporal artery-middle cerebral artery anastomosis in patients with moyamoya disease complaining of severe headache. J. Neurosurg. 2012, 116, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Seol, H.J.; Wang, K.-C.; Kim, S.-K.; Hwang, Y.-S.; Kim, K.J.; Cho, B.-K. Headache in pediatric moyamoya disease: Review of 204 consecutive cases. J. Neurosurg. 2005, 103, 439–442. [Google Scholar] [CrossRef]
- Aihara, Y.; Kashiwase, S.; Chiba, K.; Yamaguchi, K.; Okada, Y.; Kimura, T.; Kawamata, T. Aspirin use and platelet aggregation in ischemic onset-type pediatric moyamoya patients with intractable headaches (moya-ache). Child’s Nerv. Syst. 2021, 37, 1649–1657. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.-C.; Oh, C.W.; Kwon, O.-K.; Hwang, G.; Bang, J.S.; Kang, H.-S.; Kim, J.E.; Lee, S.H.; Chung, Y.-S. Epilepsy after bypass surgery in adult moyamoya disease. Neurosurgery 2011, 68, 1227–1232; discussion 1232. [Google Scholar] [CrossRef]
- Bauer, J.; Bös, M.; Reuber, M. Treatment strategies for focal epilepsy. Expert Opin. Pharmacother. 2009, 10, 743–753. [Google Scholar] [CrossRef]
- Loikas, D.; Linnér, L.; Sundström, A.; Wettermark, B.; von Euler, M. Post-stroke epilepsy and antiepileptic drug use in men and women. Basic Clin. Pharmacol. Toxicol. 2021, 129, 148–157. [Google Scholar] [CrossRef]
- Miyamoto, S.; Yoshimoto, T.; Hashimoto, N.; Okada, Y.; Tsuji, I.; Tominaga, T.; Nakagawara, J.; Takahashi, J.C. Effects of extracranial-intracranial bypass for patients with hemorrhagic moyamoya disease: Results of the Japan Adult Moyamoya Trial. Stroke 2014, 45, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.C.; Funaki, T.; Houkin, K.; Inoue, T.; Ogasawara, K.; Nakagawara, J.; Kuroda, S.; Yamada, K.; Miyamoto, S. Significance of the Hemorrhagic Site for Recurrent Bleeding: Prespecified Analysis in the Japan Adult Moyamoya Trial. Stroke 2016, 47, 37–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.; Oh, C.W.; Bang, J.S.; Kim, J.E.; Cho, W.-S. Moyamoya Disease: Treatment and Outcomes. J. Stroke 2016, 18, 21–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kan, P.; Srinivasan, V.M.; Srivatsan, A.; Kaufmann, A.B.; Cherian, J.; Burkhardt, J.-K.; Johnson, J.; Duckworth, E.A.M. Double-barrel STA-MCA bypass for cerebral revascularization: Lessons learned from a 10-year experience. J. Neurosurg. 2021, 1–9. [Google Scholar] [CrossRef]
- Woitzik, J.; Horn, P.; Vajkoczy, P.; Schmiedek, P. Intraoperative control of extracranial-intracranial bypass patency by near-infrared indocyanine green videoangiography. J. Neurosurg. 2005, 102, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Raper, D.M.S.; Rutledge, W.C.; Winkler, E.A.; Meisel, K.; Callen, A.L.; Cooke, D.L.; Abla, A.A. Controversies and Advances in Adult Intracranial Bypass Surgery in 2020. Oper. Neurosurg. 2020, 20, 1–7. [Google Scholar] [CrossRef]
- Mallory, G.W.; Bower, R.S.; Nwojo, M.E.; Taussky, P.; Wetjen, N.M.; Varzoni, T.C.; Hanel, R.A.; Meyer, F.B. Surgical outcomes and predictors of stroke in a North American white and African American moyamoya population. Neurosurgery 2013, 73, 982–984. [Google Scholar] [CrossRef] [Green Version]
- Burke, G.M.; Burke, A.M.; Sherma, A.K.; Hurley, M.C.; Batjer, H.H.; Bendok, B.R. Moyamoya disease: A summary. Neurosurg. Focus 2009, 26, E11. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, J.; Vajkoczy, P.; Hecht, N. Indocyanine green videoangiography for recipient vessel stratification in superficial temporal artery-middle cerebral artery bypass surgery. J. Neurosurg. 2020, 1, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Zhang, X.; Tan, C.; Han, Z.; Su, Y.; Duan, R.; Shi, G.; Shao, J.; Cao, P.; He, S.; et al. Intraoperative transit-time ultrasonography combined with FLOW800 predicts the occurrence of cerebral hyperperfusion syndrome after direct revascularization of Moyamoya disease: A preliminary study. Acta Neurochir. 2021, 163, 563–571. [Google Scholar] [CrossRef]
- Kapu, R.; Symss, N.P.; Cugati, G.; Pande, A.; Vasudevan, C.M.; Ramamurthi, R. Multiple burr hole surgery as a treatment modality for pediatric moyamoya disease. J. Pediatr. Neurosci. 2010, 5, 115–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dusick, J.R.; Gonzalez, N.R.; Martin, N.A. Clinical and angiographic outcomes from indirect revascularization surgery for Moyamoya disease in adults and children: A review of 63 procedures. Neurosurgery 2011, 68, 34–43, discussion 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizoi, K.; Kayama, T.; Yoshimoto, T.; Nagamine, Y. Indirect revascularization for moyamoya disease: Is there a beneficial effect for adult patients? Surg. Neurol. 1996, 45, 541–549. [Google Scholar] [CrossRef]
- Blauwblomme, T.; Lemaitre, H.; Naggara, O.; Calmon, R.; Kossorotoff, M.; Bourgeois, M.; Mathon, B.; Puget, S.; Zerah, M.; Brunelle, F.; et al. Cerebral Blood Flow Improvement after Indirect Revascularization for Pediatric Moyamoya Disease: A Statistical Analysis of Arterial Spin-Labeling MRI. AJNR Am. J. Neuroradiol. 2016, 37, 706–712. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.K.; Park, E.K.; Kim, J.; Kang, H.-C.; Kim, D.-S.; Shim, K.-W. The feasibility of performing multiple burr hole surgery in pediatric moyamoya patients as a response to failed mEDAS. Child’s Nerv. Syst. 2021, 37, 2233–2238. [Google Scholar] [CrossRef]
- Goren, O.; Hendrix, P.; Peled, A.; Kimchi, G.; Zauberman, J.; Griessenauer, C.; Feldman, Z. Encephaloduroarteriosynangiosis with Dural Inversion for Moyamoya Disease in a Pediatric and Adult Population-a Single-Center 20-Year Experience. World Neurosurg. 2021, 149, e16–e21. [Google Scholar] [CrossRef]
- Mukerji, N.; Steinberg, G.K. Burr holes for Moyamoya. World Neurosurg. 2014, 81, 29–31. [Google Scholar] [CrossRef]
- Fung, L.-W.E.; Thompson, D.; Ganesan, V. Revascularisation surgery for paediatric moyamoya: A review of the literature. Child’s Nerv. Syst. 2005, 21, 358–364. [Google Scholar] [CrossRef]
- Scott, R.M.; Smith, J.L.; Robertson, R.L.; Madsen, J.R.; Soriano, S.G.; Rockoff, M.A. Long-term outcome in children with moyamoya syndrome after cranial revascularization by pial synangiosis. J. Neurosurg. 2004, 100, 142–149. [Google Scholar] [CrossRef]
- Kim, S.-K.; Seol, H.J.; Cho, B.-K.; Hwang, Y.-S.; Lee, D.S.; Wang, K.-C. Moyamoya disease among young patients: Its aggressive clinical course and the role of active surgical treatment. Neurosurgery 2004, 54, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, D.; Wang, S.; Zhao, Y.; Teo, M.; Wang, R.; Cao, Y.; Ye, X.; Kang, S.; Zhao, J.-Z. Clinical features and long-term outcomes of moyamoya disease: A single-center experience with 528 cases in China. J. Neurosurg. 2015, 122, 392–399. [Google Scholar] [CrossRef]
- Abla, A.A.; Gandhoke, G.; Clark, J.C.; Oppenlander, M.E.; Velat, G.J.; Zabramski, J.M.; Albuquerque, F.C.; Nakaji, P.; Spetzler, R.F.; Wanebo, J.E. Surgical outcomes for moyamoya angiopathy at barrow neurological institute with comparison of adult indirect encephaloduroarteriosynangiosis bypass, adult direct superficial temporal artery-to-middle cerebral artery bypass, and pediatric bypass: 154 reva. Neurosurgery 2013, 73, 430–439. [Google Scholar] [CrossRef]
- Uchino, H.; Kuroda, S.; Hirata, K.; Shiga, T.; Houkin, K.; Tamaki, N. Predictors and clinical features of postoperative hyperperfusion after surgical revascularization for moyamoya disease: A serial single photon emission CT/positron emission tomography study. Stroke 2012, 43, 2610–2616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujimura, M.; Mugikura, S.; Kaneta, T.; Shimizu, H.; Tominaga, T. Incidence and risk factors for symptomatic cerebral hyperperfusion after superficial temporal artery-middle cerebral artery anastomosis in patients with moyamoya disease. Surg. Neurol. 2009, 71, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.-K.; Oh, H.; Lee, H.-C.; Cho, W.-S.; Kim, J.E.; Park, J.W.; Choi, H.; Park, H.-P. Effect of Sevoflurane Postconditioning on the Incidence of Symptomatic Cerebral Hyperperfusion After Revascularization Surgery in Adult Patients with Moyamoya Disease. World Neurosurg. 2020, 134, e991–e1000. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.G.; Luo, Q.; Jia, J.B.; Yu, J.L. Cerebral hyperperfusion syndrome after revascularization surgery in patients with moyamoya disease. Br. J. Neurosurg. 2013, 27, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, M.; Inoue, T.; Shimizu, H.; Saito, A.; Mugikura, S.; Tominaga, T. Efficacy of prophylactic blood pressure lowering according to a standardized postoperative management protocol to prevent symptomatic cerebral hyperperfusion after direct revascularization surgery for moyamoya disease. Cerebrovasc. Dis. 2012, 33, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Kronenburg, A.; van den Berg, E.; van Schooneveld, M.M.; Braun, K.P.J.; Calviere, L.; van der Zwan, A.; Klijn, C.J.M. Cognitive Functions in Children and Adults with Moyamoya Vasculopathy: A Systematic Review and Meta-Analysis. J. Stroke 2018, 20, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Funaki, T.; Takahashi, J.C.; Miyamoto, S. Late Cerebrovascular Events and Social Outcome after Adolescence: Long-term Outcome of Pediatric Moyamoya Disease. Neurol. Med. Chir. 2018, 58, 240–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phi, J.H.; Wang, K.-C.; Cho, B.-K.; Lee, M.S.; Lee, J.-H.; Yu, K.-S.; Hahm, B.-J.; Kim, S.-K. Long-term social outcome in children with moyamoya disease who have reached adulthood. J. Neurosurg. Pediatr. 2011, 8, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Ando, T.; Shimada, Y.; Fujiwara, S.; Yoshida, K.; Kobayashi, M.; Kubo, Y.; Terasaki, K.; Ando, S.; Ogasawara, K. Revascularisation surgery improves cognition in adult patients with moyamoya disease. J. Neurol. Neurosurg. Psychiatry 2020, 91, 332–334. [Google Scholar] [CrossRef] [PubMed]
- Zeifert, P.D.; Karzmark, P.; Bell-Stephens, T.E.; Steinberg, G.K.; Dorfman, L.J. Neurocognitive Performance After Cerebral Revascularization in Adult Moyamoya Disease. Stroke 2017, 48, 1514–1517. [Google Scholar] [CrossRef]
- Hara, S.; Kudo, T.; Hayashi, S.; Inaji, M.; Tanaka, Y.; Maehara, T.; Ishii, K.; Nariai, T. Improvement in cognitive decline after indirect bypass surgery in adult moyamoya disease: Implication of (15)O-gas positron emission tomography. Ann. Nucl. Med. 2020, 34, 467–475. [Google Scholar] [CrossRef]
- Ha, E.J.; Kim, K.H.; Wang, K.-C.; Phi, J.H.; Lee, J.Y.; Choi, J.W.; Cho, B.-K.; Yang, J.; Byun, Y.H.; Kim, S.-K. Long-Term Outcomes of Indirect Bypass for 629 Children With Moyamoya Disease: Longitudinal and Cross-Sectional Analysis. Stroke 2019, 50, 3177–3183. [Google Scholar] [CrossRef]
- Abhinav, K.; Furtado, S.V.; Nielsen, T.H.; Iyer, A.; Gooderham, P.A.; Teo, M.; Lee, J.; Han, S.S.; Steinberg, G.K. Functional Outcomes After Revascularization Procedures in Patients With Hemorrhagic Moyamoya Disease. Neurosurgery 2020, 86, 257–265. [Google Scholar] [CrossRef]
- Li, J.; Ge, P.; Zhang, Q.; Lin, F.; Wang, R.; Zhang, Y.; Zhang, D.; Wang, W.; Zhao, J. Hyperhomocysteinemia is a risk factor for postoperative ischemia in adult patients with moyamoya disease. Neurosurg. Rev. 2021. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Chen, X.; Yu, J.; Li, X.-Q. Risk factors for postoperative stroke in adults patients with moyamoya disease: A systematic review with meta-analysis. BMC Neurol. 2019, 19, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teo, M.; Furtado, S.; Kaneko, O.F.; Azad, T.D.; Madhugiri, V.; Do, H.M.; Steinberg, G.K. Validation and Application for the Berlin Grading System of Moyamoya Disease in Adult Patients. Neurosurgery 2020, 86, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-W.; Han, C.; Zhao, F.; Qiao, P.-G.; Wang, H.; Bao, X.-Y.; Zhang, Z.-S.; Yang, W.-Z.; Li, D.-S.; Duan, L. Collateral Circulation in Moyamoya Disease: A New Grading System. Stroke 2019, 50, 2708–2715. [Google Scholar] [CrossRef] [PubMed]
- Hervé, D.; Kossorotoff, M.; Bresson, D.; Blauwblomme, T.; Carneiro, M.; Touze, E.; Proust, F.; Desguerre, I.; Alamowitch, S.; Bleton, J.-P.; et al. French clinical practice guidelines for Moyamoya angiopathy. Rev. Neurol. 2018, 174, 292–303. [Google Scholar] [CrossRef]


| Stage I | Narrowing of Terminal ICA |
| Stage II | Initiation of moyamoya vessels in basal carotid circulation, dilation of intracerebral arteries |
| Stage III | Intensification of moyamoya vessels, severe carotid stenosis, defection of ACA and MCA |
| Stage IV | Minimization of moyamoya vessels, defection of PCA |
| Stage V | Further reduction of moyamoya, disappearance of major cerebral arteries |
| Stage VI | Disappearance of moyamoya collaterals and ICA, cerebral blood supply comes from external carotid arteries via leptomeningeal anastomoses |
| Houkin’s Score | Houkin’s Grading | |||
|---|---|---|---|---|
| Main Artery | Findings | Score | Score | Grade |
| ICA | Normal | 0 | 0–1 | 1 |
| C1 stenosis | 1 | |||
| Discontinuity of C1 signal | 2 | |||
| Invisible | 3 | |||
| MCA | Normal | 0 | 2–4 | 2 |
| M1 stenosis | 1 | |||
| Discontinuity of M1 signal | 2 | |||
| Invisible | 3 | |||
| ACA | Normal A2 and its distal signal | 0 | 5–7 | 3 |
| A2 and its distal signal decrease or loss | 1 | |||
| Invisible | 2 | |||
| PCA | Normal P2 and its distal signal | 0 | 8–10 | 4 |
| P2 and its distal signal decrease or loss | 1 | |||
| Invisible | 2 | |||
| Technique Focus | Advantages (A)/Disadvantages (D) |
|---|---|
| Brain CT Brain parenchyma damage | A: Easily accessible in the acute phase; Short acquisition time D: Poor spatial resolution; No information about vessels |
| CT Angiography Vessel imaging | A: Non-invasive technique; Good spatial resolution; Short acquisition time; Widely available D: Radioexposition; Contrast administration; Poor temporal resolution (without dynamic acquisition) |
| CT Perfusion Cerebral perfusion | A: Good temporal and spatial resolution; Short acquisition time; Acetazolamide can also be used to assess the CerebroVascular reactivity D: Whole-brain perfusion technology not widely available; Radioexposition; Potential underestimation of CBF in patients with EC-IC collaterals |
| Brain MRI Brain parenchyma damage | A: Non-invasive; Very good tissue and spatial resolution D: Magnetic field limitations; Claustrophobia; Long acquisition time |
| DSC-MRI Cerebral perfusion | A: Non-invasive; No exposure to ionizing radiation D: Requires contrast administration; Not fully standardized; Extensive collaterals can prolong arterial transit delays (causing inaccurate assessment of perfusion) |
| ASL-MRI Cerebral perfusion | A: Non-invasive; No exposure to ionizing radiation or contrast administration; Easy assessment and performance on children D: Not fully standardized; Extensive collaterals can prolong arterial transit delays (causing inaccurate assessment of perfusion); Drug challenge (acetazolamide) with potential side effects |
| MR Angiography Vessel imaging | A: Non-invasive; No contrast administration; Good spatial resolution in first and second-degree branches D: Relatively long acquisition time; Motion artifacts |
| Vessel Wall Imaging Vessel wall inflammation or remodeling | A: Differential diagnosis from other steno-occlusive diseases (vasculitides, atherosclerosis, dissections, etc.) D: Not validated for follow-up |
| DSA Vessel imaging | A: High spatial resolution; High temporal resolution with hemodynamic evaluation; Gold standard for vessel disease D: Invasive; Contrast administration |
| Transcranial (Color-Coded) Duplex Ultrasound Hemodynamicscerebrovascular reactivity and reserve | A: Non-invasive; Bedside executable; Repeatable; Low cost D: Dependent on acoustic window quality; Diagnostic and grading criteria are not validated in MMA; Operator-dependent |
| 15O-PET Cerebral hemodynamic statuscerebrovascular reactivity and reserve (15O-water PET) | A: Non-invasive; Quantitative measurement of hemodynamic impairment; Useful for follow-up D: Long acquisition time; Not widely accessible; Highly expensive; Radioexposition |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canavero, I.; Vetrano, I.G.; Zedde, M.; Pascarella, R.; Gatti, L.; Acerbi, F.; Nava, S.; Ferroli, P.; Parati, E.A.; Bersano, A. Clinical Management of Moyamoya Patients. J. Clin. Med. 2021, 10, 3628. https://doi.org/10.3390/jcm10163628
Canavero I, Vetrano IG, Zedde M, Pascarella R, Gatti L, Acerbi F, Nava S, Ferroli P, Parati EA, Bersano A. Clinical Management of Moyamoya Patients. Journal of Clinical Medicine. 2021; 10(16):3628. https://doi.org/10.3390/jcm10163628
Chicago/Turabian StyleCanavero, Isabella, Ignazio Gaspare Vetrano, Marialuisa Zedde, Rosario Pascarella, Laura Gatti, Francesco Acerbi, Sara Nava, Paolo Ferroli, Eugenio Agostino Parati, and Anna Bersano. 2021. "Clinical Management of Moyamoya Patients" Journal of Clinical Medicine 10, no. 16: 3628. https://doi.org/10.3390/jcm10163628
APA StyleCanavero, I., Vetrano, I. G., Zedde, M., Pascarella, R., Gatti, L., Acerbi, F., Nava, S., Ferroli, P., Parati, E. A., & Bersano, A. (2021). Clinical Management of Moyamoya Patients. Journal of Clinical Medicine, 10(16), 3628. https://doi.org/10.3390/jcm10163628









