Usefulness of Easy-to-Use Risk Scoring Systems Rated in the Emergency Department to Predict Major Adverse Outcomes in Hospitalized COVID-19 Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Definition of the Risk Scoring Systems Assessed
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 14 June 2021).
- Henry, B.M.; de Oliveira, M.H.S.; Benoit, S.; Plebani, M.; Lippi, G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): A meta-analysis. Clin. Chem. Lab. Med. 2020, 58, 1021–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Soto-Mota, A.; Marfil-Garza, B.A.; Martínez Rodríguez, E.; Barreto Rodríguez, J.O.; López Romo, A.E.; Alberti Minutti, P.; Alejandre Loya, J.V.; Pérez Talavera, F.E.; Ávila Cervera, F.J.; Velazquez Burciaga, A.; et al. The low-harm score for predicting mortality in patients diagnosed with COVID-19: A multicentric validation study. J. Am. Coll. Emerg. Physicians. Open 2020, 1, 1436–1443. [Google Scholar] [CrossRef]
- Ji, D.; Zhang, D.; Xu, J.; Chen, Z.; Yang, T.; Zhao, P.; Chen, G.; Cheng, G.; Wang, Y.; Bi, J.; et al. Prediction for Progression Risk in Patients with COVID-19 Pneumonia: The CALL Score. Clin. Infect. Dis. 2020, 71, 1393–1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bello-Chavolla, O.Y.; Bahena-López, J.P.; Antonio-Villa, N.E.; Vargas-Vázquez, A.; González-Díaz, A.; Márquez-Salinas, A.; Fermín-Martínez, C.A.; Naveja, J.J.; Aguilar-Salinas, C.A. Predicting Mortality Due to SARS-CoV-2: A Mechanistic Score Relating Obesity and Diabetes to COVID-19 Outcomes in Mexico. J. Clin. Endocrinol. Metab. 2020, 105, 2752–2761. [Google Scholar] [CrossRef] [PubMed]
- Mancilla-Galindo, J.; Vera-Zertuche, J.M.; Navarro-Cruz, A.R.; Segura-Badilla, O.; Reyes-Velázquez, G.; Tepepa-López, F.J.; Aguilar-Alonso, P.; Vidal-Mayo, J.J.; Kammar-García, A. Development and Validation of the Patient History COVID-19 (PH-Covid19) Scoring System: A Multivariable Prediction Model of Death in Mexican Patients with COVID-19. Epidemiol. Infect. 2020, 148, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- González-Pacheco, H.; Bojalil, R.; Amezcua-Guerra, L.M.; Sandoval, J.; Eid-Lidt, G.; Arias-Mendoza, A.; Azar-Manzur, F.; Álvarez-Sangabriel, A.; Altamirano-Castillo, A.; Briseño-Cruz, J.L.; et al. Derivation and validation of a simple inflammation-based risk score system for predicting in-hospital mortality in acute coronary syndrome patients. J. Cardiol. 2019, 73, 416–424. [Google Scholar] [CrossRef]
- Zahorec, R. Ratio of neutrophil to lymphocyte counts--Rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl. Lek. Listy. 2001, 102, 5–14. [Google Scholar]
- Fardet, L.; Galicier, L.; Lambotte, O.; Marzac, C.; Aumont, C.; Chahwan, D.; Coppo, P.; Hejblum, G. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014, 66, 2613–2620. [Google Scholar] [CrossRef]
- World Health Organization. COVID-19: Surveillance, Case Investigation and Epidemiological Protocols, 16 December 2020. World Health Organization. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Surveillance_Case_Definition-2020.2 (accessed on 10 April 2021).
- World Health Organization. Clinical Management of Severe Acute Respiratory Infection (SARI) When COVID-19 Disease is Suspected: Interim Guidance, 13 March 2020. World Health Organization. Available online: https://apps.who.int/iris/handle/10665/331446 (accessed on 2 May 2021).
- Tuty Kuswardhani, R.A.; Henrina, J.; Pranata, R.; Anthonius Lim, M.; Lawrensia, S.; Suastika, K. Charlson comorbidity index and a composite of poor outcomes in COVID-19 patients: A systematic review and meta-analysis. Diabetes. Metab. Syndr. 2020, 14, 2103–2109. [Google Scholar] [CrossRef]
- Amezcua-Guerra, L.M.; Audelo, K.; Guzmán, J.; Santiago, D.; González-Flores, J.; García-Ávila, C.; Torres, Z.; Baranda-Tovar, F.; Tavera-Alonso, C.; Sandoval, J.; et al. A simple and readily available inflammation-based risk scoring system on admission predicts the need for mechanical ventilation in patients with COVID-19. Inflamm. Res. 2021, 70, 731–742. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Xiang, P.; Pu, L.; Xiong, H.; Li, C.; Zhang, M.; Tan, J.; Xu, Y.; Song, R.; et al. Neutrophil-to-lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. J. Transl. Med. 2020, 18, 206. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. HLH across speciality collaboration, UK. COVID-19: Consider cytokine storm syndromes and immunosuppres.sion. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Daryabor, G.; Atashzar, M.R.; Kabelitz, D.; Meri, S.; Kalantar, K. The Effects of Type 2 Diabetes Mellitus on Organ Metabolism and the Immune System. Front. Immunol. 2020, 11, 1582. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Greco, M.; Zanella, A.; Albano, G.; Antonelli, M.; Bellani, G.; Bonanomi, E.; Cabrini, L.; Carlesso, E.; Castelli, G.; et al. Risk Factors Associated with Mortality among Patients with COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern. Med. 2020, 180, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
- Cariou, B.; Hadjadj, S.; Wargny, M.; Pichelin, M.; Al-Salameh, A.; Allix, I.; Amadou, C.; Arnault, G.; Baudoux, F.; Bauduceau, B.; et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: The CORONADO study. Diabetologia 2020, 63, 1500–1515. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, Z.; Wang, S.; Ye, Y.; Luo, D.; Wan, L.; Yu, A.; Sun, L.; Tesfaye, S.; Meng, Q.; et al. The impact of type 2 diabetes and its management on the prognosis of patients with severe COVID-19. J. Diabetes 2020, 12, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Yang, Y.; Wang, F.; Ren, H.; Zhang, S.; Shi, X.; Yu, X.; Dong, K. Clinical characteristics and outcomes of patients with severe COVID-19 with diabetes. BMJ Open. Diabetes Res. Care 2020, 8, e001343. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, D.; Cheng, B.; Chen, J.; Peng, A.; Yang, C.; Liu, C.; Xiong, M.; Deng, A.; Zhang, Y.; et al. Clinical Characteristics and Outcomes of Patients with Diabetes and COVID-19 in Association with Glucose-Lowering Medication. Diabetes Care 2020, 43, 1399–1407. [Google Scholar] [CrossRef]
- Hui, Y.; Li, Y.; Tong, X.; Wang, Z.; Mao, X.; Huang, L.; Zhang, D. The risk factors for mortality of diabetic patients with severe COVID-19: A retrospective study of 167 severe COVID-19 cases in Wuhan. PLoS ONE 2020, 15, e0243602. [Google Scholar] [CrossRef] [PubMed]
- Karczewski, J.; Śledzińska, E.; Baturo, A.; Jończyk, I.; Maleszko, A.; Samborski, P.; Begier-Krasińska, B.; Dobrowolska, A. Obesity and inflammation. Eur. Cytokine Netw. 2018, 29, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Amezcua-Guerra, L.M. Brief annotations on cytokine release syndrome and interleukin-6 therapeutic blockage in SARS-CoV-2/COVID-19. Arch. Cardiol. Mex. 2020, 90, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Simonnet, A.; Chetboun, M.; Poissy, J.; Raverdy, V.; Noulette, J.; Duhamel, A.; Labreuche, J.; Mathieu, D.; Pattou, F.; Jourdain, M. High Prevalence of Obesity in Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) Requiring Invasive Mechanical Ventilation. Obesity 2020, 28, 1195–1199. [Google Scholar] [CrossRef]
- Földi, M.; Farkas, N.; Kiss, S.; Zádori, N.; Váncsa, S.; Szakó, L.; Dembrovszky, F.; Solymár, M.; Bartalis, E.; Szakács, Z.; et al. Obesity is a risk factor for developing critical condition in COVID-19 patients: A systematic review and meta-analysis. Obes. Rev. 2020, 21, e13095. [Google Scholar] [CrossRef]
- Petrilli, C.M.; Jones, S.A.; Yang, J.; Rajagopalan, H.; O’Donnell, L.; Chernyak, Y.; Tobin, K.A.; Cerfolio, R.J.; Francois, F.; Horwitz, L.I. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: Prospective cohort study. BMJ 2020, 369, m1966. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.L.; Vittinghoff, E.; Olgin, J.E.; Pletcher, M.J.; Marcus, G.M. Body Weight Changes During Pandemic-Related Shelter-in-Place in a Longitudinal Cohort Study. JAMA Netw. Open 2021, 4, e212536. [Google Scholar] [CrossRef]
- Du, P.; Li, D.; Wang, A.; Shen, S.; Ma, Z.; Li, X. A Systematic Review and Meta-Analysis of Risk Factors Associated with Severity and Death in COVID-19 Patients. Can. J. Infect. Dis. Med. Microbiol. 2021, 2021, 6660930. [Google Scholar] [CrossRef]
- Piroth, L.; Cottenet, J.; Mariet, A.S.; Bonniaud, P.; Blot, M.; Tubert-Bitter, P.; Quantin, C. Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: A nationwide, population-based retrospective cohort study. Lancet Respir. Med. 2021, 9, 251–259. [Google Scholar] [CrossRef]
- Savla, S.R.; Prabhavalkar, K.S.; Bhatt, L.K. Cytokine storm associated coagulation complications in COVID-19 patients: Pathogenesis and Management. Expert. Rev. Anti. Infect. Ther. 2021, 1–17. [Google Scholar] [CrossRef]
- Ierardi, A.M.; Gaibazzi, N.; Tuttolomondo, D.; Fusco, S.; La Mura, V.; Peyvandi, F.; Aliberti, S.; Blasi, F.; Cozzi, D.; Carrafiello, G.; et al. Deep vein thrombosis in COVID-19 patients in general wards: Prevalence and association with clinical and laboratory variables. Radiol. Med. 2021, 126, 722–728. [Google Scholar] [CrossRef] [PubMed]
- García-Ortega, A.; Oscullo, G.; Calvillo, P.; López-Reyes, R.; Méndez, R.; Gómez-Olivas, J.D.; Bekki, A.; Fonfría, C.; Trilles-Olaso, L.; Zaldívar, E.; et al. Incidence, risk factors, and thrombotic load of pulmonary embolism in patients hospitalized for COVID-19 infection. J. Infect. 2021, 82, 261–269. [Google Scholar] [CrossRef]
- Dujardin, R.W.G.; Hilderink, B.N.; Haksteen, W.E.; Middeldorp, S.; Vlaar, A.P.J.; Thachil, J.; Müller, M.C.A.; Juffermans, N.P. Biomarkers for the prediction of venous thromboembolism in critically ill COVID-19 patients. Thromb. Res. 2020, 196, 308–312. [Google Scholar] [CrossRef]
- Al-Samkari, H.; Karp Leaf, R.S.; Dzik, W.H.; Carlson, J.C.T.; Fogerty, A.E.; Waheed, A.; Goodarzi, K.; Bendapudi, P.K.; Bornikova, L.; Gupta, S.; et al. COVID-19 and coagulation: Bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood 2020, 136, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Violi, F.; Ceccarelli, G.; Cangemi, R.; Alessandri, F.; D’Ettorre, G.; Oliva, A.; Pastori, D.; Loffredo, L.; Pignatelli, P.; Ruberto, F.; et al. Hypoalbuminemia, coagulopathy, and vascular disease in COVID-19. Circ. Res. 2020, 127, 400–401. [Google Scholar] [CrossRef] [PubMed]
- Violi, F.; Ceccarelli, G.; Loffredo, L.; Alessandri, F.; Cipollone, F.; D’ardes, D.; D’Ettorre, G.; Pignatelli, P.; Venditti, M.; Mastroianni, C.M.; et al. Albumin Supplementation Dampens Hypercoagulability in COVID-19: A Preliminary Report. Thromb. Haemost. 2021, 1, 102–105. [Google Scholar] [CrossRef]
- Loomba, R.S.; Aggarwal, G.; Villarreal, E.G.; Farias, J.S.; Flores, S.; Lavie, C.J.; Aggarwal, S. Factors associated with deep venous thrombosis in patients infected with coronavirus disease 2019: A meta-analysis. Blood Coagul. Fibrinolysis 2021, 32, 23–28. [Google Scholar] [CrossRef]
- Middleton, E.A.; He, X.Y.; Denorme, F.; Campbell, R.A.; Ng, D.; Salvatore, S.P.; Mostyka, M.; Baxter-Stoltzfus, A.; Borczuk, A.C.; Loda, M.; et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood 2020, 10, 1169–1179. [Google Scholar] [CrossRef]
- Skendros, P.; Mitsios, A.; Chrysanthopoulou, A.; Mastellos, D.C.; Metallidis, S.; Rafailidis, P.; Ntinopoulou, M.; Sertaridou, E.; Tsironidou, V.; Tsigalou, C.; et al. Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J. Clin. Invest. 2020, 130, 6151–6157. [Google Scholar] [CrossRef]
- Ng, H.; Havervall, S.; Rosell, A.; Aguilera, K.; Parv, K.; von Meijenfeldt, F.A.; Lisman, T.; Mackman, N.; Thålin, C.; Phillipson, M. Circulating Markers of Neutrophil Extracellular Traps Are of Prognostic Value in Patients With COVID-19. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 988–994. [Google Scholar] [CrossRef]
- Amezcua-Guerra, L.M.; Rojas-Velasco, G.; Brianza-Padilla, M.; Vázquez-Rangel, A.; Márquez-Velasco, R.; Baranda-Tovar, F.; Springall, R.; Gonzalez-Pacheco, H.; Juárez-Vicuña, Y.; Tavera-Alonso, C.; et al. Presence of antiphospholipid antibodies in COVID-19: Case series study. Ann. Rheum. Dis. 2021, 80, e73. [Google Scholar] [CrossRef]
- Castillo-Martínez, D.; Torres, Z.; Amezcua-Guerra, L.M.; Pineda, C. Are antiphospholipid antibodies just a common epiphenomenon or are they causative of immune-mediated coagulopathy in COVID-19? Clin. Rheumatol. 2021, 7, 3015–3019. [Google Scholar] [CrossRef] [PubMed]
- Mora-Ramírez, M.; Estevez-Garcia, I.O.; Irigoyen-Camacho, M.E.; Bojalil, R.; Gonzalez-Pacheco, H.; Amezcua-Guerra, L.M. Hyperuricemia on Admission Predicts Short-Term Mortality due to Myocardial Infarction in a Population with High Prevalence of Cardiovascular Risk Factors. Rev. Invest. Clin. 2017, 69, 247–253. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Noncommunicable Diseases, 13 April 2021. World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 5 May 2021).
- Clark, K.E.N.; Nevin, W.D.; Mahungu, T.; Lachmann, H.; Singh, A. Assessment of the Haemophagocytic lymphohistiocytosis HScore in patients with COVID-19. Clin. Infect. Dis. 2020, ciaa1463. [Google Scholar] [CrossRef] [PubMed]
| COVID-19 Patients (n = 157) | |
|---|---|
| Age in years, mean ± SD | 55 ± 12 |
| Male sex, n (%) | 105 (66.3) |
| Body mass index ≥ 30 kg/m2, n (%) | 46 (29.2) |
| Current smoking, n (%) | 30 (19.1) |
| Coexisting conditions, n (%) | |
| Diabetes mellitus | 57 (36.3) |
| Hypertension | 73 (46.4) |
| Dyslipidemia | 21 (13.3) |
| Coronary artery disease | 14 (8.9) |
| Stroke | 5 (3.1) |
| Chronic heart failure | 9 (5.7) |
| Chronic kidney disease | 19 (12.1) |
| Chronic obstructive pulmonary disease | 7 (4.4) |
| Autoimmune diseases | 9 (5.7) |
| Organ transplant | 7 (4.4) |
| Cancer | 4 (2.5) |
| Charlson comorbidity index, median (IQR) | 2 (1 to 4) |
| COVID-19 Patients (n = 157) | |
|---|---|
| Days of symptom onset, median (IQR) | 7.0 (4.7 to 9.0) |
| Clinical data | |
| Temperature > 37.3 °C, n (%) | 57 (36.3) |
| Respiratory rate, breaths/min | 26.0 ± 11.8 |
| Heart rate, beats/min | 96.2 ± 19.8 |
| Systolic blood pressure, mmHg | 124.7 ± 21.0 |
| Diastolic blood pressure, mmHg | 75.9 ± 13.5 |
| Oxygen saturation at room air, % | 79.5 ± 13.1 |
| Classified as severe COVID-19, n (%) | 125 (79.6) |
| Laboratory values | |
| White cell count (×103 per mm3), median (IQR) | 8.9 (6.1 to 12.3) |
| Neutrophils (×103 per mm3), median (IQR) | 7.7 (4.8 to 11.0) |
| Lymphocytes (×103 per mm3), median (IQR) | 0.8 (0.6 to 1.1) |
| Platelets (×103 per mm3), median (IQR) | 207 (164 to 275) |
| Hemoglobin, g/dL, median (IQR) | 14.7 (13.2 to 16.0) |
| Albumin, g/dL, median (IQR) | 3.4 (3.1 to 3.8) |
| Serum creatinine, mg/dL, median (IQR) | 1.0 (0.8 to 1.4) |
| Troponin I, ng/mL, median (IQR) | 12.8 (6.1 to 63.8) |
| Creatine kinase, U/L, median (IQR) | 105 (49 to 199) |
| D-dimer, ng/mL, median (IQR) | 390 (228 to 666) |
| Fibrinogen, mg/dL, median (IQR) | 5.3 (4.4 to 6.1) |
| C-reactive protein, mg/L, median (IQR) | 145 (61 to 256) |
| Ferritin, μg/L, median (IQR) | 590 (270 to 1101) |
| Interleukin 6, pg/mL, median (IQR) | 14.9 (4.5 to 73.5) |
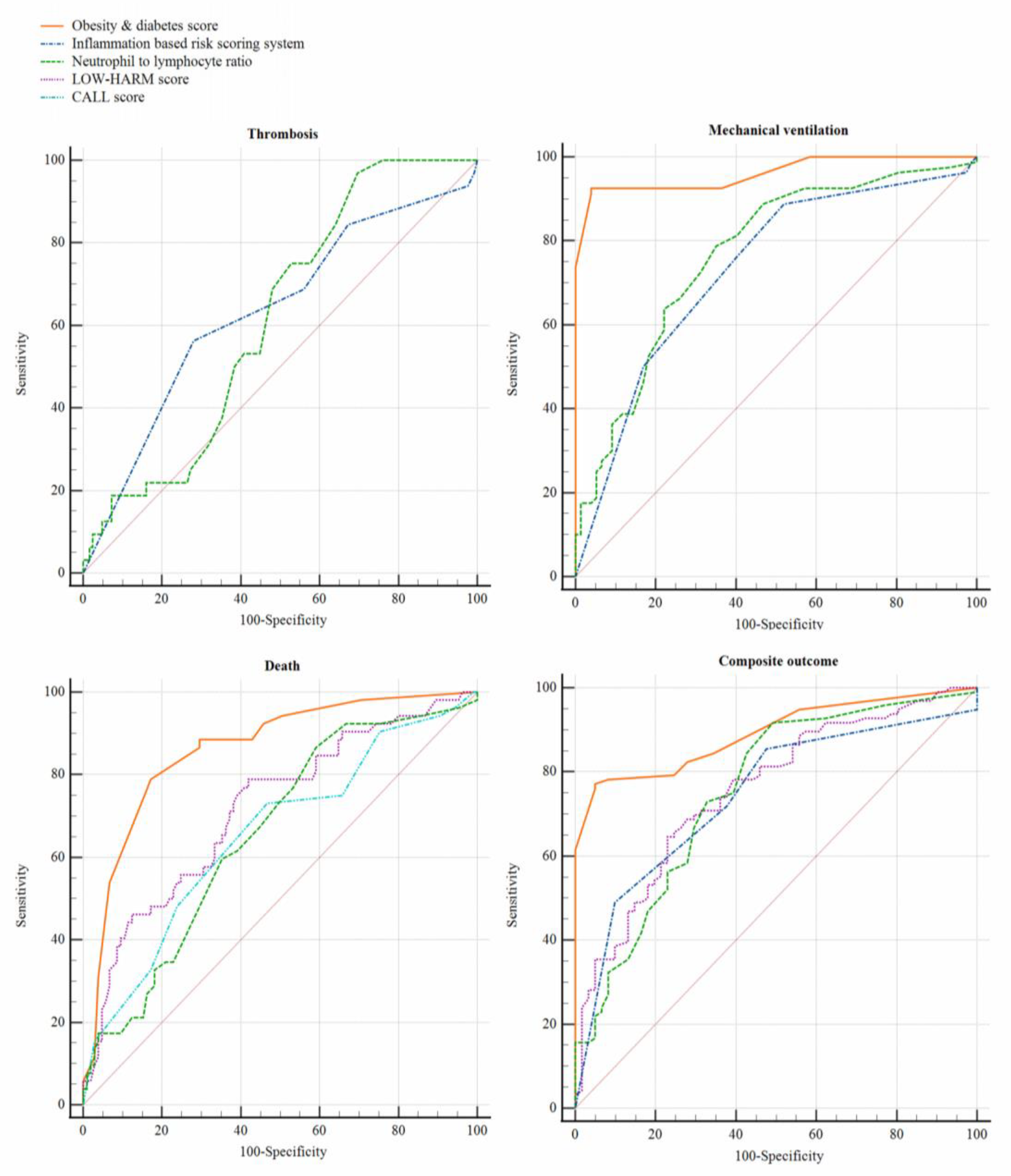
| Risk Scoring System | Thrombosis (n = 32) | Mechanical Ventilation (n = 80) | Death (n = 52) | Composite Outcome (n = 96) |
|---|---|---|---|---|
| Charlson comorbidity index | 0.52 (0.41 to 0.63) | 0.50 (0.40 to 0.59) | 0.60 (0.51 to 0.70) | 0.52 (0.43 to 0.61) |
| LOW-HARM score | 0.58 (0.47 to 0.68) | 0.72 (0.64 to 0.80) | 0.71 (0.63 to 0.80) | 0.75 (0.67 to 0.83) |
| CALL score | 0.52 (0.41 to 0.63) | 0.61 (0.52 to 0.70) | 0.65 (0.56 to 0.74) | 0.60 (0.51 to 0.69) |
| Obesity and Diabetes score | 0.59 (0.48 to 0.70) | 0.96 (0.93 to 0.99) | 0.86 (0.79 to 0.92) | 0.89 (0.84 to 0.94) |
| PH-Covid19 score | 0.52 (0.40 to 0.63) | 0.56 (0.47 to 0.65) | 0.64 (0.55 to 0.73) | 0.59 (0.50 to 0.68) |
| Inflammation-based risk scoring system | 0.63 (0.52 to 0.74) | 0.73 (0.65 to 0.81) | 0.60 (0.51 to 0.70) | 0.74 (0.66 to 0.82) |
| Neutrophil-lymphocyte ratio | 0.61 (0.51 to 0.70) | 0.76 (0.69 to 0.84) | 0.65 (0.56 to 0.74) | 0.75 (0.67 to 0.83) |
| HScore | 0.54 (0.43 to 0.64) | 0.53 (0.44 to 0.62) | 0.53 (0.43 to 0.62) | 0.55 (0.46 to 0.64) |
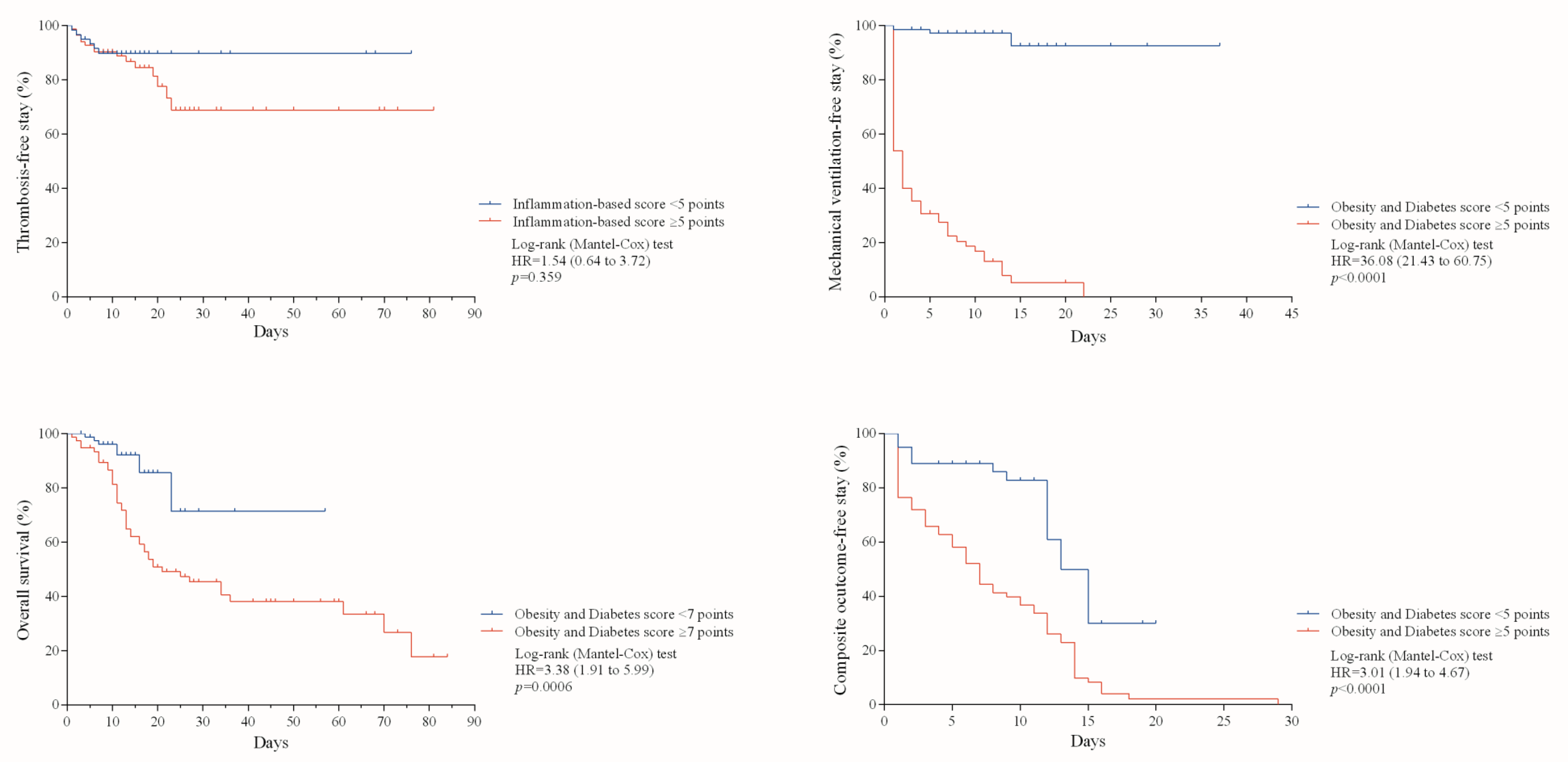
| Thrombosis | Mechanical Ventilation | Death | Composite Outcome | |
|---|---|---|---|---|
| Scoring system (optimal cutoff point by the Youden’s index) | Inflammation-based risk scoring system (≥5 points) | Obesity and Diabetes score (≥5 points) | Obesity and Diabetes score (≥7 points) | Obesity and Diabetes score (≥5 points) |
| Sensitivity | 56.2% (37.8 to 73.1) | 92.5% (83.8 to 96.9) | 78.8% (64.9 to 88.4) | 77.0% (67.1 to 84.7) |
| Specificity | 72.0% (63.1 to 79.4) | 96.1% (88.2 to 98.9) | 82.8% (73.9 to 89.2) | 95.0% (85.4 to 98.7) |
| Positive predictive value | 33.9% (21.8 to 48.3) | 96.1% (88.2 to 98.9) | 69.4% (55.9 to 80.4) | 96.1% (88.2 to 98.9) |
| Negative predictive value | 86.5% (78.1 to 92.1) | 92.5% (83.8 to 96.9) | 88.7% (80.4 to 93.9) | 72.5% (61.2 to 81.6) |
| Positive likelihood ratio | 2.0 (1.3 to 3.0) | 23.7 (7.8 to 72.1) | 4.6 (2.9 to 7.1) | 15.6 (5.1 to 47.5) |
| Negative likelihood ratio | 0.6 (0.4 to 0.9) | 0.08 (0.04 to 0.17) | 0.2 (0.1 to 0.4) | 0.2 (0.1 to 0.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Flores, J.; García-Ávila, C.; Springall, R.; Brianza-Padilla, M.; Juárez-Vicuña, Y.; Márquez-Velasco, R.; Sánchez-Muñoz, F.; Ballinas-Verdugo, M.A.; Basilio-Gálvez, E.; Castillo-Salazar, M.; et al. Usefulness of Easy-to-Use Risk Scoring Systems Rated in the Emergency Department to Predict Major Adverse Outcomes in Hospitalized COVID-19 Patients. J. Clin. Med. 2021, 10, 3657. https://doi.org/10.3390/jcm10163657
González-Flores J, García-Ávila C, Springall R, Brianza-Padilla M, Juárez-Vicuña Y, Márquez-Velasco R, Sánchez-Muñoz F, Ballinas-Verdugo MA, Basilio-Gálvez E, Castillo-Salazar M, et al. Usefulness of Easy-to-Use Risk Scoring Systems Rated in the Emergency Department to Predict Major Adverse Outcomes in Hospitalized COVID-19 Patients. Journal of Clinical Medicine. 2021; 10(16):3657. https://doi.org/10.3390/jcm10163657
Chicago/Turabian StyleGonzález-Flores, Julieta, Carlos García-Ávila, Rashidi Springall, Malinalli Brianza-Padilla, Yaneli Juárez-Vicuña, Ricardo Márquez-Velasco, Fausto Sánchez-Muñoz, Martha A. Ballinas-Verdugo, Edna Basilio-Gálvez, Mauricio Castillo-Salazar, and et al. 2021. "Usefulness of Easy-to-Use Risk Scoring Systems Rated in the Emergency Department to Predict Major Adverse Outcomes in Hospitalized COVID-19 Patients" Journal of Clinical Medicine 10, no. 16: 3657. https://doi.org/10.3390/jcm10163657
APA StyleGonzález-Flores, J., García-Ávila, C., Springall, R., Brianza-Padilla, M., Juárez-Vicuña, Y., Márquez-Velasco, R., Sánchez-Muñoz, F., Ballinas-Verdugo, M. A., Basilio-Gálvez, E., Castillo-Salazar, M., Cásarez-Alvarado, S., Hernández-Diazcouder, A., Sánchez-Gloria, J. L., Sandoval, J., González-Pacheco, H., Tavera-Alonso, C., Rojas-Velasco, G., Baranda-Tovar, F., & Amezcua-Guerra, L. M. (2021). Usefulness of Easy-to-Use Risk Scoring Systems Rated in the Emergency Department to Predict Major Adverse Outcomes in Hospitalized COVID-19 Patients. Journal of Clinical Medicine, 10(16), 3657. https://doi.org/10.3390/jcm10163657








