Laparoscopic Liver Resection Enhanced by an Intervention-Guided Fluorescence Imaging Technique Using Sodium Fluorescein
Abstract
:1. Introduction
2. Materials and Methods
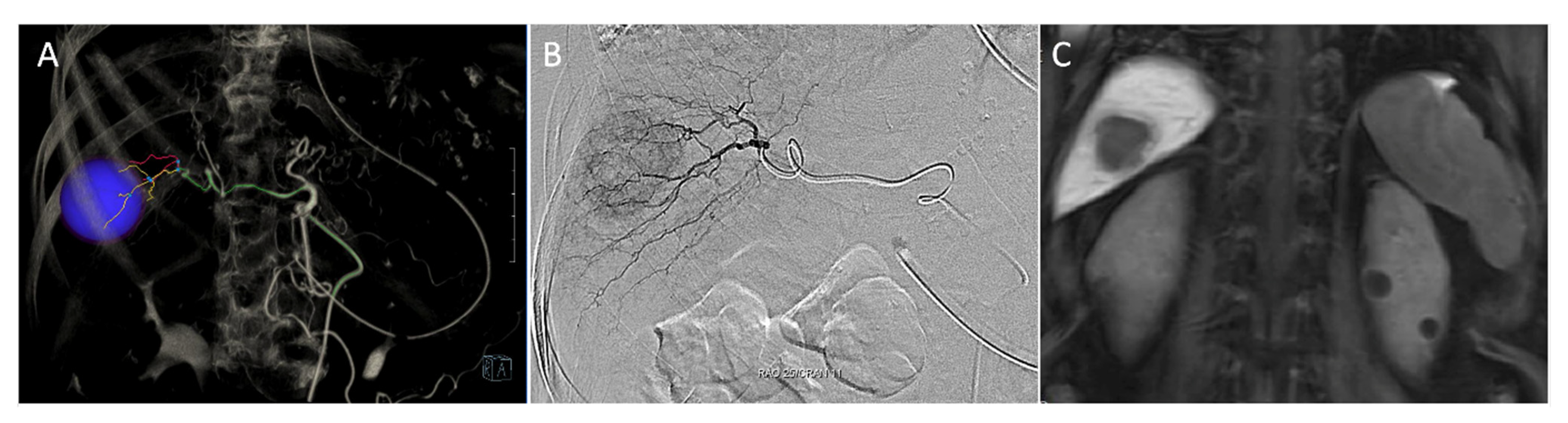
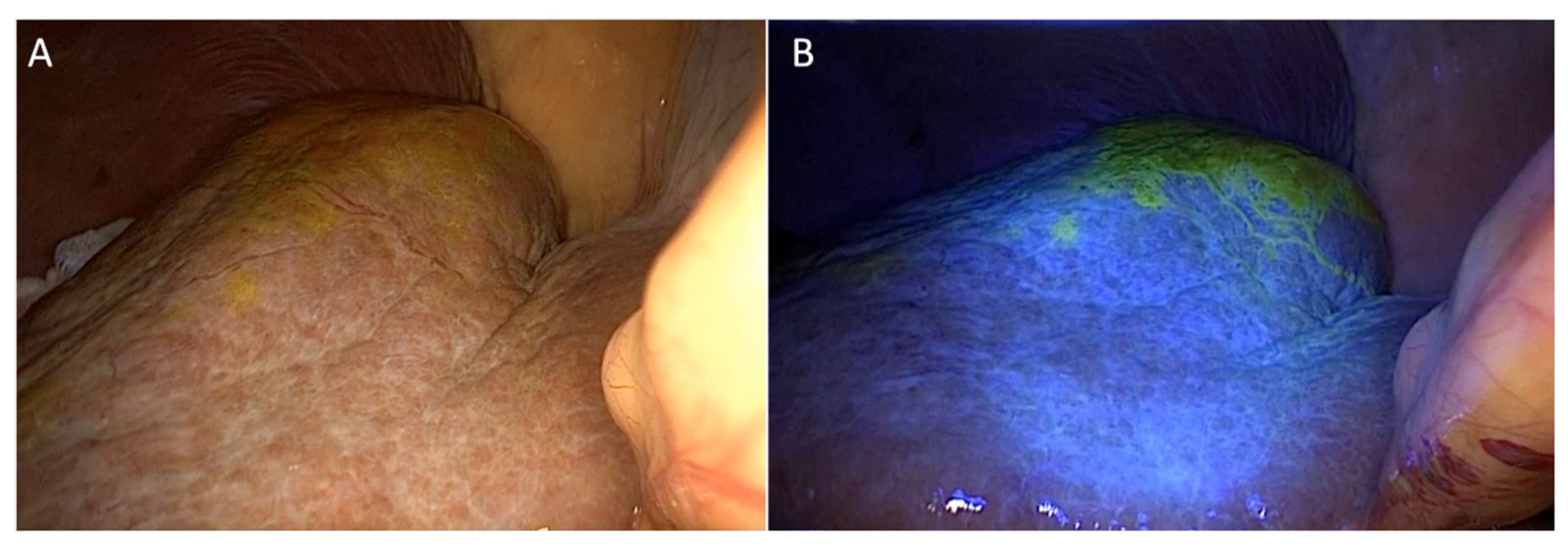
2.1. Detection of Localization under Fluorescent Imaging
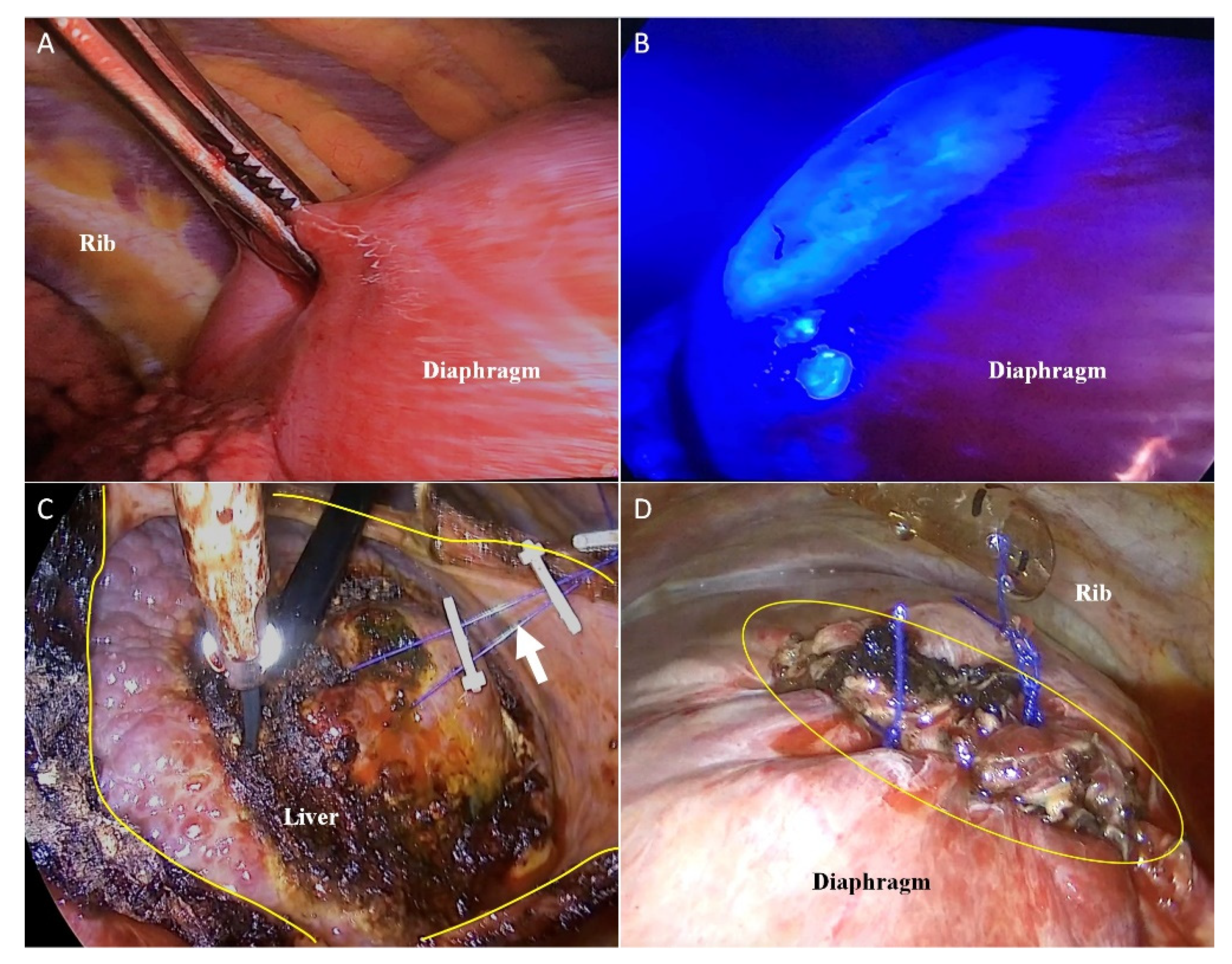
2.2. VTLR
2.3. Comparison of Clinicopathologic Outcomes with Those of Internal Controls
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alkhalili, E.; Berber, E. Laparoscopic liver resection for malignancy: A review of the literature. World J. Gastroenterol. 2014, 20, 13599–13606. [Google Scholar] [CrossRef]
- Cai, X. Laparoscopic liver resection: The current status and the future. Hepatobiliary Surg. Nutr. 2018, 7, 98–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaillard, M.; Tranchart, H.; Dagher, I. Laparoscopic liver resections for hepatocellular carcinoma: Current role and limitations. World. J. Gastroenterol. 2014, 20, 4892–4899. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, F.; Wei, Y.; Li, B. Outcomes of laparoscopic repeat liver resection for recurrent liver cancer: A system review and meta-analysis. Medicine 2019, 98, e17533. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.Y.; Kim, K.H.; Jung, D.H.; Yu, A.; Lee, S.G. Oncological and surgical results of laparoscopic versus open liver resection for HCC less than 5 cm: Case-matched analysis. Surg. Endosc. 2015, 29, 2628–2634. [Google Scholar] [CrossRef] [PubMed]
- Kruger, J.A.; Coelho, F.F.; Perini, M.V.; Herman, P. Laparoscopic transthoracic liver resection. Arq. Bras. Cir. Dig. 2014, 27, 288–290. [Google Scholar] [CrossRef] [Green Version]
- Ho, C.M.; Wakabayashi, G.; Nitta, H.; Ito, N.; Hasegawa, Y.; Takahara, T. Systematic review of robotic liver resection. Surg. Endosc. 2013, 27, 732–739. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.M.; Kwon, C.H.; Joh, J.W.; Kang, E.S.; Park, J.B.; Lee, J.H.; Kim, S.J.; Paik, S.W.; Lee, S.K.; Kim, D.W. ABO-incompatible living donor liver transplantation is suitable in patients without ABO-matched donor. J. Hepatol. 2013, 59, 1215–1222. [Google Scholar] [CrossRef]
- Sacak, B.; Tosun, U.; Egemen, O.; Sakiz, D.; Ugurlu, K. Microvascular anastomosis using fibrin glue and venous cuff in rat carotid artery. J. Plast. Surg. Hand Surg. 2015, 49, 72–76. [Google Scholar] [CrossRef]
- Ishizawa, T.; Saiura, A.; Kokudo, N. Clinical application of indocyanine green-fluorescence imaging during hepatectomy. Hepatobiliary Surg. Nutr. 2016, 5, 322–328. [Google Scholar] [CrossRef] [Green Version]
- Ueno, M.; Hayami, S.; Sonomura, T.; Tanaka, R.; Kawai, M.; Hirono, S.; Okada, K.I.; Yamaue, H. Indocyanine green fluorescence imaging techniques and interventional radiology during laparoscopic anatomical liver resection (with video). Surg. Endosc. 2018, 32, 1051–1055. [Google Scholar] [CrossRef] [PubMed]
- Mehdorn, A.S.; Beckmann, J.H.; Braun, F.; Becker, T.; Egberts, J.H. Usability of Indocyanine Green in Robot-Assisted Hepatic Surgery. J. Clin. Med. 2021, 10, 456. [Google Scholar] [CrossRef] [PubMed]
- Ishizawa, T.; Zuker, N.B.; Kokudo, N.; Gayet, B. Positive and negative staining of hepatic segments by use of fluorescent imaging techniques during laparoscopic hepatectomy. Arch. Surg. 2012, 147, 393–394. [Google Scholar] [CrossRef] [PubMed]
- Ishizawa, T.; Gumbs, A.A.; Kokudo, N.; Gayet, B. Laparoscopic segmentectomy of the liver: From segment I to VIII. Ann. Surg. 2012, 256, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Park, S.; Park, S.H.; Jung, S.W.; Choe, J.W.; Sul, J.Y.; Jang, Y.J.; Mok, Y.J.; Kim, J.H. Sentinel Node Mapping Using a Fluorescent Dye and Visible Light During Laparoscopic Gastrectomy for Early Gastric Cancer: Result of a Prospective Study From a Single Institute. Ann. Surg. 2017, 265, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.Y.; Lee, C.M.; Song, T.J.; Han, H.J.; Kim, S. A new fluorescence imaging technique for visualizing hepatobiliary structures using sodium fluorescein: Result of a preclinical study in a rat model. Surg. Endosc. 2018, 32, 2076–2083. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.W.; Lee, H.Y.; Lee, C.M.; Jung, S.P.; Kim, W.Y.; Woo, S.U.; Lee, J.B.; Son, G.S. Sentinel lymph node detection using fluorescein and blue light-emitting diodes in patients with breast carcinoma: A single-center prospective study. Asian J. Surg. 2020, 43, 220–226. [Google Scholar] [CrossRef]
- Aoki, T.; Yasuda, D.; Shimizu, Y.; Odaira, M.; Niiya, T.; Kusano, T.; Mitamura, K.; Hayashi, K.; Murai, N.; Koizumi, T.; et al. Image-guided liver mapping using fluorescence navigation system with indocyanine green for anatomical hepatic resection. World J. Surgery 2008, 32, 1763–1767. [Google Scholar] [CrossRef]
- Miyata, A.; Ishizawa, T.; Tani, K.; Shimizu, A.; Kaneko, J.; Aoki, T.; Sakamoto, Y.; Sugawara, Y.; Hasegawa, K.; Kokudo, N. Reappraisal of a Dye-Staining Technique for Anatomic Hepatectomy by the Concomitant Use of Indocyanine Green Fluorescence Imaging. J. Am. Coll. Surg. 2015, 221, E27–E36. [Google Scholar] [CrossRef]
- Inoue, Y.; Arita, J.; Sakamoto, T.; Ono, Y.; Takahashi, M.; Takahashi, Y.; Kokudo, N.; Saiura, A. Anatomical Liver Resections Guided by 3-Dimensional Parenchymal Staining Using Fusion Indocyanine Green Fluorescence Imaging. Ann. Surg. 2015, 262, 105–111. [Google Scholar] [CrossRef]
- Morise, Z.; Ciria, R.; Cherqui, D.; Chen, K.H.; Belli, G.; Wakabayashi, G. Can we expand the indications for laparoscopic liver resection? A systematic review and meta-analysis of laparoscopic liver resection for patients with hepatocellular carcinoma and chronic liver disease. J. Hepatobiliary Pancreat. Sci. 2015, 22, 342–352. [Google Scholar] [CrossRef] [PubMed]


| Patients Who Underwent Intervention-Guided Fluorescence Imaging Technique (n = 24) | |
|---|---|
| Age | 55.3 (49–63) |
| Sex ratio (Male: Female) | 2:1 |
| Liver disease | |
| Hepatitis B | 21 (87.5%) |
| Hepatitis C | 1 (4.2%) |
| Alcoholic hepatitis | 2 (8.3%) |
| ICG 15 (%) | 12.4 (8.9–15.2) |
| AFP (ng/mL) | 166 (3.2–200) |
| Platelets, ×103/mm3 | 143 (121–182) |
| INR | 1.05 (0.89–1.38) |
| Total bilirubin (mg/dl) | 1.03 (0.8–1.3) |
| Albumin (g/dL) | 3.88 (3.7–4.2) |
| CTP score | |
| A | 20 (83.3%) |
| B | 4 (16.7%) |
| Tumor location | |
| IV | 6 (6 LLR) |
| V | 5 (5 LLR) |
| VI | 4 (4 LLR) |
| VII | 5 (3 LLR, 2 VTLR) |
| VIII | 4 (2 LLR, 2 VTLR) |
| IFIT (n = 24) | Internal Controls (n = 29) | p | |
|---|---|---|---|
| Operation time (min) | 221 (143–275) | 265 (200–300) | <0.001 |
| Time to the first semi-fluid diet (days) | 2.4 (1–4) | 2.8 (1–5) | 0.222 |
| Transfusion a | 3 (13%) | 4 (13.8%) | 0.758 |
| Blood loss (cc) | 200 (10–1100) | 215 (5–1300) | 0.438 |
| Hospital stay (days) | 10.2 (6–14) | 10.0 (6–15) | 0.556 |
| Resection margin (cm) | 1.03 (0.3–2.0) | 1.01(0.2–3.0) | 0.587 |
| Tumor size | 2.73 (0.70–3.40) | 2.51 (0.5–3.5) | 0.412 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-M.; Jeong, M.-Y.; Yoon, S.-Y. Laparoscopic Liver Resection Enhanced by an Intervention-Guided Fluorescence Imaging Technique Using Sodium Fluorescein. J. Clin. Med. 2021, 10, 3663. https://doi.org/10.3390/jcm10163663
Lee C-M, Jeong M-Y, Yoon S-Y. Laparoscopic Liver Resection Enhanced by an Intervention-Guided Fluorescence Imaging Technique Using Sodium Fluorescein. Journal of Clinical Medicine. 2021; 10(16):3663. https://doi.org/10.3390/jcm10163663
Chicago/Turabian StyleLee, Chang-Min, Min-Young Jeong, and Sam-Youl Yoon. 2021. "Laparoscopic Liver Resection Enhanced by an Intervention-Guided Fluorescence Imaging Technique Using Sodium Fluorescein" Journal of Clinical Medicine 10, no. 16: 3663. https://doi.org/10.3390/jcm10163663
APA StyleLee, C.-M., Jeong, M.-Y., & Yoon, S.-Y. (2021). Laparoscopic Liver Resection Enhanced by an Intervention-Guided Fluorescence Imaging Technique Using Sodium Fluorescein. Journal of Clinical Medicine, 10(16), 3663. https://doi.org/10.3390/jcm10163663






