Application of a Global Multiparameter Scoring System for the Prenatal Prediction of Coarctation of the Aorta
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
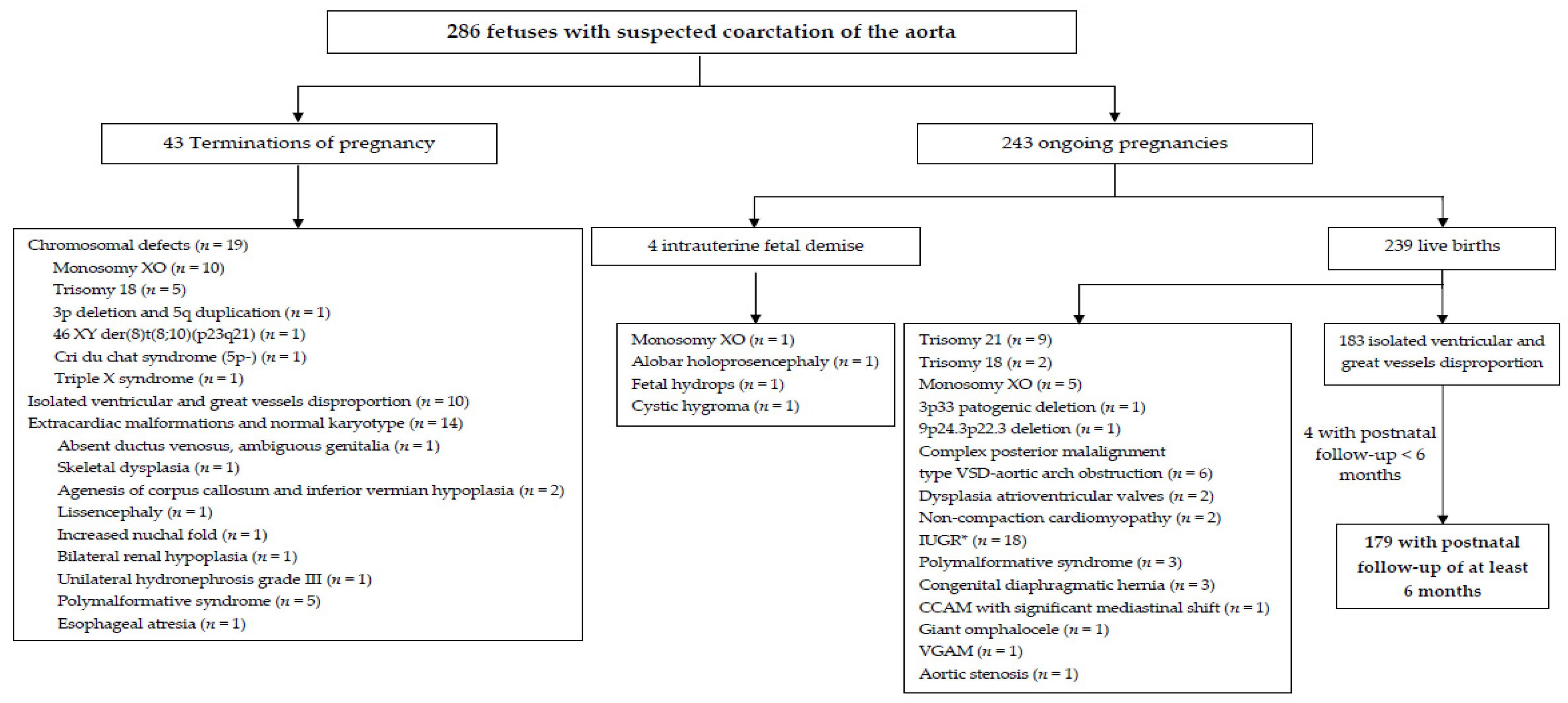
3. Results
3.1. Prenatal Prediction of CoAo at First Diagnostic Echocardiography
3.2. Prospective Application of the Global Scoring System
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharland, G.K.; Chan, K.Y.; Allan, L.D. Coarctation of the aorta: Difficulties in prenatal diagnosis. Br. Heart J. 1994, 71, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, E. Coarctation of the aorta from fetus to adult: Curable condition or life long disease process? Heart 2005, 91, 1495–1502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wren, C.; Reinhardt, Z.; Khawaja, K. Twenty-year trends in diagnosis of life-threatening neonatal cardiovascular malformations. Arch. Dis. Child. Fetal Neonatal Ed. 2008, 93, F33–F35. [Google Scholar] [CrossRef]
- Mellander, M.; Sunnegardh, J. Failure to diagnose critical heart malformation in newborns before discharge: An increasing problem? Acta Paediatr. 2006, 95, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Garne, E.; Stoll, C.; Clementi, M. Euroscan group. Evaluation of prenatal diagnosis of congenital heart diseases by ultrasound: Experience from 20 European registries. Ultrasound Obstet. Gynecol. 2001, 17, 386–391. [Google Scholar] [CrossRef]
- Galindo, A.; Herraiz, I.; Escribano, D.; Lora, D.; Melchor, J.C.; de la Cruz, J. Prenatal detection of congenital heart defects: A survey on clinical practice in Spain. Fetal Diagn. Ther. 2011, 29, 287–295. [Google Scholar] [CrossRef]
- Brown, D.L.; Durfee, S.M.; Hornberger, L.K. Ventricular discrepancy as a sonographic sign of coarctation of the fetal aorta: How reliable is it? J. Ultrasound Med. 1997, 16, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Hornberger, L.; Sahn, D.; Kleinman, C.; Copel, J.; Silverman, N. Antenatal diagnosis of coarctation of the aorta: A multicenter experience. J. Am. Coll. Cardiol. 1994, 23, 417–423. [Google Scholar] [CrossRef] [Green Version]
- Benacerraf, B.; Saltzman, D.; Sanders, S. Sonographic sign suggesting the prenatal diagnosis of coarctation of the aorta. J. Ultrasound Med. 1989, 8, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Allan, L.; Chita, S.; Anderson, R.; Fagg, N.; Crawford, D.; Tynan, M. Coarctation of the aorta in prenatal life: An echocardiographic, anatomical, and functional study. Br. Heart J. 1988, 59, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.; Won, H.; Lee, P.; Kim, A.; Park, I. Clinical implication of isolated right dominant heart in the fetus. Prenat. Diagn. 2007, 27, 695–698. [Google Scholar] [CrossRef] [PubMed]
- Kirk, J.; Comstock, C.; Lee, W.; Smith, R.; Riggs, T.; Weinhouse, E. Fetal cardiac asymmetry: A marker for congenital heart disease. Obstet. Gynecol. 1999, 93, 189–192. [Google Scholar] [CrossRef]
- Gómez-Montes, E.; Herráiz, I.; Mendoza, A.; Escribano, D.; Galindo, A. Prediction of coarctation of the aorta in the second half of pregnancy. Ultrasound Obstet. Gynecol. 2013, 41, 298–305. [Google Scholar] [CrossRef]
- Gomez-Montes, E.; Herraiz, I.; Gomez-Arriaga, P.I.; Escribano, D.; Mendoza, A.; Galindo, A. Gestational age-specific scoring systems for the prediction of coarctation of the aorta. Prenat. Diagn. 2014, 34, 1198–1206. [Google Scholar] [CrossRef]
- Sharland, G.; Allan, L. Normal fetal cardiac measurement derived by cross-sectional echocardiography. Ultrasound Obstet. Gynecol. 1992, 2, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Stos, B.; Le Bidois, J.; Fermont, L.; Bonnet, D. Is antenatal diagnosis of coarctation of the aorta possible? Arch. Mal. Coeur Vaiss. 2007, 100, 428–432. [Google Scholar] [PubMed]
- Familiari, A.; Morlando, M.; Khalil, A.; Sonesson, S.E.; Scala, C.; Rizzo, G.; Del Sordo, G.; Vassallo, C.; Elena Flacco, M.; Manzoli, L.; et al. Risk Factors for Coarctation of the Aorta on Prenatal Ultrasound: A Systematic Review and Meta-Analysis. Circulation 2017, 135, 772–785. [Google Scholar] [CrossRef]
- Anuwutnavin, S.; Satou, G.; Chang, R.K.; DeVore, G.R.; Abuel, A.; Sklansky, M. Prenatal Sonographic Predictors of Neonatal Coarctation of the Aorta. J. Ultrasound Med. 2016, 35, 2353–2364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Lei, W.; Liu, J.; Yang, B.; Li, H.; Huang, D. The distolic and systolic velocity-time integral ratio of the aortic isthmus is a sensitive indicator of artic coarctacion in fetuses. J. Am. Soc. Echocardiogr. 2019, 32, 1470–1476. [Google Scholar] [CrossRef]
- Morgan, C.T.; Mueller, B.; Thakur, V.; Guerra, V.; Jull, C.; Mertens, L.; Friedberg, M.; Golding, F.; Seed, M.; Miner, S.E.S.; et al. Improving Prenatal Diagnosis of Coarctation of the Aorta. Can. J. Cardiol. 2019, 35, 453–461. [Google Scholar] [CrossRef]
- Arya, B.; Bhat, A.; Vernon, M.; Conwell, J.; Lewin, M. Utility of novel fetal echocardiographic morphometric measures of the aortic arch in the diagnosis of neonatal coarctation of the aorta. Prenat. Diagn. 2016, 36, 127–134. [Google Scholar] [CrossRef]
- Beattie, M.; Peyvandi, S.; Ganesan, S.; Moon-Grady, A. Toward improving the fetal diagnosis of coarctation of the aorta. Pediatr. Cardiol. 2017, 38, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Marginean, C.; Marginean, C.O.; Muntean, I.; Toganel, R.; Voidazan, S.; Gozar, L. The role of ventricular disproportion, aortic, and ductal isthmus ultrasound measurements for the diagnosis of fetal aortic coarctation, in the third trimester of pregnancy. Med. Ultrason 2015, 17, 475–481. [Google Scholar]
- Toole, B.J.; Schlosser, B.; McCracken, C.E.; Stauffer, N.; Border, W.L.; Sachdeva, R. Importance of Relationship between Ductus and Isthmus in Fetal Diagnosis of Coarctation of Aorta. Echocardiography 2016, 33, 771–777. [Google Scholar] [CrossRef]
- Vigneswaran, T.V.; Zidere, V.; Chivers, S.; Charakida, M.; Akolekar, R.; Simpson, J.M. Impact of prospective measurement of outflow tracts in the prediction of coarctation of the aorta. Ultrasound Obstet. Gynecol. 2020, 56, 850–856. [Google Scholar] [CrossRef]
- Gabbay-Benziv, R.; Cetinkaya Demir, B.; Crimmins, S.; Esin, S.; Turan, O.M.; Turan, S. Retrospective case series examining the clinical significance of subjective fetal cardiac ventricular disproportion. Int. J. Gynaecol. Obstet. 2016, 135, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Franklin, O.; Burch, M.; Manning, N.; Sleeman, K.; Gould, S.; Archer, N. Prenatal diagnosis of coarctation of the aorta improves survival and reduces morbidity. Heart 2002, 87, 67–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houshmandi, M.M.; Eckersley, L.; Fruitman, D.; Mills, L.; Power, A.; Hornberger, L.K. Fetal Diagnosis is Associated with Improved Perioperative Condition of Neonates Requiring Surgical Intervention for Coarctation. Pediatr. Cardiol. 2021. [Google Scholar] [CrossRef]
- Matsui, H.; Mellander, M.; Roughton, M.; Jicinska, H.; Gardiner, H.M. Morphological and physiological predictors of fetal aortic coarctation. Circulation 2008, 118, 1793–1801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jowett, V.; Aparicio, P.; Santhakumaran, S.; Seale, A.; Jicinska, H.; Gardiner, H.M. Sonographic predictors of surgery in fetal coarctation of the aorta. Ultrasound Obstet. Gynecol. 2012, 40, 47–54. [Google Scholar] [CrossRef]
- Patel, C.; Weeks, B.; Copel, J.; Fahey, J.; Song, X.; Shabanova, V. Fetal echocardiographic measures to improve the prenatal diagnosis of coarctation of the aorta. Pediatr. Cardiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Freeman, K.; Kronmal, R.; Clouse, M.; Conwell, J.; Bhat, A.; Young, L.; Lewin, M.; Arya, B. Validation of Prenatal Aortic Arch Angle Measurements in the Diagnosis of Neonatal Coarctation of the Aorta. Pediatr. Cardiol. 2021, 16, 601–604. [Google Scholar] [CrossRef]
- Fricke, K.; Liuba, P.; Weismann, C.G. Fetal Echocardiographic Dimension Indices: Important Predictors of Postnatal Coarctation. Pediatr Cardiol 2021, 42, 517–525. [Google Scholar] [CrossRef]
- Hess, D.; Flaker, G.; Aggarwal, K.; Buchheit, L.; Hess, L. Fetal cardiac imaging. In Fetal Echocardiography; Hess, D.B.H.L., Ed.; Appleton & Lange: Stamford, CT, USA, 1999; pp. 149–194. [Google Scholar]
- Rychik, J.; Ayres, N.; Cuneo, B.; Gotteiner, N.; Hornberger, L.; Spevak, P.; Van Der Veld, M. American Society of Echocardiography guidelines and standards for performance of the fetal echocardiogram. J. Am. Soc. Echocardiogr. 2004, 17, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Pasquini, L.; Mellander, M.; Seale, A.; Matsui, H.; Roughton, M.; Ho, S.Y.; Gardiner, H.M. Z-scores of the fetal aortic isthmus and duct: An aid to assessing arch hypoplasia. Ultrasound Obstet. Gynecol. 2007, 29, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; McCrindle, B.W.; Carvalho, J.S.; Hornberger, L.K.; McCarthy, K.P.; Daubeney, P.E. Development of Z-scores for fetal cardiac dimensions from echocardiography. Ultrasound Obstet. Gynecol. 2005, 26, 599–605. [Google Scholar] [CrossRef]
- Devore, G. The use of Z-scores in the analysis of fetal cardiac dimensions. Ultrasound Obstet Gynecol 2005, 26, 596–598. [Google Scholar] [CrossRef] [PubMed]
- Committee opinion no 611: Method for estimating due date. Obstet. Gynecol. 2014, 124, 863–866. [CrossRef]
- Vena, F.; Donarini, G.; Scala, C.; Tuo, G.; Paladini, D. Redundancy of the foramen ovale flap may mimic aortic coarctation in the fetus. Ultrasound Obstet. Gynecol. 2020, 56, 857–863. [Google Scholar] [CrossRef]
- Head, C.; Jowett, V.; Sharland, G.; Simpson, J. Timing of presentation and postnatal outcome of infants suspected of having coarctation of the aorta during fetal life. Heart 2005, 91, 1070–1074. [Google Scholar] [CrossRef]
- Rizzo, G.; Arduini, D.; Capponi, A. Use of 4-dimensional sonography in the measurement of fetal great vessels in mediastinum to distinguish true from false positive coarctation of the aorta. J. Ultrasound Med. 2010, 29, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Quarello, E.; Ville, Y.; Carvalho, J. The aortic isthmus—Ductal angle: A novel measurement to diagnose fetal aortic coarctation. Ultrasound Obstet. Gynecol. 2008, 32, 262–263. [Google Scholar] [CrossRef]
- DeVore, G.R.; Haxel, C.; Satou, G.; Sklansky, M.; Pelka, M.J.; Jone, P.N.; Cuneo, B.F. Improved detection of coarctation of the aorta using speckle-tracking analysis of fetal heart on last examination prior to delivery. Ultrasound Obstet. Gynecol. 2021, 57, 282–291. [Google Scholar] [CrossRef] [PubMed]




| Predictive Parameter | Cut-Off Value | LR + (95% CI) | LR − (95% CI) |
|---|---|---|---|
| Gestational age (weeks) | ≤28 | 4.3 (2.0–8.8) | 0.3 (0.1–0.5) |
| Ascending aorta Z-score | ≤−1.5 | 2.8 (1.4–5.6) | 0.4 (0.2–0.7) |
| Aortic isthmus (3VT) Z-score | ≤−2 | 1.8 (0.8–3.8) | 0.6 (0.3–1.2) |
| Pulmonary valve/aortic valve ratio | ≥1.6 | 1.8 (1.1–3.1) | 0.4 (0.2–0.9) |
| Pre-test odds for CoAo: 41 CoAo/44 no CoAo = 0.93 Post-test odds for CoAo: Pre-test odds × LR1 × LR2 × LR3 × LR4 Post-test probability of CoAo: Post-test odds/(Post-test odds + 1) | |||
| Variable | Value |
|---|---|
| Whole study population, n | 179 |
| Mean GA at diagnosis of cardiac asymmetry ‡, weeks (SD, range) | 29.6 (6.5, 17–39) |
| Detection at the 19–22 weeks’ scan *, n (%) | 47 (26.3) |
| Detection at the 32–36 weeks’ scan *, n (%) | 84 (46.9) |
| Mean GA at diagnosis performed at unscheduled scans, weeks (SD, range) | 30.7 (6.0, 17–39) |
| Mean GA at the first fetal echocardiography, weeks (SD, range) | 30.9 (6.2, 17–40) |
| Characteristics of prenatal diagnosis, n (%) | |
| Patients referred from their local centers | 131 (73.2) |
| Suspected CHD | 118 |
| Extracardiac anomaly | 6 |
| Suspected CHD and extracardiac anomaly | 3 |
| Suspected CHD and medical history | 2 |
| Medical history | 1 |
| Others | 1 |
| Patients primarily assisting our hospital | 48 (26.8) |
| Suspected cardiac asymmetry | 43 |
| Extracardiac anomaly | 4 |
| Suspected CHD and medical history | 1 |
| Sort of pregnancy, n (%) | |
| Single | 177 (98.9) |
| Twin | 2 (1.1) |
| Other minor cardiac anomalies, n (%) | 34 (19.0) |
| PLSVC | 26 (14.5) |
| VSD | 5 (2.8) |
| ARSA | 2 (1.1) |
| Auricular extrasystoles | 1 (0.6) |
| Extracardiac abnormalities, n (%) | 24 (13.4) |
| Single umbilical artery | 7 (3.9) |
| Ductus venosus agenesis | 1 (0.6) |
| Horseshoe kidney | 2 (1.1) |
| Left isomerism | 1 (0.6) |
| Esophageal atresia | 1 (0.6) |
| Persistent intrahepatic right umbilical vein | 1 (0.6) |
| Hydronephrosis | 3 (1.7) |
| Talipes equinovarus bilateral | 3 (1.7) |
| Cerebellar hemorrhagic stroke | 1 (0.6) |
| Congenital cystic adenomatoid malformation | 2 (1.1) |
| Interrupted inferior vena cava | 1 (0.6) |
| Myelomeningocele | 1 (0.6) |
| Mean GA at birth, weeks (SD, range) | 38.7 (1.9, 27–41) |
| Mean birth weight, grams (SD, range) | 3175.3 (576.6, 930–4300) |
| Cut-Off Point | Sn (95% CI) | Sp (95% CI) | NPV (95% CI) | PPV (95% CI) | AUC (95% CI) | |
|---|---|---|---|---|---|---|
| Whole study population | ||||||
| Best balance between Sn and Sp | ≥53% | 92.3 (79.7–97.3) | 80.0 (72.6–85.8) | 97.4 (92.6–99.1) | 56.3 (44.1–67.7) | 0.93 (0.89–0.97) |
| Maximum Sn | ≥35% | 100 (91.0–100) | 72.9 (65.0–79.5) | 100 (96.4–100) | 50.6 (39.7–61.5) | |
| Maximum Sp | ≥96% | 43.6 (29.3–59.0) | 96.4 (91.9–98.5) | 86.0 (79.7–90.6) | 77.3 (56.6–89.9) | |
| Early-onset cardiac asymmetry (≤28 weeks) | ||||||
| Best balance between Sn and Sp | ≥84% | 76.5 (60.0–87.6) | 70.6 (53.8–83.2) | 75.0 (57.9–86.7) | 72.2 (56.0–84.2) | 0.82 (0.71–0.92) |
| Maximum Sn | ≥39% | 100 (89.8–100) | 52.9 (36.7–68.5) | 100 (82.4–100) | 68.0 (54.2–79.2) | |
| Maximum Sp | ≥96% | 50.0 (34.1–65.9) | 85.3 (69.9–93.6) | 63.0 (48.6–75.5) | 77.3 (56.6–89.9) | |
| Late-onset cardiac asymmetry (>28 weeks) | ||||||
| Best balance between Sn and Sp | ≥45% | 80.0 (37.6–96.4) | 86.8 (79.0–92.0) | 98.9 (94.2–99.8) | 22.2 (9.0–45.2) | 0.91 (0.85–0.98) |
| Maximum Sn | ≥35% | 100 (56.6–100) | 79.2 (70.6–85.9) | 100 (95.6–100) | 18.5 (8.2–36.7) | |
| Maximum Sp | ≥71% | 60.0 (23.1–88.2) | 89.6 (82.4–94.1) | 97.9 (92.8–99.4) | 21.4 (7.6–47.6) | |
| Study | Predictive Parameters/Scores | AUC (95% CI) | Sn (95% CI) | Sp (95% CI) | |
|---|---|---|---|---|---|
| Jowett et al. 2012 [30] (n = 37) | Z-score AoIsth (3VT or sagittal) < −2 AoIsth/DA < 0.74 Contraductal shelf Continuous diastolic flow at AoIsth | 0.52 (0.32–0.72) | 33 (17–53) | 71 (29–96) | |
| Gómez Montes et al. 2013 [13] (n = 85) | GA at diagnosis ≤28 s AAo Z-score ≤ −1.5 AoIsth Z-score (3VT) ≤ −2 PV/AV ≥ 1.6 | 0.94 (0.87–0.99) | 90 (77–96) | 75 (61–85) | |
| Gómez Montes et al. 2014 [14] (n = 115) | ≤28 weeks | AAo Z-score ≤ −1.1 AoIsth Z-score (3VT) ≤ −1.2 | 0.98 (0.94–1.00) | 91 (76–97) | 91 (62–98) |
| >28 weeks | TV/MV ≥ 1.48 MPA/AAo ≥ 1.85 | 0.84 (0.67–1.00) | 63 (31–86) | 43 (30–58) | |
| Marginean et al. 2015 [23] (n = 32) Late-onset cardiac asymmetry (32–39 weeks) | RV/LV > 1.5 AoIsth/DA < 0.7 AoIsth (3VT) < 4.2 mm Three parameters present | 56 (21–86) | 87 (66–97) | ||
| Toole et al. 2016 [24] (n = 62) | MV Z-score < −1.63 MV/TV < 0.75 AoIsth/DA < 0.5I sthmus-ductal angle < 117° At least two of these parameters present | 0.92 (0.80–1.00) | 85 (66–96) | 60 (42–76) | |
| Anuwutnavin et al. 2016 [18] (n = 31) | AAs Z-score ≤ −2 (4 points) MV Z-score VM ≤ −2 (2 points) Transverse aortic arch Z-score ≤ −2 (1 point) VSD (1 point) Score ≥ 4 points | 100 | 88 | ||
| Arya et al. 2016 [21] (n = 40) ≥28 weeks | LCCA-LSA distance > 4.5 mm AAo-DAo angle ≤ 20.31° Transverse aortic arch-DAo angle ≥ 96.15° | 95 (75–100) | 100 (83–100) | ||
| Beattie et al. 2017 [22] (n = 62) | MPA/AAo ≤ 0.65 Diastolic flow persistence at AoIsth | 87 | 53 | ||
| Patel et al. 2018 [31] (n = 27) | LCCA-LSA distance (mean in CoAo 5.3 mm) DT/LCCA-LSCA index (mean in CoAo 0.59) BA-LCCA distance (mean in CoAo 3.2 mm) | ||||
| Wang et al. 2019 [19] (n = 69) | AoIsth Z-score (sagittal) ≤ −3.7 VTID/VTIS > 0.56 | 0.96 (0.88–0.99) | |||
| Morgan et al. 2019 [20] (n = 107) | GA at diagnosis Transverse aortic arch (mm) AAo Z-scorePeak AAo Doppler velocity | 0.92 (0.87–0.97) | 89 | 82 | |
| Vigneswaran et al. 2020 [25] (n = 149) | AoIsth Z-score (3VT) DA Z-score | ||||
| Freeman et al. 2021 [32] (n = 35) | AAo-DAo angle Transverse aortic arch-DAo angle AAo | ||||
| Fricke et al. 2021 [33] (n = 65) | CSA index < 0.78 AoIsth/DA × MV/TV < 0.37 | 0.94 (0.85–1) - | 92 100 | 97 95 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Montes, E.; Herraiz García, I.; Escribano Abad, D.; Rodríguez Calvo, J.; Villalaín González, C.; Galindo Izquierdo, A. Application of a Global Multiparameter Scoring System for the Prenatal Prediction of Coarctation of the Aorta. J. Clin. Med. 2021, 10, 3690. https://doi.org/10.3390/jcm10163690
Gómez-Montes E, Herraiz García I, Escribano Abad D, Rodríguez Calvo J, Villalaín González C, Galindo Izquierdo A. Application of a Global Multiparameter Scoring System for the Prenatal Prediction of Coarctation of the Aorta. Journal of Clinical Medicine. 2021; 10(16):3690. https://doi.org/10.3390/jcm10163690
Chicago/Turabian StyleGómez-Montes, Enery, Ignacio Herraiz García, David Escribano Abad, Jesús Rodríguez Calvo, Cecilia Villalaín González, and Alberto Galindo Izquierdo. 2021. "Application of a Global Multiparameter Scoring System for the Prenatal Prediction of Coarctation of the Aorta" Journal of Clinical Medicine 10, no. 16: 3690. https://doi.org/10.3390/jcm10163690
APA StyleGómez-Montes, E., Herraiz García, I., Escribano Abad, D., Rodríguez Calvo, J., Villalaín González, C., & Galindo Izquierdo, A. (2021). Application of a Global Multiparameter Scoring System for the Prenatal Prediction of Coarctation of the Aorta. Journal of Clinical Medicine, 10(16), 3690. https://doi.org/10.3390/jcm10163690







