Predictive Model of Good Clinical Outcomes in Patients Undergoing Coronary Angiography after Out-of-Hospital Cardiac Arrest: A Prospective, Multicenter Observational Study Conducted by the Korean Cardiac Arrest Research Consortium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Korean Emergency Medical Service (EMS) System
2.3. Study Population
2.4. Data Collection
2.5. Outcome Measurements
2.6. Statistical Analyses
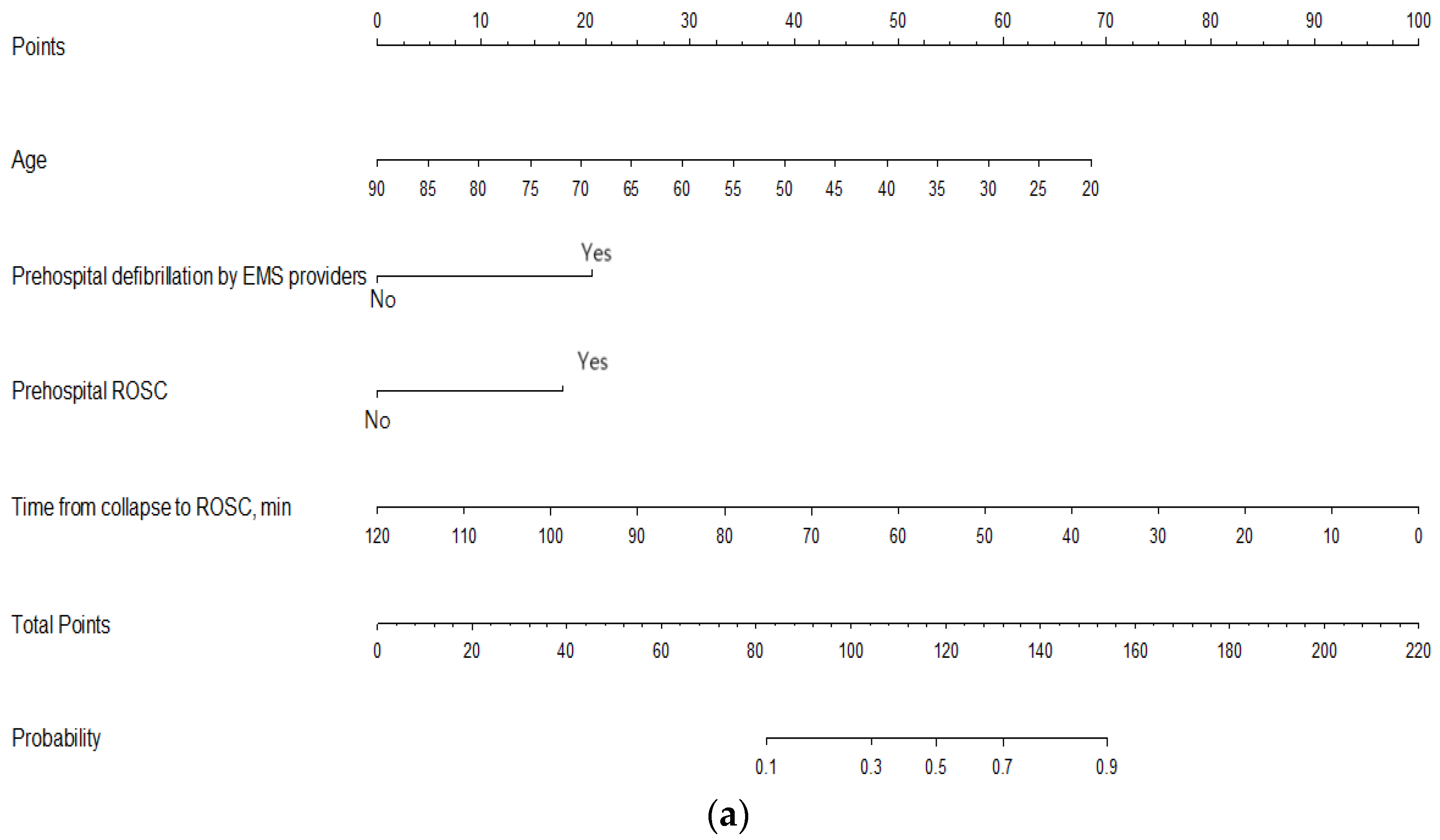
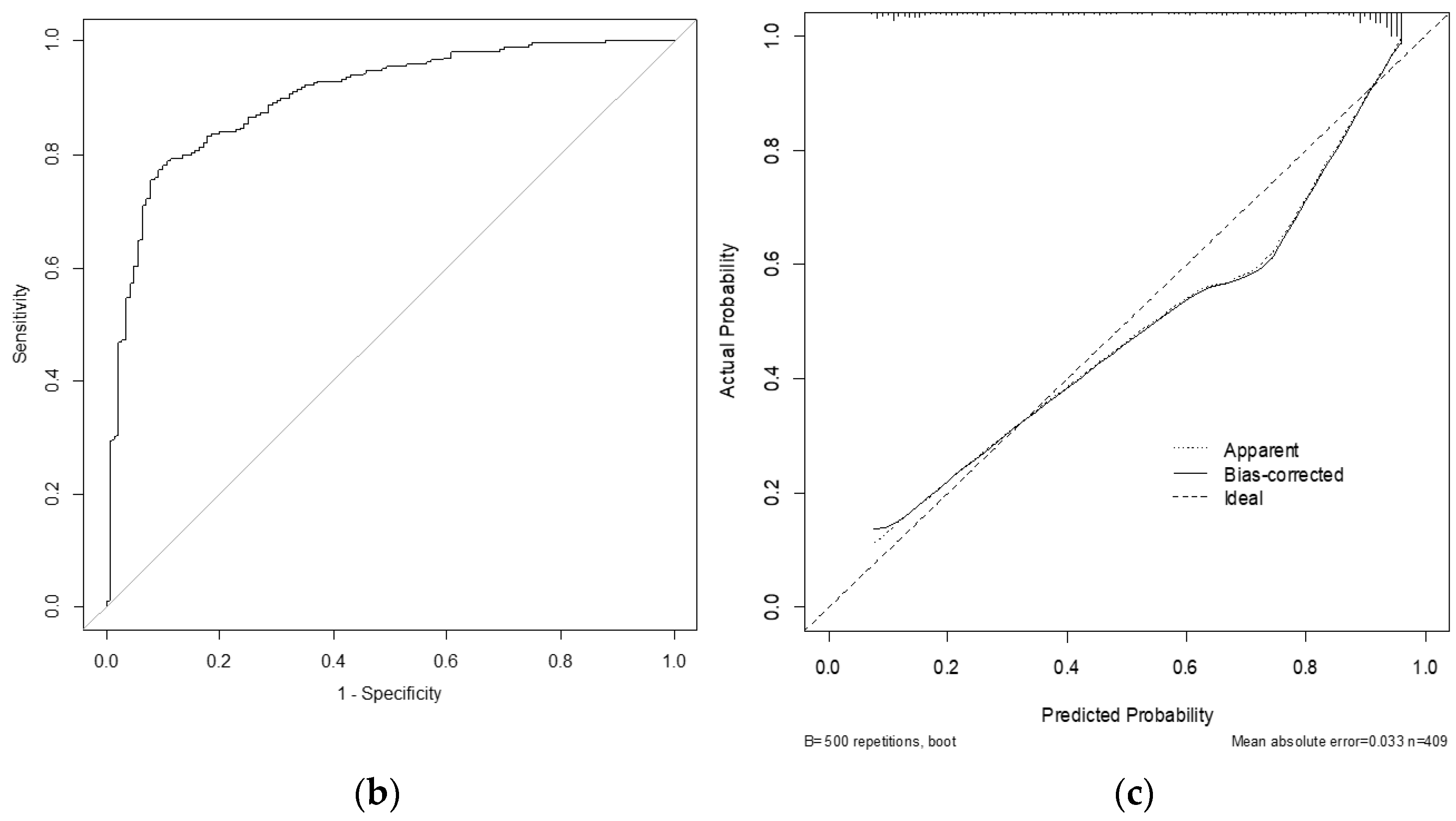
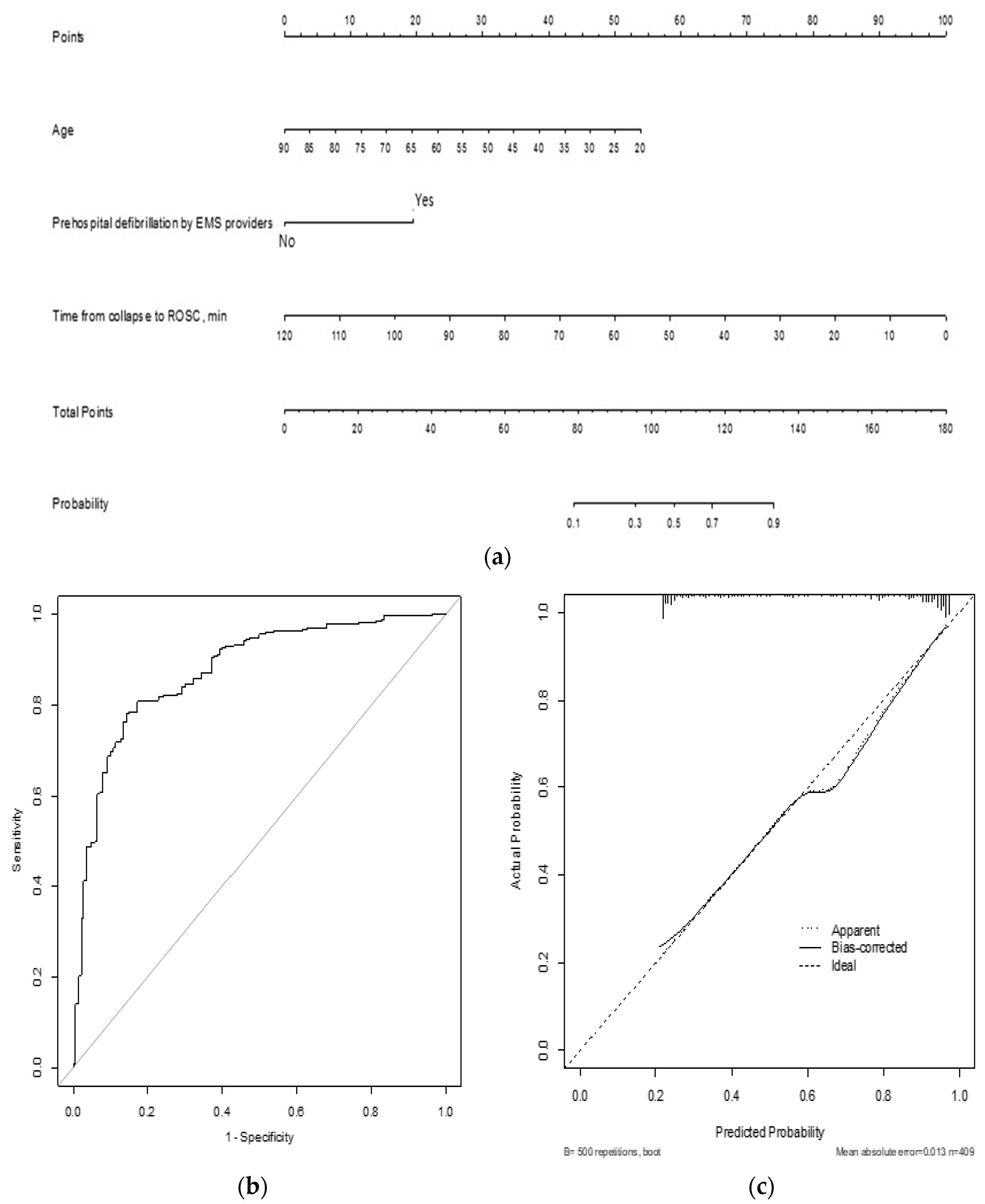
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sasson, C.; Rogers, M.A.; Dahl, J.; Kellermann, A.L. Predictors of survival from out-of-hospital cardiac arrest: A systematic review and meta-analysis. Circ. Cardiovasc. Qual. Outcomes 2010, 3, 63–81. [Google Scholar] [CrossRef] [Green Version]
- Fugate, J.E.; Brinjikji, W.; Mandrekar, J.N.; Cloft, H.J.; White, R.D.; Wijdicks, E.F.; Rabinstein, A.A. Post-cardiac arrest mortality is declining: A study of the US National Inpatient Sample 2001 to 2009. Circulation 2012, 126, 546–550. [Google Scholar] [CrossRef] [Green Version]
- Nolan, J.P.; Sandroni, C.; Bottiger, B.W.; Cariou, A.; Cronberg, T.; Friberg, H.; Genbrugge, G.; Haywood, K.; Lilja, G.; Moulaert, V.R.M.; et al. European Resuscitation Council and European Society of Intensive Care Medicine Guidelines 2021: Post resuscitation care. Resuscitation 2021, 161, 220–269. [Google Scholar] [CrossRef]
- Panchal, A.R.; Bartos, J.A.; Cabañas, J.G.; Cariou, A.; Cronberg, T.; Friberg, H.; Genbrugge, C.; Haywood, K.; Lilja, G.; Moulaert, V.R.; et al. Part 3: Adult basic and advanced life support 2020 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2020, 142, 428–429. [Google Scholar] [CrossRef]
- Garcia, S.; Drexel, T.; Bekwelem, W.; Raveendran, G.; Caldwell, E.; Hodgson, L.; Wang, Q.; Adabag, S.; Mahoney, B.; Frascone, R.; et al. Early access to the cardiac catheterization laboratory for patients resuscitated from cardiac arrest due to a shockable rhythm: The Minnesota Resuscitation Consortium Twin Cities Unified Protocol. J. Am. Heart Assoc. 2016, 5, e002670. [Google Scholar] [CrossRef] [Green Version]
- Kern, K.B.; Lotun, K.; Patel, N.; Mooney, M.R.; Hollenbeck, R.D.; McPherson, J.A.; McMullan, P.W.; Unger, B.; Hsu, C.H.; Seder, D.B. INTCAR-Cardiology Registry. Outcomes of comatose cardiac arrest survivors with and without ST-segment elevation myocardial infarction: Importance of coronary angiography. JACC Cardiovasc. Interv. 2015, 8, 1031–1040. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.S.; Shah, S.M.M.; Mubashir, A.; Khan, A.R.; Fatima, K.; Schenone, A.L.; Khosa, F.; Samady, H.; Menon, V. Early coronary angiography in patients resuscitated from out of hospital cardiac arrest without ST-segment elevation: A systematic review and meta-analysis. Resuscitation 2017, 121, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Lemkes, J.S.; Janssens, G.N.; van der Hoeven, N.W.; Jewbali, L.S.; Dubois, E.A.; Meuwissen, M.; Rijpstra, T.; Rijpstra, T.A.; Bosker, H.A.; Blans, M.J.; et al. Coronary angiography after cardiac arrest without ST-segment elevation. N. Engl. J. Med. 2019, 380, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Demsar, J.; Zupan, B.; Kattan, M.W.; Beck, J.R.; Bratko, I. Naïve Bayesian-based nomogram for prediction of prostate cancer recurrence. Stud. Health Technol. Inform. 1999, 436–441. [Google Scholar] [CrossRef]
- Kim, J.Y.; Hwang, S.O.; Shin, S.D.; Yang, H.J.; Chung, S.P.; Lee, S.W.; Song, K.J.; Hwang, S.S.; Cho, G.C.; Moon, S.W.; et al. Korean Cardiac Arrest Research Consortium (KoCARC): Rationale, development, and implementation. Clin. Exp. Emerg. Med. 2018, 5, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Moon, H.J.; Heo, N.H.; KoCARC. Association between ambulance response time and neurologic outcome in patients with cardiac arrest. Am. J. Emerg. Med. 2019, 37, 1999–2003. [Google Scholar] [CrossRef]
- Song, K.J.; Shin, S.D.; Park, C.B.; Kim, J.Y.; Kim, D.K.; Kim, C.H.; Ha, S.Y.; Marcus, E.; Bentley, J.; Bryan, M. Dispatcher-assisted bystander cardiopulmonary resuscitation in a metropolitan city: A before-after population-based study. Resuscitation 2014, 85, 34–41. [Google Scholar] [CrossRef]
- Miller, J. Reaction time analysis with outlier exclusion: Bias varies with sample size. Q. J. Exp. Psychol. A 1991, 43, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Iasonos, A.; Schrag, D.; Raj, G.V.; Panageas, K.S. How to build and interpret a nomogram for cancer prognosis. J. Clin. Oncol. 2008, 26, 1364–1370. [Google Scholar] [CrossRef]
- Koretsune, Y.; Marban, E. Mechanism of ischemic contracture in ferret hearts: Relative roles of [Ca2+] i elevation and ATP depletion. Am. J. Physiol. 1990, 258, H9–H16. [Google Scholar] [CrossRef] [PubMed]
- Fries, M.; Tang, W.; Chang, Y.T.; Wang, J.; Castillo, C.; Weil, M.H. Microvascular blood flow during cardiopulmonary resuscitation is predictive of outcome. Resuscitation 2006, 71, 248–253. [Google Scholar] [CrossRef]
- Laver, S.; Farrow, C.; Turner, D.; Nolan, J. Mode of death after admission to an intensive care unit following cardiac arrest. Intensive Care Med. 2004, 30, 2126–2128. [Google Scholar] [CrossRef] [PubMed]
- Aissaoui, N.; Bougouin, W.; Dumas, F.; Beganton, F.; Chocron, R.; Varenne, O.; Spaulding, C.; Karam, N.; Montalescot, G.; Aubry, P.; et al. Age and benefit of early coronary angiography after out-of-hospital cardiac arrest in patients presenting with shockable rhythm: Insights from the Sudden Death Expertise Center registry. Resuscitation 2018, 128, 126–131. [Google Scholar] [CrossRef]
- Mader, T.J.; Nathanson, B.H.; Millay, S.; Coute, R.A.; Clapp, M.; McNally, B. Out-of-hospital cardiac arrest outcomes stratified by rhythm analysis. Resuscitation 2012, 83, 1358–1362. [Google Scholar] [CrossRef]
- Dumas, F.; Bougouin, W.; Geri, G.; Lamhaut, L.; Rosencher, J.; Pène, F.; Chiche, J.D.; Varenne, O.; Carli, P.; Jouven, X.; et al. Emergency percutaneous coronary intervention in post-cardiac arrest patients without ST-segment elevation pattern: Insights from the PROCAT II registry. JACC Cardiovasc. Interv. 2016, 9, 1011–1018. [Google Scholar] [CrossRef]
- Wampler, D.A.; Collett, L.; Manifold, C.A.; Velasquez, C.; McMullan, J.T. Cardiac arrest survival is rare without prehospital return of spontaneous circulation. Prehosp. Emerg. Care 2012, 16, 451–455. [Google Scholar] [CrossRef]
- Morrison, L.; Visentin, L.; Kiss, A. Validation of a rule for termination of resuscitation in out-of-hospital cardiac arrest. N. Engl. J. Med. 2006, 355, 478–487. [Google Scholar] [CrossRef]
- Kim, M.J.; Ro, Y.S.; Shin, S.D.; Song, K.J.; Ahn, K.O.; Hong, S.O.; Kim, Y.T. Association of emergent and elective percutaneous coronary intervention with neurological outcome and survival after out-of-hospital cardiac arrest in patients with and without a history of heart disease. Resuscitation 2015, 97, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Camuglia, A.C.; Randhawa, V.K.; Lavi, S.; Walters, D.L. Cardiac catheterization is associated with superior outcomes for survivors of out of hospital cardiac arrest: Review and meta-analysis. Resuscitation 2014, 85, 1533–1540. [Google Scholar] [CrossRef]
- Elfwén, L.; Lagedal, R.; James, S.; Jonsson, M.; Jensen, U.; Ringh, M.; Claesson, A.; Oldgren, J.; Herlitz, J.; Rubertsson, S.; et al. Coronary angiography in out-of-hospital cardiac arrest without ST elevation on ECG—Short- and long-term survival. Am. Heart J. 2018, 200, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Dankiewicz, J.; Nielsen, N.; Annborn, M.; Cronberg, T.; Erlinge, D.; Gasche, Y.; Hassager, C.; Kjaergaard, J.; Pellis, T.; Friberg, H. Survival in patients without acute ST elevation after cardiac arrest and association with early coronary angiography: A post hoc analysis from the TTM trial. Intensive Care Med. 2015, 41, 856–864. [Google Scholar] [CrossRef]
- Bro-Jeppesen, J.; Kjaergaard, J.; Wanscher, M.; Pedersen, F.; Holmvang, L.; Lippert, F.K.; Møller, J.E.; Køber, L.; Hassager, C. Emergency coronary angiography in comatose cardiac arrest patients: Do real-life experiences support the guidelines? Eur. Heart J. Acute Cardiovasc. Care 2012, 1, 291–301. [Google Scholar] [CrossRef] [PubMed]

| Training Set (n = 496) | Validation Set (n = 227) | |||||
|---|---|---|---|---|---|---|
| Survival (n = 334) | Death (n = 162) | p-Value | Survival (n = 141) | Death (n = 86) | p-Value | |
| Age (years) | 55.3 ± 13.0 | 64.3 ± 13.3 | <0.001 | 56.1 ± 13.1 | 63.3 ± 13.8 | <0.001 |
| Sex, male | 279 (83.5) | 133 (82.1) | 0.69 | 123 (87.2) | 72 (83.7) | 0.46 |
| Medical history | ||||||
| Hypertension | 122 (39.1) | 80 (52.0) | 0.008 | 64 (46.7) | 39 (47.6) | 0.9 |
| Diabetes mellitus | 65 (21.0) | 55 (36.2) | <0.001 | 24 (17.8) | 29 (35.4) | 0.003 |
| Dyslipidemia | 23 (7.80) | 9 (6.2) | 0.53 | 11 (8.6) | 7 (9.6) | 0.81 |
| Bystander-witnessed | 275 (84.1) | 125 (77.2) | 0.06 | 108 (77.1) | 66 (76.7) | 0.95 |
| Place of arrest | 0.02 | 0.09 | ||||
| Public | 185 (44.8) | 75 (46.9) | 74 (53.6) | 41 (47.6) | ||
| Private | 122 (37.7) | 76 (47.5) | 60 (43.5) | 36 (41.9) | ||
| Ambulance | 16 (4.9) | 9 (5.6) | 4 (2.9) | 9 (10.5) | ||
| Bystander CPR | 0.008 | 0.1 | ||||
| Chest compressions | 193 (61.3) | 73 (46.8) | 83 (62.4) | 42 (50.0) | ||
| Prehospital defibrillation by bystanders | 14 (4.4) | 1 (0.6) | 0.03 | 6 (4.6) | 1 (1.2) | 0.18 |
| Primary cardiac rhythm at the scene | <0.001 | <0.001 | ||||
| Shockable | 263 (85.1) | 73 (46.5) | 113 (85.0) | 42 (51.9) | ||
| Prehospital defibrillation by EMS providers | 285 (85.6) | 81 (51.3) | <0.001 | 119 (85.6) | 45 (52.3) | <0.001 |
| Epinephrine use by EMS providers | 24 (7.2) | 21 (13.0) | 0.04 | 16 (11.4) | 15 (17.4) | 0.2 |
| Prehospital ROSC, yes | 241 (72.2) | 20 (12.4) | <0.001 | 101 (71.6) | 11 (12.8) | <0.001 |
| Primary cardiac rhythm at hospital | <0.001 | 0.27 | ||||
| Shockable | 36 (45.6) | 24 (17.52) | 11 (35.5) | 20 (28.7) | ||
| Full dose of epinephrine used during CPR in the hospital (mg) | 1.6 ± 3.3 | 7.1 ± 6.6 | <0.001 | 1.5± 2.9 | 5.7 ± 5.4 | <0.001 |
| SBP (mmHg) | 129.0 ± 39.5 | 117.5 ± 40.8 | 0.07 | 133.2 ± 38.5 | 121.3 ± 41.7 | 0.17 |
| DBP (mmHg) | 82.4 ± 22.0 | 73.4 ± 28.1 | 0.02 | 83.5± 26.5 | 79.8 ± 32.4 | 0.55 |
| HR (beats/min) | 103.1 ± 30.6 | 98.2 ±32.0 | 0.32 | 100.2 ± 31.9 | 93.3 ± 34.1 | 0.34 |
| Troponin I | 74.4 ± 650.3 | 24.5 ± 141.9 | 0.42 | 32.4 ± 254.4 | 8.5 ± 26.7 | 0.45 |
| E-CPR | 18 (6.2) | 48 (29.3) | <0.001 | 6 (4.6) | 20 (28.2) | <0.001 |
| Targeted temperature management | 132 (40.7) | 52 (32.7) | 0.17 | 44 (32.8) | 28 (33.3) | 0.61 |
| PCI | 99 (35.1) | 66 (41.0) | 0.22 | 43 (32.1) | 30 (46.2) | 0.05 |
| Vasopressor use in the hospital | 177 (54.3) | 149 (92.0) | <0.001 | 82 (60.3) | 78 (91.8) | <0.001 |
| Time from EMS call to scene arrival, min | 7.1 ± 3.6 | 8.2 ± 3.6 | 0.002 | 7.9 ± 4.7 | 8.6 ± 5.3 | 0.33 |
| Time from collapse to ED arrival, min | 30.3 ± 13.2 | 30.4 ± 15.2 | 0.93 | 31.3 ± 14.1 | 30.0 ± 13.0 | 0.51 |
| Time from collapse to ROSC, min | 21.0 ± 14.1 | 44.3 ± 25.2 | <0.001 | 21.9 ± 17.0 | 44.7 ± 24.0 | <0.001 |
| Time from ED arrival to CAG, min | 2042.5 ± 4903.8 | 204.6 ± 727.3 | <0.001 | 1781.4± 5702.3 | 978.9 ± 5196.9 | 0.29 |
| Survival Discharge | Good Neurologic Outcomes | |||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Age | 0.94 (0.90–0.98) | <0.001 | 0.95 (0.92–0.98) | <0.001 |
| Prehospital defibrillation by EMS providers | 3.52 (1.53–8.05) | 0.003 | 7.84 (3.05–20.17) | <0.001 |
| Prehospital ROSC | 2.96 (1.20–7.29) | 0.02 | ||
| Time from collapse to ROSC, min | 0.95 (0.93–0.97) | <0.001 | 0.91 (0.88–0.94) | <0.001 |
| Variables | Training Set (n = 489) | Validation Set (n = 220) | ||||
|---|---|---|---|---|---|---|
| Good Neurologic Outcome (CPC 1, 2) (n = 210) | Poor Neurologic Outcome (CPC ≥ 3) (n = 279) | p-Value | Good Neurologic Outcome (CPC 1, 2) (n = 105) | Poor Neurologic Outcome (CPC ≥ 3) (n = 115) | p-Value | |
| Age (years) | 54.6 ± 12.7 | 63.0 ± 13.6 | <0.001 | 55.0 ± 12.8 | 62.7 ± 13.5 | <0.001 |
| Sex, male | 234 (83.9) | 170 (80.9) | 0.4 | 100 (86.9) | 90 (85.7) | 0.79 |
| Medical history | ||||||
| Hypertension | 101 (38.4) | 99 (50.3) | 0.01 | 52 (46.9) | 47 (46.1) | 0.91 |
| Diabetes mellitus | 47 (18.1) | 71 (36.4) | <0.001 | 17 (15.6) | 35 (34.7) | 0.001 |
| Dyslipidemia | 22 (8.76) | 10 (5.41) | 0.18 | 11 (10.7) | 7 (7.5) | 0.45 |
| Bystander-witnessed | 232 (84.7) | 162 (77.9) | 0.06 | 87 (76.3) | 82 (78.1) | 0.75 |
| Place of arrest | 0.02 | 0.003 | ||||
| Public | 157 (57.7) | 104 (50.5) | 58 (51.8) | 54 (51.5) | ||
| Private | 103 (37.9) | 89 (43.2) | 51 (45.5) | 41 (39.1) | ||
| Ambulance | 12 (4.4) | 13 (6.3) | 3 (2.7) | 10 (9.5) | ||
| Bystander CPR | <0.001 | 0.01 | ||||
| Chest compression | 174 (65.4) | 91 (45.5) | 73 (66.4) | 50 (49.5) | ||
| Prehospital defibrillation by bystanders | 13 (4.9) | 2 (1.0) | 0.02 | 5 (4.6) | 2 (2.0) | 0.31 |
| Primary cardiac rhythm at the scene | <0.001 | <0.001 | ||||
| Shockable | 233 (90.3) | 99 (48.7) | 100 (92.6) | 53 (53.00) | ||
| Prehospital defibrillation by EMS providers | 250 (89.9) | 111 (53.6) | <0.001 | 103 (91.2) | 58 (55.2) | <0.001 |
| Epinephrine use by EMS providers | 16 (5.7) | 29 (13.8) | 0.002 | 12 (10.4) | 18 (17.1) | 0.15 |
| Prehospital ROSC, yes | 226 (81.0) | 34 (16.2) | <0.001 | 93 (80.9) | 18 (17.1) | <0.001 |
| Primary cardiac rhythm at hospital | <0.001 | 0.005 | ||||
| Shockable | 23 (53.5) | 37 (21.9) | 10 (71.4) | 20 (24.1) | ||
| Full dose of epinephrine used during CPR in the hospital (mg) | 1.0 ± 2.8 | 6.2 ± 6.1 | <0.001 | 1.2 ± 2.9 | 5.0 ± 5.2 | <0.001 |
| SBP (mmHg) | 129.4 ± 36.4 | 122.0 ± 44.8 | 0.19 | 133.7 ± 39.3 | 122.9 ± 41.1 | 0.2 |
| DBP (mmHg) | 83.7± 20.5 | 74.7 ± 27.8 | 0.01 | 85.2 ± 27.9 | 78.6 ± 29.1 | 0.28 |
| HR (beats/min) | 101.8 ± 28.7 | 102.6 ± 34.1 | 0.86 | 99.3 ± 29.7 | 95.7 ± 35.7 | 0.61 |
| Troponin I | 85.8 ± 705.6 | 21.6 ± 127.6 | 0.28 | 39.8 ± 283.6 | 7.4 ± 24.4 | 0.31 |
| E-CPR | 9 (3.9) | 55 (26.1) | <0.001 | 3 (2.7) | 22 (25.0) | <0.001 |
| Targeted temperature management | 103 (38.0) | 82 (40.0) | 0.63 | 37 (33.6) | 34 (33.3) | 0.96 |
| PCI | 85 (36.8) | 76 (36.9) | 0.98 | 35 (30.4) | 38 (45.8) | 0.03 |
| Vasopressor use in the hospital | 137 (50.4) | 184 (88.0) | <0.001 | 59 (53.6) | 95 (90.5) | <0.001 |
| Time from EMS call to scene arrival, min | 6.9 ± 3.3 | 8.1 ± 3.9 | <0.001 | 7.6 ± 4.9 | 8.8 ± 5.0 | 0.08 |
| Time from collapse to ED arrival, min | 30.2 ± 13.4 | 30.5 ± 14.7 | 0.84 | 30.9 ± 14.3 | 30.7 ± 13.3 | 0.91 |
| Time from collapse to ROSC, min | 18.3 ± 11.7 | 42.0 ± 24.1 | <0.001 | 19.6 ± 16.5 | 42.6 ± 22.9 | <0.001 |
| Time from ED arrival to CAG, min | 2184.0 ± 4916.1 | 483.7 ± 2535.2 | <0.001 | 2218.1 ± 6287.7 | 828.4 ± 4676.9 | 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beom, J.H.; Park, I.; You, J.S.; Roh, Y.H.; Kim, M.J.; Park, Y.S.; on behalf of the Korean Cardiac Arrest Research Consortium Investigators. Predictive Model of Good Clinical Outcomes in Patients Undergoing Coronary Angiography after Out-of-Hospital Cardiac Arrest: A Prospective, Multicenter Observational Study Conducted by the Korean Cardiac Arrest Research Consortium. J. Clin. Med. 2021, 10, 3695. https://doi.org/10.3390/jcm10163695
Beom JH, Park I, You JS, Roh YH, Kim MJ, Park YS, on behalf of the Korean Cardiac Arrest Research Consortium Investigators. Predictive Model of Good Clinical Outcomes in Patients Undergoing Coronary Angiography after Out-of-Hospital Cardiac Arrest: A Prospective, Multicenter Observational Study Conducted by the Korean Cardiac Arrest Research Consortium. Journal of Clinical Medicine. 2021; 10(16):3695. https://doi.org/10.3390/jcm10163695
Chicago/Turabian StyleBeom, Jin Ho, Incheol Park, Je Sung You, Yun Ho Roh, Min Joung Kim, Yoo Seok Park, and on behalf of the Korean Cardiac Arrest Research Consortium (KoCARC) Investigators. 2021. "Predictive Model of Good Clinical Outcomes in Patients Undergoing Coronary Angiography after Out-of-Hospital Cardiac Arrest: A Prospective, Multicenter Observational Study Conducted by the Korean Cardiac Arrest Research Consortium" Journal of Clinical Medicine 10, no. 16: 3695. https://doi.org/10.3390/jcm10163695






