Pyuria as a Predictive Marker of Bacillus Calmette–Guérin Unresponsiveness in Non-Muscle Invasive Bladder Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Approval and Informed Consent
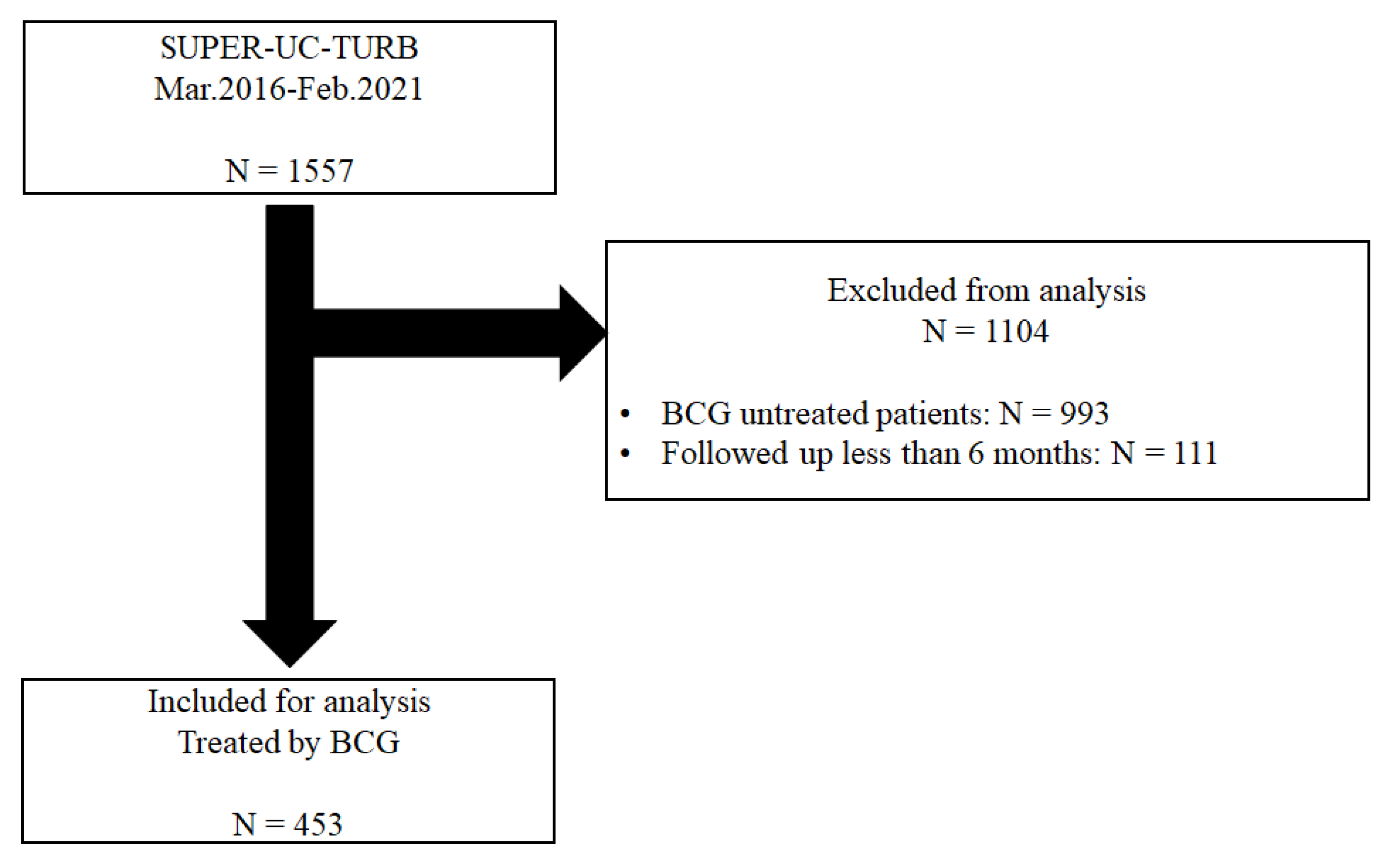
2.2. Patient Selection and Cohort Follow-Up Protocols
2.3. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Univariate and Multivariate Logistic Regression for BCG Unresponsiveness
3.3. Kaplan–Meier Curve with the Log-Rank Test for Recurrence- and Progression-Free Survival with or without Preoperative Pyuria in BCG-Treated Patients with Non-Muscle Invasive Bladder Cancer
3.4. Univariate and Multivariate Analysis Using the Cox Proportional Hazards Regression Model
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Babjuk, M.; Burger, M.; Compérat, E.M.; Gontero, P.; Mostafid, A.H.; Palou, J.; van Rhijn, B.W.G.; Rouprêt, M.; Shariat, S.F.; Sylvester, R.; et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (TaT1 and Carcinoma In Situ)—2019 Update. Eur. Urol. 2019, 76, 639–657. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.S.; Boorjian, S.A.; Chou, R.; Clark, P.E.; Daneshmand, S.; Konety, B.R.; Pruthi, R.; Quale, D.Z.; Ritch, C.R.; Seigne, J.D.; et al. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline. J. Urol. 2016, 196, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Kamat, A.M.; Sylvester, R.J.; Böhle, A.; Palou, J.; Lamm, D.L.; Brausi, M.; Soloway, M.; Persad, R.; Buckley, R.; Colombel, M.; et al. Definitions, end points, and clinical trial designs for non-muscle-invasive bladder cancer: Recommendations from the International Bladder Cancer Group. J. Clin. Oncol. 2016, 34, 1935–1944. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Kamat, A.M. Predictors of Response to Intravesical Therapy. Urol. Clin. N. Am. 2020, 47, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Pettenati, C.; Ingersoll, M.A. Mechanisms of BCG immunotherapy and its outlook for bladder cancer. Nat. Rev. Urol. 2018, 15, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.; Jung, J.H.; Jeong, C.W.; Kwak, C.; Kim, H.H.; Ku, J.H. Clinical Significance of Pre-treated Neutrophil-Lymphocyte Ratio in the Management of Urothelial Carcinoma: A Systemic Review and Meta-Analysis. Front. Oncol. 2019, 9, 1365. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Y.; Hao, X.Y.; Ma, T.M.; Dai, H.X.; Song, Y.S. The Prognostic Value of Platelet-to-Lymphocyte Ratio in Urological Cancers: A Meta-Analysis. Sci. Rep. 2017, 7, 15387. [Google Scholar] [CrossRef] [Green Version]
- Satake, N.; Ohno, Y.; Nakashima, J.; Ohori, M.; Tachibana, M. Prognostic value of preoperative pyuria in patients with non-muscle-invasive bladder cancer. Int. J. Urol. 2015, 22, 645–649. [Google Scholar] [CrossRef]
- Jeong, C.W.; Suh, J.; Yuk, H.D.; Tae, B.S.; Kim, M.; Keam, B.; Kim, J.H.; Kim, S.Y.; Cho, J.Y.; Kim, S.H.; et al. Establishment of the Seoul National University Prospectively Enrolled Registry for Genitourinary Cancer (SUPER-GUC): A prospective, multidisciplinary, bio-bank linked cohort and research platform. Investig. Clin. Urol. 2019, 60, 235. [Google Scholar] [CrossRef]
- Suh, J.; Moon, K.C.; Jung, J.H.; Lee, J.; Song, W.H.; Kang, Y.J.; Jeong, C.W.; Kwak, C.; Kim, H.H.; Ku, J.H. BCG instillation versus radical cystectomy for high-risk NMIBC with squamous/glandular histologic variants. Sci. Rep. 2019, 9, 15268. [Google Scholar] [CrossRef] [Green Version]
- Jeon, B.J.; Tae, B.S.; Choi, H.; Bae, J.H.; Kim, J.W.; Park, H.S.; Park, J.Y. Preoperative sterile pyuria as a prognostic biomarker for intravesical recurrence in upper urinary tract urothelial carcinoma. Investig. Clin. Urol. 2020, 61, 51–58. [Google Scholar] [CrossRef]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef] [Green Version]
- Nepple, K.G.; Lightfoot, A.J.; Rosevear, H.M.; O’Donnell, M.A.; Lamm, D.L. Bacillus Calmette-Guréin with or without interferon α-2b and megadose versus recommended daily allowance vitamins during induction and maintenance intravesical treatment of nonmuscle invasive bladder cancer. J. Urol. 2010, 184, 1915–1919. [Google Scholar] [CrossRef]
- Li, R.; Tabayoyong, W.B.; Guo, C.C.; González, G.M.N.; Navai, N.; Grossman, H.B.; Dinney, C.P.; Kamat, A.M. Prognostic Implication of the United States Food and Drug Administration-defined BCG-unresponsive Disease. Eur. Urol. 2019, 75, 8–10. [Google Scholar] [CrossRef]
- Kikuchi, E.; Hayakawa, N.; Fukumoto, K.; Shigeta, K.; Matsumoto, K. Bacillus Calmette–Guérin-unresponsive non-muscle-invasive bladder cancer: Its definition and future therapeutic strategies. Int. J. Urol. 2020, 27, 108–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamat, A.M.; Li, R.; O’Donnell, M.A.; Black, P.C.; Roupret, M.; Catto, J.W.; Comperat, E.; Ingersoll, M.A.; Witjes, W.P.; McConkey, D.J.; et al. Predicting Response to Intravesical Bacillus Calmette-Guérin Immunotherapy: Are We There Yet? A Systematic Review. Eur. Urol. 2018, 73, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Faba, Ó.R.; Pisano, F.; Krajewski, W.; Breda, A.; Palou, J. Salvage Therapies for Non–muscle-invasive Bladder Cancer: Who Will Respond to Bacillus Calmette-Guérin? Predictors and Nomograms. Urol. Clin. N. Am. 2020, 47, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Poli, G.; Cochetti, G.; Boni, A.; Egidi, M.G.; Brancorsini, S.; Mearini, E. Characterization of inflammasome-related genes in urine sediments of patients receiving intravesical BCG therapy. Urol. Oncol. Semin. Orig. Investig. 2017, 35, 674.e19–674.e24. [Google Scholar] [CrossRef] [PubMed]
- Kardoust Parizi, M.; Shariat, S.F.; Margulis, V.; Mori, K.; Lotan, Y. Value of tumour-infiltrating immune cells in predicting response to intravesical BCG in patients with non-muscle-invasive bladder cancer: A systematic review and meta-analysis. BJU Int. 2021, 127, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Miyake, M.; Tatsumi, Y.; Matsumoto, H.; Nagao, K.; Matsuyama, H.; Inamoto, T.; Azuma, H.; Yasumoto, H.; Shiina, H.; Fujimoto, K. Outcomes of subsequent non-muscle-invasive bladder cancer treated with intravesical Bacillus Calmette-Guérin after radical nephroureterectomy for upper urinary tract urothelial carcinoma. BJU Int. 2018, 121, 764–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elawdy, M.M.; Osman, Y.; Taha, D.E.; Zahran, M.H.; El-Halwagy, S.; Garba, M.; El-Harraz, A.M. Risk factors and prognosis of intravesical recurrence after surgical management of upper tract urothelial carcinoma: A 30-year single centre experience. Arab J. Urol. 2017, 15, 216–222. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; Jeong, C.W.; Kwak, C.; Kim, H.H.; Ku, J.H. Novel nomograms to predict recurrence and progression in primary non-muscle-invasive bladder cancer: Validation of predictive efficacy in comparison with European Organization of Research and Treatment of Cancer scoring system. World J. Urol. 2019, 37, 1867–1877. [Google Scholar] [CrossRef]
- Azuma, T.; Nagase, Y.; Oshi, M. Pyuria predicts poor prognosis in patients with non-muscle-invasive bladder cancer. Clin. Genitourin. Cancer 2013, 11, 331–336. [Google Scholar] [CrossRef]
- Bhardwaj, S.S.; Camacho, F.; Derrow, A.; Fleischer, A.B.; Feldman, S.R. Statistical Significance and Clinical Relevance. Arch. Dermatol. 2004, 140, 1520–1523. [Google Scholar] [CrossRef]
- Poletajew, S.; Gajewska, D.; Kaczmarek, K.; Krajewski, W.; Łykowski, M.; Sondka-Migdalska, J.; Borowik, M.; Buraczyński, P.; Dzięgała, M.; Przudzik, M.; et al. Preoperative pyuria predicts the presence of high-grade bladder carcinoma in patients with bladder tumors. Cent. Eur. J. Urol. 2020, 73, 423. [Google Scholar] [CrossRef]
- Sui, X.; Lei, L.; Chen, L.; Xie, T.; Li, X. Inflammatory microenvironment in the initiation and progression of bladder cancer. Oncotarget 2017, 8, 93279–93294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, G.; Yoshida, T.; Yanishi, M.; Saito, R.; Murota, T.; Kawa, G.; Kinoshita, H.; Matsuda, T. Preoperative Pyuria Predicts for Intravesical Recurrence in Patients With Urothelial Carcinoma of the Upper Urinary Tract After Radical Nephroureterectomy Without a History of Bladder Cancer. Clin. Genitourin. Cancer 2020, 18, e167–e173. [Google Scholar] [CrossRef]
- Kim, B.S.; Tae, B.S.; Ku, J.H.; Kwak, C.; Kim, H.H.; Jeong, C.W. Rate and association of lower urinary tract infection with recurrence after transurethral resection of bladder tumor. Investig. Clin. Urol. 2018, 59, 10–17. [Google Scholar] [CrossRef] [PubMed]

| BCG-Effective | BCG-Unresponsive | p-Value | |
|---|---|---|---|
| Number of Patients | N = 283 | N = 170 | |
| Age, mean ± SD (IQR) | 68.2 ± 9.8 (62.0–77.0) | 67.7 ± 12.3 (62.0–76.0) | 0.629 |
| Previous TUR history, n (%) | 68 (24.0) | 52 (30.6) | 0.155 |
| Female, n (%) | 41 (14.5) | 23 (13.5) | 0.885 |
| Previous BCG treatment history, n (%) | 23 (8.1) | 20 (11.8) | 0.266 |
| Upper Tract Urothelial Carcinoma history, n (%) | 28 (9.9) | 28 (16.5) | 0.056 |
| Microscopic haematuria, n (%) | 143 (52.8) | 95 (57.2) | 0.418 |
| Pyuria, n (%) | 97 (35.9) | 77 (46.4) | 0.039 |
| BCG dose reduction, n (%) | 11 (3.9) | 7 (4.1) | 0.882 |
| Pathologic T stage, n (%) | 0.950 | ||
| Ta | 162 (57.2) | 96 (56.5) | |
| T1 | 121 (42.8) | 74 (43.5) | |
| Tumour size (>3 cm), n (%) | 97 (35.0) | 74 (44.6) | 0.057 |
| Multiple tumours, n (%) | 160 (58.2) | 119 (71.7) | <0.001 |
| Concomitant CIS, n (%) | 63 (22.3) | 37 (21.8) | 0.995 |
| Histologic variants, n (%) | 13 (4.6) | 7 (4.1) | 0.998 |
| Univariate | Multivariable | |||||
|---|---|---|---|---|---|---|
| Variables | Odds Ratio | Confidence Interval | p-Value | Odds Ratio | Confidence Interval | p-Value |
| Age | 0.799 | |||||
| Age < 65 | Ref. | |||||
| 65 ≤ Age ≤ 75 | 0.89 | 0.51–1.54 | ||||
| Age > 75 | 1.02 | 0.60–1.74 | ||||
| Previous TUR history | 1.39 | 0.91–2.13 | 0.126 | |||
| Sex (Female) | 0.92 | 0.53–1.60 | 0.777 | |||
| Previous BCG treatment history | 1.51 | 0.80–2.84 | 0.203 | |||
| Upper Tract Urothelial Carcinoma history | 1.80 | 1.02–3.15 | 0.041 | 1.86 | 1.04–3.32 | 0.035 |
| BCG dose reduction (half) | 1.05 | 0.40–2.76 | 0.921 | |||
| Pathologic T stage (T1) | 1.03 | 0.70–1.51 | 0.872 | |||
| Pyuria (more than W5) | 1.54 | 1.04–2.29 | 0.031 | 1.51 | 1.01–2.27 | 0.047 |
| Large tumour (3 cm) | 1.49 | 1.01–2.21 | 0.046 | 1.39 | 0.136 | |
| Multiple tumour | 1.82 | 1.20–2.75 | 0.005 | 1.80 | 1.18–2.75 | <0.001 |
| Concomitant CIS | 0.97 | 0.61–1.54 | 0.902 | |||
| Univariate | Multivariable | |||||
|---|---|---|---|---|---|---|
| Variables | Hazard Ratio | Confidence Interval | p-Value | Hazard Ratio | Confidence Interval | p-Value |
| Age | 0.834 | |||||
| Age < 65 | Ref. | |||||
| 65 ≤ Age ≤ 75 | 0.88 | 0.57–1.37 | ||||
| Age > 75 | 0.95 | 0.63–1.44 | ||||
| Previous TUR history | 1.09 | 0.78–1.52 | 0.614 | |||
| Sex (Female) | 0.94 | 0.60–1.48 | 0.802 | |||
| Previous BCG treatment history | 1.22 | 0.76–1.94 | 0.414 | |||
| Upper Tract Urothelial Carcinoma history | 1.36 | 0.92–2.04 | 0.143 | |||
| BCG dose reduction (half) | 1.21 | 0.57–2.58 | 0.624 | |||
| Pathologic T stage (T1) | 1.18 | 0.86–1.60 | 0.302 | |||
| Pyuria (more than W5) | 1.47 | 1.08–2.00 | 0.013 | 1.31 | 0.95–1.80 | 0.095 |
| Large tumour | 1.51 | 1.11–2.06 | 0.009 | 1.36 | 0.98–1.87 | 0.063 |
| Multiple tumour | 1.59 | 1.13–2.24 | 0.008 | 1.51 | 1.07–2.15 | 0.019 |
| Concomitant CIS | 0.97 | 0.68–1.41 | 0.902 | |||
| Univariate | Multivariable | |||||
|---|---|---|---|---|---|---|
| Variables | Hazard Ratio | Confidence Interval | p-Value | Hazard Ratio | Confidence Interval | p-Value |
| Age | 0.866 | |||||
| Age < 65 | Ref. | |||||
| 65 ≤ Age ≤ 75 | 0.66 | 0.15–2.97 | ||||
| Age > 75 | 0.81 | 0.20–3.25 | ||||
| Previous TUR history | 0.64 | 0.21–1.97 | 0.435 | |||
| Sex (Female) | 0.49 | 0.06–3.80 | 0.498 | |||
| Previous BCG treatment history | 1.62 | 0.36–7.33 | 0.529 | |||
| Upper Tract Urothelial Carcinoma history | 0.56 | 0.07–4.31 | 0.578 | |||
| BCG dose reduction (half) | 2.40 | 0.31–18.59 | 0.402 | |||
| Pathologic T stage (T1) | 3.33 | 1.02–10.89 | 0.046 | 0.157 | ||
| Pyuria (more than W5) | 3.52 | 1.08–11.44 | 0.036 | 4.51 | 1.22–16.66 | 0.024 |
| Large tumour | 2.34 | 0.74–7.38 | 0.147 | |||
| Multiple tumour | 6.32 | 0.82–48.93 | 0.078 | 5.97 | 0.77–46.28 | 0.087 |
| Concomitant CIS | 2.20 | 0.72–6.72 | 0.167 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suh, J.; Yuk, H.D.; Jeong, C.W.; Kwak, C.; Kim, H.H.; Ku, J.H. Pyuria as a Predictive Marker of Bacillus Calmette–Guérin Unresponsiveness in Non-Muscle Invasive Bladder Cancer. J. Clin. Med. 2021, 10, 3764. https://doi.org/10.3390/jcm10173764
Suh J, Yuk HD, Jeong CW, Kwak C, Kim HH, Ku JH. Pyuria as a Predictive Marker of Bacillus Calmette–Guérin Unresponsiveness in Non-Muscle Invasive Bladder Cancer. Journal of Clinical Medicine. 2021; 10(17):3764. https://doi.org/10.3390/jcm10173764
Chicago/Turabian StyleSuh, Jungyo, Hyeong Dong Yuk, Chang Wook Jeong, Cheol Kwak, Hyeon Hoe Kim, and Ja Hyeon Ku. 2021. "Pyuria as a Predictive Marker of Bacillus Calmette–Guérin Unresponsiveness in Non-Muscle Invasive Bladder Cancer" Journal of Clinical Medicine 10, no. 17: 3764. https://doi.org/10.3390/jcm10173764
APA StyleSuh, J., Yuk, H. D., Jeong, C. W., Kwak, C., Kim, H. H., & Ku, J. H. (2021). Pyuria as a Predictive Marker of Bacillus Calmette–Guérin Unresponsiveness in Non-Muscle Invasive Bladder Cancer. Journal of Clinical Medicine, 10(17), 3764. https://doi.org/10.3390/jcm10173764






