Controversy and Consideration of Refractive Surgery in Patients with Heritable Disorders of Connective Tissue
Abstract
1. Introduction
2. Osteogenesis Imperfecta
3. Ehlers Danlos Syndrome
4. Marfan Syndrome
5. Loeys-Dietz Syndrome
6. Epidermolysis Bullosa
7. Stickler Syndrome
8. Wagner Syndrome
9. Pseudoxanthoma Elasticum
10. Refractive Surgery Considerations and Consultation
11. Specific Recommendations
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Randleman, J.B.; Woodward, M.; Lynn, M.J.; Stulting, R.D. Risk Assessment for Ectasia after Corneal Refractive Surgery. Ophthalmology 2008, 115, 37–50.e4. [Google Scholar] [CrossRef]
- Kamiya, K.; Hagishima, M.; Fujimura, F.; Shimizu, K. Factors affecting corneal hysteresis in normal eyes. Graefe’s Arch. Clin. Exp. Ophthalmol. 2008, 246, 1491–1494. [Google Scholar] [CrossRef]
- Świerkowska, J.; Gajecka, M. Genetic factors influencing the reduction of central corneal thickness in disorders affecting the eye. Ophthalmic Genet. 2017, 38, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Thomas, I.H.; DiMeglio, L.A. Advances in the Classification and Treatment of Osteogenesis Imperfecta. Curr. Osteoporos. Rep. 2016, 14, 1–9. [Google Scholar] [CrossRef]
- Treurniet, S.; Burger, P.; Ghyczy, E.A.; Verbraak, F.D.; Tafili, K.R.C.; Micha, D.; Bravenboer, N.; Ralston, S.H.; de Vries, R.; Moll, A.C.; et al. Ocular characteristics and complications in patients with osteogenesis imperfecta: A systematic review. Acta Ophthalmol. 2021. [Google Scholar] [CrossRef]
- Brady, A.F.; Demirdas, S.; Fournel-Gigleux, S.; Ghali, N.; Giunta, C.; Kapferer-Seebacher, I.; Kosho, T.; Mendoza-Londono, R.; Pope, M.F.; Rohrbach, M.; et al. The Ehlers-Danlos syndromes, rare types. Am. J. Med. Genet. C Semin. Med. Genet. 2017, 175, 70–115. [Google Scholar] [CrossRef]
- De Paepe, A.; Malfait, F. The Ehlers-Danlos syndrome, a disorder with many faces. Clin. Genet. 2012, 82, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Esfandiari, H.; Mohammad-Rabei, H.; Mets, M.B. Management strategies of ocular abnormalities in patients with marfan syndrome: Current perspective. J. Ophthalmic Vis. Res. 2019, 14, 71–77. [Google Scholar] [CrossRef]
- Bitterman, A.D.; Sponseller, P.D. Marfan Syndrome. J. Am. Acad. Orthop. Surg. 2017, 25, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Busch, C.; Voitl, R.; Goergen, B.; Zemojtel, T.; Gehle, P.; Salchow, D.J. Ocular findings in Loeys-Dietz syndrome. Br. J. Ophthalmol. 2018, 102, 1036–1040. [Google Scholar] [CrossRef]
- Mahdavi, M.; Javadi, M.-A. External Ocular Manifestations in Autosomal Dominant Dystrophic Epidermolysis Bullosa; a Case Report. J. Ophthalmic Vis. Res. 2008, 3, 70–73. [Google Scholar] [PubMed]
- Fine, J.-D.; Johnson, L.B.; Weiner, M.; Stein, A.; Cash, S.; Deleoz, J.; Devries, D.T.; Suchindran, C. Eye involvement in inherited epidermolysis bullosa: Experience of the National Epidermolysis Bullosa Registry. Am. J. Ophthalmol. 2004, 138, 254–262. [Google Scholar] [CrossRef]
- Huang, L.; Chen, C.; Wang, Z.; Sun, L.; Li, S.; Zhang, T.; Luo, X.; Ding, X. Mutation Spectrum and De Novo Mutation Analysis in Stickler Syndrome Patients with High Myopia or Retinal Detachment. Genes 2020, 11, 882. [Google Scholar] [CrossRef] [PubMed]
- Boysen, K.B.; La Cour, M.; Kessel, L. Ocular complications and prophylactic strategies in Stickler syndrome: A systematic literature review. Ophthalmic Genet. 2020, 41, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Jewsbury, H.; Fry, A.E.; Watts, P.; Nas, V.; Morgan, J. Congenital glaucoma in Wagner syndrome. J. AAPOS 2014, 18, 291–293. [Google Scholar] [CrossRef]
- Graemiger, R.A.; Niemeyer, G.; Schneeberger, S.A.; Messmer, E.P. Wagner Vitreoretinal Degeneration Follow-up of the Original Pedigree. Ophthalmology 1995, 102, 1830–1839. [Google Scholar] [CrossRef]
- Jen, M.; Nallasamy, S. Ocular manifestations of genetic skin disorders. Clin. Dermatol. 2016, 34, 242–275. [Google Scholar] [CrossRef]
- Egliem, M.; Zaeytijd, J.E.; Finger, R.P.; Holz, F.G.; Leroy, B.P.; Issa, P.E. An update on the ocular phenotype in patients with pseudoxanthoma elasticum. Front. Genet. 2013, 4, 14. [Google Scholar] [CrossRef]
- Louie, A.; Meyerle, C.; Francomano, C.; Srikumaran, D.; Merali, F.; Doyle, J.J.; Bower, K.; Bloom, L.; Boland, M.V.; Mahoney, N.; et al. Survey of Ehlers‒Danlos Patients’ ophthalmic surgery experiences. Mol. Genet. Genom. Med. 2020, 8, e1155. [Google Scholar] [CrossRef]
- Tatar, M.G.; Kantarci, F.A.; Yildirim, A.; Uslu, H.; Colak, H.N.; Goker, H.; Gürler, B. Risk Factors in Post-LASIK Corneal Ectasia. J. Ophthalmol. 2014, 2014, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Keleş, A.; Doğuizi, S.; Şahin, N.M.; Koç, M.; Aycan, Z. Anterior Segment Findings in Patients with Osteogenesis Imperfecta: A Case-Control Study. Cornea 2020, 39, 935–939. [Google Scholar] [CrossRef]
- MedlinePlus. Osteogenesis Imperfecta. Available online: https://medlineplus.gov/genetics/condition/osteogenesis-imperfecta/#frequency (accessed on 22 July 2021).
- Subramanian, S.; Viswanathan, V.K. Osteogenesis Imperfecta Continuing Education Activity. Available online: https://www.ncbi.nlm.nih.gov/books/NBK536957/?report=printable (accessed on 19 July 2021).
- Lagrou, L.M.; Gilbert, J.; Hannibal, M.; Caird, M.S.; Thomas, I.; Moroi, S.E.; Bohnsack, B.L. Altered corneal biomechanical properties in children with osteogenesis imperfecta. J. AAPOS 2018, 22, 183–187.e1. [Google Scholar] [CrossRef]
- Warman, M.L.; Cormier-Daire, V.; Hall, C.; Krakow, D.; Lachman, R.; LeMerrer, M.; Mortier, G.; Mundlos, S.; Nishimura, G.; Rimoin, D.L.; et al. Nosology and classification of genetic skeletal disorders: 2010 revision. Am. J. Med. Genet. A 2011, 155, 943–968. [Google Scholar] [CrossRef]
- Pesudovs, K. Orbscan mapping in Ehlers-Danlos syndrome. J. Cataract Refract. Surg. 2004, 30, 1795–1798. [Google Scholar] [CrossRef]
- Kobayashi, A.A.; Higashide, T.; Yokogawa, H.; Yamazaki, N.; Masaki, T.; Sugiyama, K. In vivo laser confocal microscopy findings of a cornea with osteogenesis imperfecta. Clin. Ophthalmol. 2014, 8, 429–433. [Google Scholar] [CrossRef][Green Version]
- Gorovoy, M.S.; Gorovoy, I.R.; Ullman, S.; Gorovoy, J.B. Descemet Stripping Automated Endothelial Keratoplasty for Spontaneous Descemet Membrane Detachment in a Patient with Osteogenesis Imperfecta. Cornea 2012, 31, 832–835. [Google Scholar] [CrossRef]
- Polat, N.; Ulucan, P.B. Nontraumatic Descemet Membrane Detachment with Tear in Osteogenesis Imperfecta. Ophthalmol. Ther. 2015, 4, 59–63. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hald, J.; Folkestad, L.; Swan, C.Z.; Wanscher, J.; Schmidt, M.; Gjørup, H.; Haubek, D.; Leonhard, C.-H.; Larsen, D.; Hjortdal, J.; et al. Osteogenesis imperfecta and the teeth, eyes, and ears—A study of non-skeletal phenotypes in adults. Osteoporos. Int. 2018, 29, 2781–2789. [Google Scholar] [CrossRef]
- Evereklioglu, C.; Madenci, E.; Bayazit, Y.A.; Yilmaz, K.; Balat, A.; Bekir, N.A. Central corneal thickness is lower in osteogenesis imperfecta and negatively correlates with the presence of blue sclera. Ophthalmic Physiol. Opt. 2002, 22, 511–515. [Google Scholar] [CrossRef]
- Magalhaes, O.A.; Rohenkohl, H.C.; de Souza, L.T.; Schuler-Faccini, L.; Félix, T.M. Collagen I Defect Corneal Profiles in Osteogenesis Imperfecta. Cornea 2018, 37, 1561–1565. Available online: www.corneajrnl.com (accessed on 19 July 2021). [CrossRef]
- MedlinePlus. Ehlers-Danlos Syndrome. Available online: https://medlineplus.gov/ehlersdanlossyndrome.html (accessed on 22 July 2021).
- Beighton, P. Serious ophthalmological complications in the Ehlers-Danlos syndrome. Br. J. Ophthalmol. 1970, 54, 263–268. [Google Scholar] [CrossRef]
- Ticho, U.; Ivry, M.; Merin, S. Brittle cornea, blue sclera, and red hair syndrome (the brittle cornea syndrome). Br. J. Ophthalmol. 1980, 64, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ben Margines, J.; Jacobs, D.; Rabinowitz, Y.S.; Hanser, E.M.; Chauhan, T.; Chung, D.; Bykhovskaya, Y.; Gaster, R.N.; Aldave, A.J. Corneal Perforation After Corneal Cross-Linking in Keratoconus Associated with Potentially Pathogenic ZNF469 Mutations. Cornea 2019, 38, 1033–1039. [Google Scholar] [CrossRef]
- Whitaker, J.K.; Alexander, P.; Chau, D.Y.; Tint, N.L. Severe conjunctivochalasis in association with classic type Ehlers-Danlos syndrome. BMC Ophthalmol. 2012, 12, 47. [Google Scholar] [CrossRef]
- Gharbiya, M.; Moramarco, A.; Castori, M.; Parisi, F.; Celletti, C.; Marenco, M.; Mariani, I.; Grammatico, P.; Camerota, F. Ocular Features in Joint Hypermobility Syndrome/Ehlers-Danlos Syndrome Hypermobility Type: A Clinical and In Vivo Confocal Microscopy Study. Am. J. Ophthalmol. 2012, 154, 593–600.e1. [Google Scholar] [CrossRef] [PubMed]
- Cameron, J.A. Corneal Abnormalities in Ehlers-Danlos Syndrome Type VI. Cornea 1993, 12, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.M.B.; Spencer, H.L.; Daly, S.B.; Manson, F.; Zeef, L.A.; Urquhart, J.; Zoppi, N.; Bonshek, R.; Tosounidis, I.; Mohan, M.; et al. Mutations in PRDM5 in Brittle Cornea Syndrome Identify a Pathway Regulating Extracellular Matrix Development and Maintenance. Am. J. Hum. Genet. 2011, 88, 767–777. [Google Scholar] [CrossRef]
- McDermott, M.L.; Holladay, J.T.; Liu, D.; Puklin, J.E.; Shin, D.H.; Cowden, J.W. Corneal topography in Ehlers-Danlos syndrome. J. Cataract Refract. Surg. 1998, 24, 1212–1215. [Google Scholar] [CrossRef]
- Segev, F.; Héon, E.; Cole, W.G.; Wenstrup, R.J.; Young, F.; Slomovic, A.R.; Rootman, D.S.; Whitaker-Menezes, D.; Chervoneva, I.; Birk, D.E. Structural Abnormalities of the Cornea and Lid Resulting from Collagen V Mutations. Investig. Opthalmology Vis. Sci. 2006, 47, 565–573. [Google Scholar] [CrossRef]
- Colombi, M.; Dordoni, C.; Venturini, M.; Ciaccio, C.; Morlino, S.; Chiarelli, N.; Zanca, A.; Calzavara-Pinton, P.; Zoppi, N.; Castori, M.; et al. Spectrum of mucocutaneous, ocular and facial features and delineation of novel presentations in 62 classical Ehlers-Danlos syndrome patients. Clin. Genet. 2017, 92, 624–631. [Google Scholar] [CrossRef]
- Ihme, A.; Risteli, L.; Krieg, T.; Risteli, J.; Feldmann, U.; Kruse, K.; Müller, P.K.; Ristell, J. Biochemical characterization of variants of the Ehlers-Danlos syndrome type VI. Eur. J. Clin. Investig. 1983, 13, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Ramappa, M.; Wilson, M.E.; Rogers, R.C.; Trivedi, R. Brittle cornea syndrome: A case report and comparison with Ehlers Danlos syndrome. J. AAPOS 2014, 18, 509–511. [Google Scholar] [CrossRef]
- Byers, P.H.; Belmont, J.; Black, J.; De Backer, J.; Frank, M.; Jeunemaitre, X.; Johnson, D.; Pepin, M.; Robert, L.; Sanders, L.; et al. Diagnosis, natural history, and management in vascular Ehlers-Danlos syndrome. Am. J. Med. Genet. C Semin. Med. Genet. 2017, 175, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Demirdas, S.; Dulfer, E.; Robert, L.; Kempers, M.; Van Beek, D.; Micha, D.; Van Engelen, B.; Hamel, B.; Schalkwijk, J.; Loeys, B.; et al. Recognizing the tenascin-X deficient type of Ehlers–Danlos syndrome: A cross-sectional study in 17 patients. Clin. Genet. 2017, 91, 411–425. [Google Scholar] [CrossRef]
- Dietz, H.; McKusick, V.A. Marfan Syndrome. Available online: https://rarediseases.org/rare-diseases/marfan-syndrome/ (accessed on 22 July 2021).
- Gehle, P.; Goergen, B.; Pilger, D.; Ruokonen, P.; Robinson, P.N.; Salchow, D.J. Biometric and structural ocular manifestations of Marfan syndrome. PLoS ONE 2017, 12, e0183370. [Google Scholar] [CrossRef] [PubMed]
- Loeys, B.; Chen, J.; Neptune, E.R.; Judge, D.; Podowski, M.; Holm, T.; Meyers, J.; Leitch, C.C.; Katsanis, N.; Sharifi, N.; et al. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat. Genet. 2005, 37, 275–281. [Google Scholar] [CrossRef]
- Loughborough, W.W.; Minhas, K.S.; Rodrigues, J.; Lyen, S.M.; Burt, H.E.; Manghat, N.; Brooks, M.J.; Stuart, G.; Hamilton, M.C.K. Cardiovascular Manifestations and Complications of Loeys-Dietz Syndrome: CT and MR Imaging Findings. Radiographics 2018, 38, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Hoornaert, K.P.; Vereecke, I.; Dewinter, C.; Rosenberg, T.; A Beemer, F.; Leroy, J.G.; Bendix, L.; Björck, E.; Bonduelle, M.; Boute, O.; et al. Stickler syndrome caused by COL2A1 mutations: Genotype–phenotype correlation in a series of 100 patients. Eur. J. Hum. Genet. 2010, 18, 872–880. [Google Scholar] [CrossRef]
- Goldenberg, P.C. Stickler Syndrome. Available online: https://rarediseases.org/rare-diseases/stickler-syndrome/ (accessed on 21 July 2021).
- Araújo, J.R.; Tavares-Ferreira, J.; Silva, S.E.; Rocha, P.; Brandão, E.; Faria, P.A.; Falcão-Reis, F.; Rocha-Sousa, A. WAGNER syndrome: Anatomic, functional and genetic characterization of a Portuguese family. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 256, 163–171. [Google Scholar] [CrossRef]
- MedlinePlus. Wagner Syndrome. Available online: https://medlineplus.gov/genetics/condition/wagner-syndrome/#:~:text=Wagner%20syndrome%20is%20a%20rare,individuals%20are%20from%20the%20Netherlands (accessed on 21 July 2021).
- Finger, R.P.; Charbel Issa, P.; Ladewig, M.S.; Götting, C.; Szliska, C.; Scholl, H.P.; Holz, F.G. Pseudoxanthoma Elasticum: Genetics, Clinical Manifestations and Therapeutic Approaches. Surv. Ophthalmol. 2009, 54, 272–285. [Google Scholar] [CrossRef]
- Germain, D.P. Pseudoxanthoma elasticum. Orphanet J. Rare Dis. 2017, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Beene, L.C.; Traboulsi, E.I.; Seven, I.; Ford, M.R.; Roy, A.S.; Butler, R.S.; Dupps, W.J. Corneal Deformation Response and Ocular Geometry: A Noninvasive Diagnostic Strategy in Marfan Syndrome. Am. J. Ophthalmol. 2016, 161, 56–64.e1. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Scheibenberger, D.; Frings, A.; Steinberg, J.; Schüler, H.; Druchkiv, V.; Katz, T.; Von Kodolitsch, Y.; Linke, S. Ocular manifestation in Marfan syndrome: Corneal biomechanical properties relate to increased systemic score points. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 256, 1159–1163. [Google Scholar] [CrossRef] [PubMed]
- Sultan, G.; Baudouin, C.; Auzerie, O.; Jean, M.D.S.; Goldschild, M.; Pisella, P.-J. Cornea in Marfan disease: Orbscan and in vivo confocal microscopy analysis. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1757–1764. [Google Scholar]
- Nahum, Y.; Spierer, A. Ocular features of Marfan syndrome: Diagnosis and management. Isr. Med. Assoc. J. 2008, 10, 179–181. [Google Scholar] [PubMed]
- Maumenee, I.H. The eye in the Marfan syndrome. Birth Defects Orig. Artic. Ser. 1982, 18, 515–524. [Google Scholar] [PubMed]
- Kinori, M.; Wehrli, S.; Kassem, I.S.; Azar, N.F.; Maumenee, I.H.; Mets, M.B. Biometry Characteristics in Adults and Children with Marfan Syndrome: From the Marfan Eye Consortium of Chicago. Am. J. Ophthalmol. 2017, 177, 144–149. [Google Scholar] [CrossRef]
- Konradsen, T.R.; Zetterström, C. A descriptive study of ocular characteristics in Marfan syndrome. Acta Ophthalmol. 2013, 91, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Salchow, D.J.; Gehle, P. Ocular manifestations of Marfan syndrome in children and adolescents. Eur. J. Ophthalmol. 2019, 29, 38–43. [Google Scholar] [CrossRef]
- MedlinePlus. Loeys-Dietz Syndrome. Available online: https://medlineplus.gov/genetics/condition/loeys-dietz-syndrome/ (accessed on 21 July 2021).
- Loeys, B.L.; Dietz, H.C. Loeys-Dietz Syndrome. In GeneReviews®; Adam, M.P., Ardinger, H.H., Pagon, R.A., Eds.; University of Washington: Seattle, WA, USA, 2008. [Google Scholar]
- Van Hemelrijk, C.; Renard, M.; Loeys, B. The Loeys–Dietz syndrome: An update for the clinician. Curr. Opin. Cardiol. 2010, 25, 546–551. [Google Scholar] [CrossRef]
- Van De Laar, I.M.B.H.; Van Der Linde, D.; Oei, E.H.G.; Bos, P.K.; Bessems, J.H.; Bierma-Zeinstra, S.M.; Van Meer, B.L.; Pals, G.; Oldenburg, R.A.; Bekkers, J.A.; et al. Phenotypic spectrum of the SMAD3-related aneurysms–osteoarthritis syndrome. J. Med. Genet. 2012, 49, 47–57. [Google Scholar] [CrossRef] [PubMed]
- MacCarrick, G.; Black, J.H.; Bowdin, S.; El-Hamamsy, I.; Frischmeyer-Guerrerio, P.A.; Guerrerio, A.L.; Sponseller, P.D.; Loeys, B.; Dietz, H.C. Loeys–Dietz syndrome: A primer for diagnosis and management. Genet. Med. 2014, 16, 576–587. [Google Scholar] [CrossRef] [PubMed]
- Laimer, M.; Prodinger, C.; Bauer, J.W. Hereditäre Epidermolysen. JDDG J. Ger. Soc. Dermatol. 2015, 13, 1125–1134. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Dogru, M.; Tsubota, K. Ocular Surface Findings in Hallopeau-Siemens Subtype of Dystrophic Epidermolysis Bullosa Report of a Case and Literature Review. Cornea 2005, 24, 474–479. [Google Scholar] [CrossRef]
- Hung Chow Lee, B.W.; Tan, J.; Radjenovic, M.; Tat, L.; Murrell, D.F.; Coroneo, M.T. Prospective study of the ocular manifestations in epidermolysis bullosa and autoimmune blistering diseases identifies dry eye disease. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4707. Available online: https://iovs.arvojournals.org/article.aspx?articleid=2744191 (accessed on 5 July 2021).
- Tong, L.; Hodgkins, P.R.; Denyer, J.; Brosnahan, D.; Harper, J.; Russell-Eggitt, I.; I Taylor, D.S.; Atherton, D. The eye in epidermolysis bullosa. Br. J. Ophthalmol. 1999, 83, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Robin, N.H.; Moran, R.T.; Ala-Kokko, L. Stickler Syndrome. In GeneReviews®; Adam, M.P., Ardinger, H.H., Pagon, R.A., Eds.; University of Washington: Seattle, WA, USA, 1993–2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1302/ (accessed on 22 July 2021).
- Zhou, L.; Xiao, X.; Li, S.; Jia, X.; Wang, P.; Sun, W.; Zhang, F.; Li, J.; Li, T.; Zhang, Q. Phenotypic characterization of patients with early-onset high myopia due to mutations in COL2A1 or COL11A1: Why not Stickler syndrome? Mol. Vis. 2018, 24, 560–573. [Google Scholar]
- Boothe, M.; Morris, R.; Robin, N. Stickler Syndrome: A Review of Clinical Manifestations and the Genetics Evaluation. J. Pers. Med. 2020, 10, 105. [Google Scholar] [CrossRef]
- Kloeckener-Gruissem, B.; Amstutz, C. VCAN-Related Vitreoretinopathy. Available online: https://www.ncbi.nlm.nih.gov/books/ (accessed on 19 July 2021).
- Jansen, L. Degeneratio Hyaloideo-Retinalis Hereditaria. Ophthalmologica 1962, 144, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Georgalas, I.; Tservakis, I.; Papaconstantinou, D.; Kardara, M.; Koutsandrea, C.; Ladas, I. Pseudoxanthoma elasticum, ocular manifestations, complications and treatment. Clin. Exp. Optom. 2011, 94, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.H.-S.; Vargas-Mora, P.; Aranibar, L. Xantogranuloma juvenil: Una entidad con amplio espectro clínico. Actas Dermo-Sifiliogr. 2020, 111, 725–733. [Google Scholar] [CrossRef]
- Gliem, M.; Birtel, J.; Müller, P.L.; Hendig, D.; Faust, I.; Herrmann, P.; Holz, F.G.; Adamus, G.; Charbel Issa, P. Acute Retinopathy in Pseudoxanthoma Elasticum. JAMA Ophthalmol. 2019, 137, 1165–1173. [Google Scholar] [CrossRef]
- Naouri, M.; Boisseau, C.; Bonicel, P.; Daudon, P.; Bonneau, D.; Chassaing, N.; Martin, L. Manifestations of pseudoxanthoma elasticum in childhood. Br. J. Dermatol. 2009, 161, 635–639. [Google Scholar] [CrossRef]
- Orssaud, C.; Roche, O.; Dufier, J.-L.; Germain, D.P. Visual Impairment in Pseudoxanthoma Elasticum: A Survey of 40 Patients. Ophthalmic Genet. 2015, 36, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Lauzirika, G.; Garcia-Gonzalez, M.; Bolivar, G.; Hernández-Verdejo, J.L.; Sánchez, V.B.; Gros-Otero, J.; Teus, M.A. Measurement of the Intraocular Pressure Elevation During Laser-Assisted In Situ Keratomileusis Flap Creation Using a Femtosecond Laser Platform. Transl. Vis. Sci. Technol. 2021, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Strohmaier, C.; Runge, C.; Seyeddain, O.; Emesz, M.; Nischler, C.; Dexl, A.; Grabner, G.; Reitsamer, H.A. Profiles of Intraocular Pressure in Human Donor Eyes during Femtosecond Laser Procedures—A Comparative Study. Investig. Opthalmology Vis. Sci. 2013, 54, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Vetter, J.M.; Holzer, M.P.; Teping, C.; Weingärtner, W.E.; Gericke, A.; Stoffelns, B.; Pfeiffer, N.; Sekundo, W. Intraocular Pressure During Corneal Flap Preparation: Comparison Among Four Femtosecond Lasers in Porcine Eyes. J. Refract. Surg. 2011, 27, 427–433. [Google Scholar] [CrossRef]
- Kanellopoulos, J. Long-term safety and efficacy follow-up of prophylactic higher fluence collagen cross-linking in high myopic laser-assisted in situ keratomileusis. Clin. Ophthalmol. 2012, 6, 1125–1130. [Google Scholar] [CrossRef][Green Version]
- Kanellopoulos, A.J. Comparison of Sequential vs Same-Day Simultaneous Collagen Cross-Linking and Topography-Guided PRK for Treatment of Keratoconus. J. Refract. Surg. 2009, 25, S812–S818. [Google Scholar] [CrossRef]
- Gatinel, D.; Hoang-Xuan, T.; Azar, D.T. Volume estimation of excimer laser tissue ablation for correction of spherical myopia and hyperopia. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1445–1449. [Google Scholar]
- Santhiago, M.; Giacomin, N.; Smadja, D.; Bechara, S. Ectasia risk factors in refractive surgery. Clin. Ophthalmol. 2016, 10, 713–720. [Google Scholar] [CrossRef]
- Li, M.; Yang, D.; Zhao, Y.; Yang, W.; Shang, J.; Zhou, X.; Yao, P.; Yang, D.; Lin, X.; Zhou, X. Impact of ablation ratio on 5-year postoperative posterior corneal stability after refractive surgery: SMILE and FS-LASIK. Eye Vis. 2020, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, J.F.; Fernández-Vega, L.; Fernandes, P.; Gonzalez-Meijome, J.M.; Montés-Micó, R. Collagen copolymer toric posterior chamber phakic intraocular lens for myopic astigmatism. J. Cataract Refract. Surg. 2010, 36, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Lim, D.H.; Nam, S.W.; Yang, C.M.; Chung, E.S.; Chung, T.-Y. Ten-year clinical outcomes after implantation of a posterior chamber phakic intraocular lens for myopia. J. Cataract Refract. Surg. 2019, 45, 1555–1561. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, J.F.; Palacios, A.; Montés-Micó, R. Myopic Phakic STAAR Collamer Posterior Chamber Intraocular Lenses for Keratoconus. J. Refract. Surg. 2008, 24, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Güell, J.L.; Morral, M.; Gris, O.; Gaytan, J.; Sisquella, M.; Manero, F. Five-Year Follow-up of 399 Phakic Artisan–Verisyse Implantation for Myopia, Hyperopia, and/or Astigmatism. Ophthalmology 2008, 115, 1002–1012. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, M.; Dick, B.H. Iris-fixated toric phakic intraocular lens: Three-year follow-up. J. Cataract Refract. Surg. 2006, 32, 1301–1306. [Google Scholar] [CrossRef]
- Ruckhofer, J.; Seyeddain, O.; Dexl, A.K.; Grabner, G.; Stoiber, J. Correction of myopic astigmatism with a foldable iris-claw toric phakic intraocular lens: Short-term follow-up. J. Cataract Refract. Surg. 2012, 38, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Konstantopoulos, A.; Liu, Y.-C.; Teo, E.P.; Nyein, C.L.; Yam, G.H.; Mehta, J.S. Corneal Stability of LASIK and SMILE When Combined with Collagen Cross-Linking. Transl. Vis. Sci. Technol. 2019, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Brar, S.; Gautam, M.; Sute, S.S.; Ganesh, S. Refractive surgery with simultaneous collagen cross-linking for borderline corneas—A review of different techniques, their protocols and clinical outcomes. Indian J. Ophthalmol. 2020, 68, 2744–2756. [Google Scholar] [CrossRef]
- Sorkin, N.; Varssano, D. Corneal Collagen Crosslinking: A Systematic Review. Ophthalmologica 2014, 232, 10–27. [Google Scholar] [CrossRef]
- Pawiroranu, S.; Herani, D.N.; Setyowati, R.; Mahayana, I.T. Outcomes of corneal collagen cross linking prior to photorefractive keratectomy in prekeratoconus. Ann. Res. Hosp. 2017, 1, 1. [Google Scholar] [CrossRef]
- Kymionis, G.D.; Kontadakis, G.; Kounis, G.A.; Portaliou, D.M.; Karavitaki, A.E.; Magarakis, M.; Yoo, S.; Pallikaris, I.G. Simultaneous Topography-Guided PRK Followed by Corneal Collagen Cross-Linking for Keratoconus. J. Refract. Surg. 2009, 25, 807–811. [Google Scholar] [CrossRef]
- Labiris, G.; Kaloghianni, E.; Koukoula, S.; Zissimopoulos, A.; Kozobolis, V.P. Corneal melting after collagen cross-linking for keratoconus: A case report. J. Med. Case Rep. 2011, 5, 152. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, K.; Shimizu, K.; Kobashi, H.; Igarashi, A.; Komatsu, M.; Nakamura, A.; Kojima, T.; Nakamura, T. Three-year follow-up of posterior chamber toric phakic intraocular lens implantation for the correction of high myopic astigmatism in eyes with keratoconus. Br. J. Ophthalmol. 2015, 99, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Moshirfar, M.; Grégoire, F.J.; Mirzaian, G.; Whitehead, G.F.; Kang, P.C. Use of Verisyse iris-supported phakic intraocular lens for myopia in keratoconic patients. J. Cataract Refract. Surg. 2006, 32, 1227–1232. [Google Scholar] [CrossRef]
- Al-Amri, A.M. 5-year follow-up of combined non-topography guided photorefractive keratectomy and corneal collagen cross linking for keratoconus. Int. J. Ophthalmol. 2018, 11, 48–52. [Google Scholar] [CrossRef]
- Hopping, G.; Somani, A.N.; Vaidyanathan, U.; Liu, H.; Barnes, J.R.; Ronquillo, Y.; Hoopes, P.C.; Moshirfar, M. Myopic regression and recurrent Salzmann nodule degeneration after laser in situ keratomileusis in Ehlers Danlos Syndrome. Am. J. Ophthalmol. Case Rep. 2020, 19, 100729. [Google Scholar] [CrossRef]
- Sandvik, G.F.; Vanem, T.T.; Rand-Hendriksen, S.; Cholidis, S.; Sæthre, M.; Drolsum, L.; Saethre, M. Ten-year reinvestigation of ocular manifestations in Marfan syndrome. Clin. Exp. Ophthalmol. 2019, 47, 212–218. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, S.; Dadia, S.; Bharti, N.; Bharti, S. Bilateral Toric Phakic Intraocular Lens Implantation for Correction of High Myopic Astigmatism in a Patient with Marfan Syndrome with Lens Coloboma: A Case Report. Case Rep. Ophthalmol. 2021, 12, 208–213. [Google Scholar] [CrossRef]
- Bower, K.S.; Woreta, F. Update on contraindications for laser-assisted in situ keratomileusis and photorefractive keratectomy. Curr. Opin. Ophthalmol. 2014, 25, 251–257. [Google Scholar] [CrossRef]
- Yesilirmak, N.; Davis, Z.; Yoo, S.H. Refractive Surgery (SMILE vs. LASIK vs. Phakic IOL). Int. Ophthalmol. Clin. 2016, 61, 1–2. Available online: www.internat-ophthalmology.com (accessed on 19 July 2021).
- Ruiz-Moreno, J.M.; Alió, J.L.; Pérez-Santonja, J.J.; de la Hoz, F. Retinal Detachment in Phakic Eyes with Anterior Chamber Intraocular Lenses to Correct Severe Myopia. Am J Ophthalmol. 1999, 127, 270–275. [Google Scholar] [CrossRef]
- Martinezcastillo, V.; Boixadera, A.; Verdugo, A.; Elies, D.; Coret, A.; Garciaarumi, J. Rhegmatogenous Retinal Detachment in Phakic Eyes After Posterior Chamber Phakic Intraocular Lens Implantation for Severe Myopia. Ophthalmology 2005, 112, 580–585.e1. [Google Scholar] [CrossRef] [PubMed]
| OI Type Prevalence: 1 in 15,000 [22] | Inheritance Pattern | Gene | Severity | Phenotype | Ocular Manifestations | Refractive Surgery Recommendations |
|---|---|---|---|---|---|---|
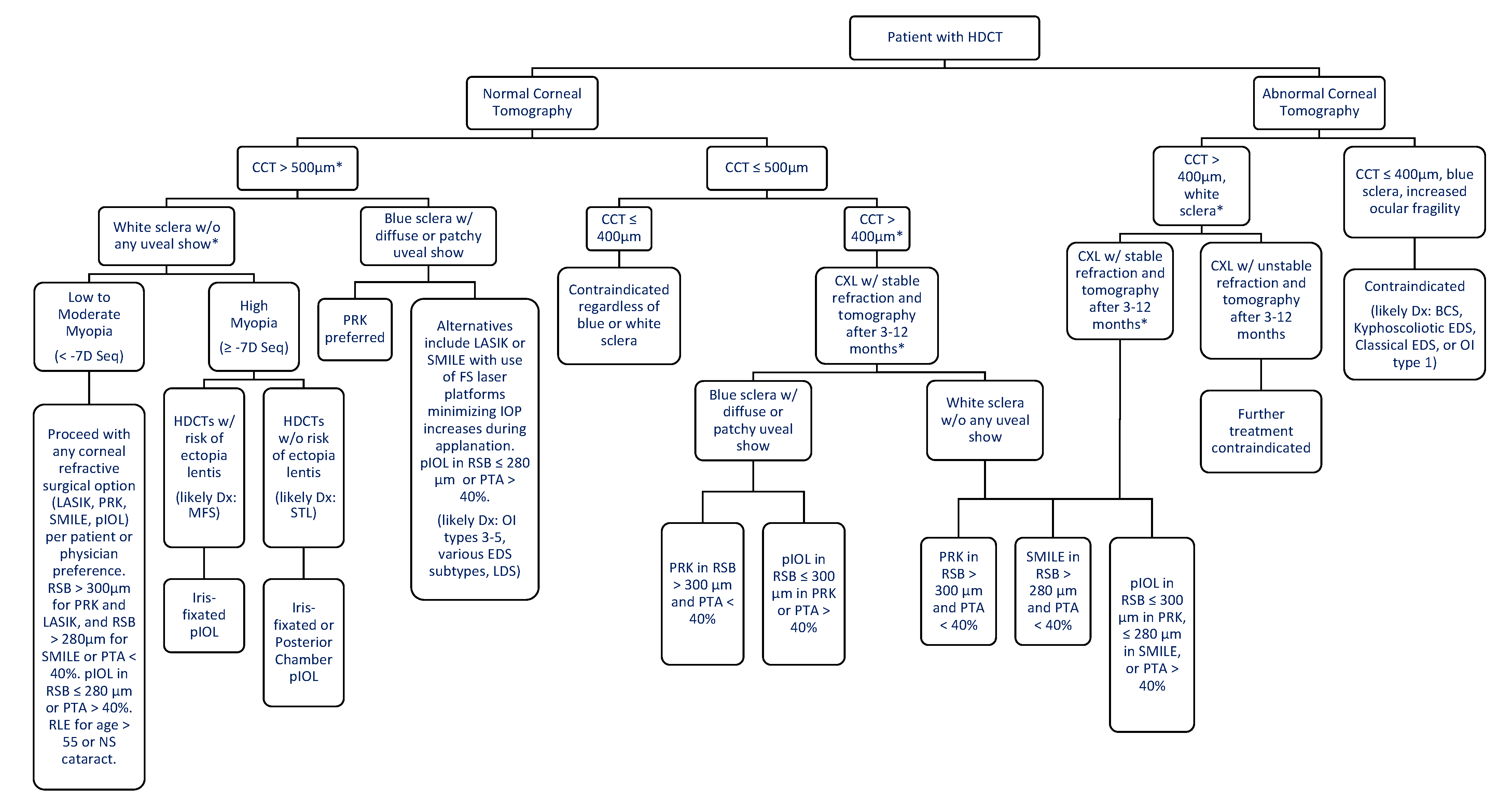
| 1 [4,5,21,23,24,25] | AD | COL1A1/2 | Mild | Osteoporosis, fractures, conductive deafness, mild stunting, +/− dentinogenesis imperfecta | Blue sclera, ocular rigidity, significantly lower CCT, corneal hysteresis, corneal resistance factor, global rupture, absence of Bowman’s Layer, glaucoma | Follow the framework in Figure 1 for patients with CCT > 400 µm. Surgery is contraindicated in patients with blue sclera and CCT ≤ 400 µm. |
| X-linked | PLS3 | |||||
| 2 [4,5,23,25] | AD (dominant-negative inheritance), AR | COL1A1/2, CRTAP, LEPREI1, PPIB, BMP1 | Severe (perinatal lethal form) | Accordion femur, delayed skull ossification, blue sclera | N/A | N/A |
| 3 [4,5,21,23,25] | AD (dominant-negative inheritance), AR | COL1A1/2, CRTAP, LEPREI1, PPIB, FKBP10, SERPINH1, SERINF1, WNT1 | Severe | Moderate to severe bone fragility, coxa vara, multiple fractures, marked long bone deformities, early-onset scoliosis, triangular facies, frontal bossing, extreme short stature | Blue sclera in infancy → white sclera in adolescence, absence of Bowman’s Layer, thinner CCT | Select the most appropriate refractive surgery if the minimum criteria are fulfilled according to the framework in Figure 1. Use femtosecond with lowest IOP increase (e.g., Visumax) for LASIK or SMILE procedures. |
| 4 [4,5,23,25] | AD, AR | COL1A1/2, CRTAP, FKBP10, SP7, SERPINF1, WNT1, TMEM38B | Moderate | Moderate to severe bone fragility, deformity of long bones and spinal column, moderate to severe growth stunting, +/− dentinogenesis imperfecta | +/− Blue sclera (rare), thinner CCT | Select the most appropriate refractive surgery if the minimum criteria are fulfilled according to the framework in Figure 1. Use femtosecond with lowest IOP increase (e.g., Visumax) for LASIK or SMILE procedures. |
| 5 [4,5,23,25] | AD | IFTM5 | Mild to Moderate | Calcification of interosseous membrane, hypertrophic callus | +/− Blue sclera (rare) | Select the most appropriate refractive surgery if the minimum criteria are fulfilled according to the framework in Figure 1. Use femtosecond with lowest IOP increase (e.g., Visumax) for LASIK or SMILE procedures. |
| EDS Subtype Prevalence: 1 in 5000 [33] | Inheritance Pattern | Gene | Phenotype | Ocular Manifestations | Refractive Surgery Recommendations |
|---|---|---|---|---|---|
| Classical EDS [26,37] | AD | COL5A1, COL1A1 | Skin hyperextensibility, atrophic scarring, generalized joint hypermobility | Blue sclera, epicanthal folds, ptosis, deep-set eyes, myopia, decreased CCT, steep cornea, conjunctivochalasis | Follow the framework in Figure 1 for patients with CCT > 400 µm. Surgery is contraindicated in patients with blue sclera and CCT ≤ 400 µm. |
| Classical-like EDS [6,26] | AR | TNXB | Skin hyperextensibility, no atrophic scarring, easy bruising, and generalized joint hypermobility +/− recurrent dislocations | Strabismus, subconjunctival hemorrhage has been reported, astigmatism | Select the most appropriate refractive surgery if the minimum criteria are fulfilled according to the framework in Figure 1. Use femtosecond with lowest IOP increase (e.g., Visumax) for LASIK or SMILE procedures. |
| Cardiac-valvular EDS [6,26] | AR | COL1A2 | Progressive cardiac-valvular problems, skin hyperextensibility, joint hypermobility | Myopia, +/− blue sclera, astigmatism | Select the most appropriate refractive surgery if the minimum criteria are fulfilled according to the framework in Figure 1. Use femtosecond with lowest IOP increase (e.g., Visumax) for LASIK or SMILE procedures. |
| Vascular EDS [6,26] | AD | COL3A1, COL1A1 | Arterial ruptures, sigmoid colon perforations, uterine ruptures, and carotid-cavernous sinus fistulas | Globe protrusion, decreased CCT, rare cases reported of keratoconus | Follow the framework in Figure 1 for patients with CCT > 400 µm. Surgery is contraindicated in patients with blue sclera and CCT ≤ 400 µm. |
| Hypermobile EDS [6,26,38] | AD | unknown | Generalized joint hypermobility, skin hyperextensibility, bilateral piezogenic papules of the heel, abdominal hernias, atrophic scarring, pelvic organ prolapse, aortic root dilation, mitral valve prolapse | Dry eyes, steep cornea, myopia, vitreous abnormalities | Select the most appropriate refractive surgery if the minimum criteria are fulfilled according to the framework in Figure 1. Consultation with retina before SMILE or PRK. Dry eyes and a steep cornea may contraindicate for LASIK but can be considered for pIOL if there is high myopia |
| Arthrochalasia EDS [6,26] | AD | COL1A1, COL1A2 | Congenital hip dislocations, severe generalized joint hypermobility, skin hyperextensibility | +/− Blue sclera, +/− lens dislocation | Select the most appropriate refractive surgery if the minimum criteria are fulfilled according to the framework in Figure 1. Use femtosecond with lowest IOP increase (e.g., Visumax) for LASIK or SMILE. PRK for those with blue sclera. An iris fixated pIOL can be used for those with lens dislocation. |
| Dermatosparaxis EDS [6,26] | AR | ADAMTS2 | Skin fragility, redundant skin with increased palmar wrinkling, short limbs, severe bruisability | Congenital or early progressive myopia, glaucoma | Select the most appropriate refractive surgery if the minimum criteria are fulfilled according to the framework in Figure 1. Use femtosecond with lowest IOP increase (e.g., Visumax) for LASIK or SMILE. An alternative is pIOL for those with high myopia. |
| Kyphoscoliotic EDS [6,26,39] | AR | PLOD1, KBP14 | Muscle hypotonia, kyphoscoliosis, generalized joint hypermobility | Ocular fragility, keratoconus, blue sclera, myopia, microcornea, decreased CCT, absent Bowman’s membrane | Refractive surgery is contraindicated |
| Brittle Cornea Syndrome [6,26,35,36,40] | AR | ZNF469, PRDM5 | Joint hypermotility, kyphoscoliosis, hyperlaxity of the skin, +/− red hair, conductive hearing loss | Ocular fragility, blue sclera, keratoconus and keratoglobus, megalocornea, myopia, very reduced CCT | Refractive surgery is contraindicated |
| Spondylodysplastic EDS [6,26] | AR | B4GALT7, B3GALT6, SLC39A13 | Short stature, muscle hypotonia, bowed limbs | Hypermetropia, strabismus, corneal clouding, microcornea, glaucoma, refractive errors, +/− blue sclera, astigmatism | Select the most appropriate refractive surgery if the minimum criteria are fulfilled according to the framework in Figure 1. Use femtosecond with lowest IOP increase (e.g., Visumax) for LASIK or SMILE. |
| Musculocontractural EDS [6,26] | AR | CHST14, DSE | Multiple congenital contractures, skin hyperextensibility, skin fragility with atrophic scars | Myopia, hyperopia, astigmatism, strabismus, microcornea, glaucoma, retinal detachment | Select the most appropriate refractive surgery if the minimum criteria are fulfilled according to the framework in Figure 1. Use femtosecond with lowest IOP increase (e.g., Visumax) for LASIK or SMILE. |
| Myopathic EDS [6,26] | AD or AR | COL12A1 | Congenital muscle hypotonia, proximal joint contractures, hypermobility of distal joints | No reports of ocular manifestations | Select the most appropriate refractive surgery if the minimum criteria are fulfilled according to the framework in Figure 1. Use femtosecond with lowest IOP increase (e.g., Visumax) for LASIK or SMILE. |
| Periodontal EDS [6,26] | AD | C1R | Severe and intractable periodontitis, lack of attached gingiva, pretibial plaques | No reports of ocular manifestations | Select the most appropriate refractive surgery if the minimum criteria are fulfilled according to the framework in Figure 1. Use femtosecond with lowest IOP increase (e.g., Visumax) for LASIK or SMILE. |
| HDCT (Subtypes) * Prevalence | Inheritance Pattern | Gene (Warman) | Phenotype | Ocular Manifestations | Refractive Surgery Recommendations |
|---|---|---|---|---|---|
| Marfan Syndrome [8,49] * 1 in 5–10,0000 [48] | AD | Fibrillin-1 | Long bone overgrowth, aortic root aneurysm, hypermobility, low bone mineral density, scoliosis, pectus excavatum and carinatum, dural ectasia, foot deformities, generalized ligamentous laxity | Ectopia lentis, flattened cornea, increased axial length, astigmatism, hypoplastic iris or hypoplastic ciliary muscle, uveitis, myopia, decreased CCT | Select the most appropriate refractive surgery if the minimum criteria are fulfilled according to the framework in Figure 1. Use femtosecond with lowest IOP increase (e.g., Visumax) for LASIK or SMILE. For those with high myopia, an iris-fixated pIOL can be considered except for those with iridodonesis. |
| Loeys-Dietz Syndrome [10,50] (Types 1–5) * <1 in 100,000 [51] | AD | TGFBR1, TGFBR2, SMAD3, TGFB2 or TGFB3 | Vascular aneurysms of the cerebral, thoracic, and abdominal arterial system, skeletal abnormalities such as pectus excavatum or carinatum, scoliosis, joint laxity and craniofacial abnormalities such as cleft palate, craniosynostosis, and bifid uvula, No lens dislocation | Blue or dusky sclera, hypertelorism, myopia, cataracts, retinal detachment, retinal tortuosity, strabismus, decreased CCT | Select the most appropriate refractive surgery if the minimum criteria are fulfilled according to the framework in Figure 1. Use femtosecond with lowest IOP increase (e.g., Visumax) for LASIK or SMILE. For blue and dusky sclera PRK can be preferred. |
| Epidermolysis Bullosa [12] (Main Types: EB Simplex, Junctional EB, Dystrophic EB and Kindler Syndrome) * 0.4–4.6 per million people [12] | AD | keratin 5 or 14, Laminin-322, COLA71 or FERMT1 (KIND1) | Epithelial tissue fragility, resulting in blisters and erosions from minimal trauma | Corneal erosions, conjunctival injections blistering of eyelids, pannus formation, symblepharon, corneal scarring | Select the most appropriate refractive surgery if the minimum criteria are fulfilled according to the framework in Figure 1. Patients with dry eye and/or optimized ocular surface PRK or SMILE would be better options. Check limbal stem cells with impression cytology or high-res AS-OCT. Patients with uncontrolled ocular surface disease can be considered for pIOL. |
| Stickler Syndrome [13,52] (Types 1–6) * 1–3 in 10,000 [53] | AD | COL2A1, COL11A1, COL11A2, COL9A1, COL9A2, COL9A3, LOXL3. COL2A1, COL11A1, or COL11A2 | Conductive and sensorineural hearing loss, midfacial underdevelopment and cleft palate, spondyloepiphyseal dysplasia | High myopia, cataracts, glaucoma, retinal detachments, vitreous abnormalities | Retina consultation before selecting the most appropriate refractive surgery if the minimum criteria are fulfilled according to the framework in Figure 1. Use femtosecond with lowest IOP increase (e.g., Visumax) for LASIK or SMILE. The use of pIOL can be considered for patients with high myopia. |
| AR | COL9A1, COL9A2, COL9A3, or LOXL3 | ||||
| Wagner Syndrome [54,55] * 300 affected worldwide [55] | AD | VCAN | No systemic abnormalities | Empty vitreous, chorioretinal atrophy, myopia, night blindness, retinal detachment, presenile cataract, uveitis | Retina consultation before selecting the most appropriate refractive surgery if the minimum criteria are fulfilled according to the framework in Figure 1. Use femtosecond with lowest IOP increase (e.g., Visumax) for LASIK or SMILE. The use of pIOL can be considered for patients with high myopia. |
| Pseudoxanthoma Elasticum [17,56,57] * 1 in 25–100,000 [56] | AR | ABCC6 | Accumulation of elastic fiber in the skin, vasculature and, Bruch’s membrane of the eye | Angioid streaks, choroidal neovascularization, peau d’orange appearance on fundoscopy, optic disc drusen, choroidal atrophy with comet tails, submacular hemorrhage | Retina consultation before selecting the most appropriate refractive surgery if the minimum criteria are fulfilled according to the framework in Figure 1. Use femtosecond with lowest IOP increase (e.g., Visumax) for LASIK or SMILE. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moshirfar, M.; Barke, M.R.; Huynh, R.; Waite, A.J.; Ply, B.; Ronquillo, Y.C.; Hoopes, P.C. Controversy and Consideration of Refractive Surgery in Patients with Heritable Disorders of Connective Tissue. J. Clin. Med. 2021, 10, 3769. https://doi.org/10.3390/jcm10173769
Moshirfar M, Barke MR, Huynh R, Waite AJ, Ply B, Ronquillo YC, Hoopes PC. Controversy and Consideration of Refractive Surgery in Patients with Heritable Disorders of Connective Tissue. Journal of Clinical Medicine. 2021; 10(17):3769. https://doi.org/10.3390/jcm10173769
Chicago/Turabian StyleMoshirfar, Majid, Matthew R. Barke, Rachel Huynh, Austin J. Waite, Briana Ply, Yasmyne C. Ronquillo, and Phillip C. Hoopes. 2021. "Controversy and Consideration of Refractive Surgery in Patients with Heritable Disorders of Connective Tissue" Journal of Clinical Medicine 10, no. 17: 3769. https://doi.org/10.3390/jcm10173769
APA StyleMoshirfar, M., Barke, M. R., Huynh, R., Waite, A. J., Ply, B., Ronquillo, Y. C., & Hoopes, P. C. (2021). Controversy and Consideration of Refractive Surgery in Patients with Heritable Disorders of Connective Tissue. Journal of Clinical Medicine, 10(17), 3769. https://doi.org/10.3390/jcm10173769









