Predicting Duration of Mechanical Ventilation in Acute Respiratory Distress Syndrome Using Supervised Machine Learning
Abstract
1. Background
2. Methods
2.1. Study Design and Patient Population
2.2. MIMIC-III
2.3. eICU
2.4. Predictive Models
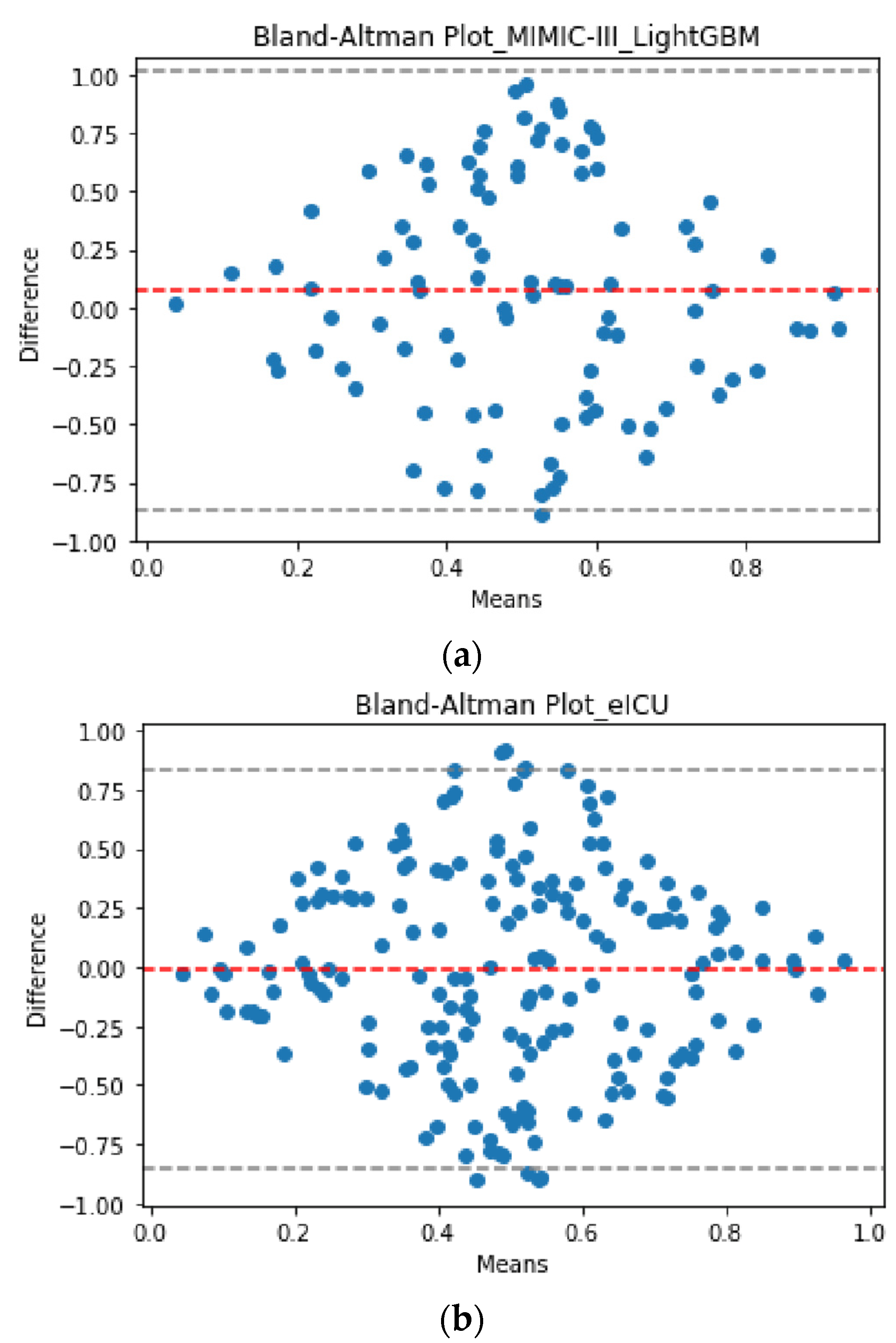
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rubenfeld, G.D.; Caldwell, E.; Peabody, E.; Weaver, J.; Martin, D.P.; Neff, M.; Stern, E.J.; Hudson, L.D. Incidence and outcomes of acute lung injury. N. Engl. J. Med. 2005, 353, 1685–1693. [Google Scholar] [CrossRef]
- Slutsky, A.S.; Villar, J.; Pesenti, A. Happy 50th birthday ARDS! Intensive Care Med. 2016, 42, 637–639. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bice, T.; Carson, S.S. Acute Respiratory Distress Syndrome: Cost (Early and Long-Term). Semin. Respir. Crit. Care Med. 2019, 40, 137–144. [Google Scholar] [CrossRef]
- Dasta, J.F.; McLaughlin, T.P.; Mody, S.H.; Piech, C.T. Daily cost of an intensive care unit day: The contribution of mechanical ventilation. Crit. Care Med. 2005, 33, 1266–1271. [Google Scholar] [CrossRef] [PubMed]
- Marti, J.; Hall, P.; Hamilton, P.; Lamb, S.; McCabe, C.; Lall, R.; Darbyshire, J.; Young, D.; Hulme, C. One-year resource utilisation, costs and quality of life in patients with acute respiratory distress syndrome (ARDS): Secondary analysis of a randomised controlled trial. J. Intensive Care 2016, 4. [Google Scholar] [CrossRef]
- ARDS Definition Task Force; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef]
- Ferguson, N.D.; Fan, E.; Camporota, L.; Antonelli, M.; Anzueto, A.; Beale, R.; Brochard, L.; Brower, R.; Esteban, A.; Gattinoni, L.; et al. The Berlin definition of ARDS: An expanded rationale, justification, and supplementary material. Intensive Care Med. 2012, 38, 1573–1582. [Google Scholar] [CrossRef] [PubMed]
- Robles, A.; Kornblith, L.Z.; Hendrickson, C.M.; Howard, B.M.; Conroy, A.S.; Moazed, F.; Calfee, C.S.; Cohen, M.J.; Callcut, R.A. Health care utilization and the cost of posttraumatic acute respiratory distress syndrome care. J. Trauma Acute Care Surg. 2018, 85, 148–154. [Google Scholar] [CrossRef]
- Sayed, M.; Riaño, D. Modelling ICU Patients to Improve Care Requirements and Outcome Prediction of Acute Respiratory Distress Syndrome: A Supervised Learning Approach. In Artificial Intelligence in Medicine: Knowledge Representation and Transparent and Explainable Systems. Lecture Notes in Artificial Intelligence (LINAI), Proceedings of the AIME 2019 International Workshops KR4HC-ProHealth/TEAAM, Poznan, Poland, 26–29 June 2019; Marcos, M., Juarez, J.M., Lenz, R., Nalepa, G.J., Nowaczyk, S., Peleg, M., Stefanowski, J., Stiglic, G., Eds.; Springer: Cham, Switzerland, 2019; Volume 11979, pp. 39–49. [Google Scholar]
- Del Sorbo, L.; Ranieri, V.M.; Ferguson, N.D. The Berlin definition met our needs: Yes. Intensive Care Med. 2016, 42, 643–647. [Google Scholar] [CrossRef]
- Villar, J.; Pérez-Méndez, L.; Kacmarek, R.M. The Berlin definition met our needs: No. Intensive Care Med. 2016, 42, 648–650. [Google Scholar] [CrossRef]
- Pirracchio, R.; Gropper, M.A. Heterogeneity in intensive care: Low severity does not mean low risk! Anesthesiology 2019, 130, 190–191. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Casas, J.B.; Connery, S.M.; Montoya, R.; Dwivedi, A.K.; Lee, S. Accuracy of early prediction of duration of mechanical ventilation by intensivists. Ann. Am. Thorac. Soc. 2014, 11, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Casas, J.B.; Dwivedi, A.K.; Connery, S.M.; Quansah, R.; Ellerbrook, L.; Galvis, J. Predictive models of prolonged mechanical ventilation yield moderate accuracy. J. Crit. Care 2015, 30, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C.; Tu, J.V.; Ho, J.E.; Levy, D.; Lee, D.S. Using methods from the data-mining and machine-learning literature for disease classification and prediction: A case study examining classification of heart failure subtypes. J. Clin. Epidemiol. 2013, 66, 398–407. [Google Scholar] [CrossRef]
- Cherifa, M.; Pirracchio, R. What every intensivist should know about Big Data and targeted machine learning in the intensive care unit. Rev. Bras. Ter. Intensive 2019, 31, 444–446. [Google Scholar] [CrossRef]
- Gutierrez, G. Artificial Intelligence in the Intensive Care Unit. Crit. Care 2020, 24, 101. [Google Scholar] [CrossRef]
- Greco, M.; Caruso, P.F.; Cecconi, M. Artificial Intelligence in the Intensive Care Unit. Semin. Respir. Crit. Care Med. 2021, 42, 2–9. [Google Scholar] [CrossRef]
- Sayed, M.; Riaño, D.; Villar, J. Novel criteria to classify ARDS severity using a machine learning approach. Crit. Care 2021, 25. [Google Scholar] [CrossRef]
- Pirracchio, R.; Petersen, M.L.; Carone, M.; Resche-Rigon, M.; Chevret, S.; van der Laan, M.J. Mortality prediction in intensive care units with the Super ICU Learner Algorithm (SICULA): A population-based study. Lancet Respir. Med. 2015, 3, 42–52. [Google Scholar] [CrossRef]
- Troché, G.; Moine, P. Is the duration of mechanical ventilation predictable? Chest 1997, 112, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Parreco, J.; Hidalgo, A.; Parks, J.J.; Kozol, R.; Rattan, R. Using artificial intelligence to predict prolonged mechanical ventilation and tracheostomy placement. J. Surg. Res. 2018, 228, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Physionet.org, ‘MIMIC-III Critical Care Database’. Available online: https://mimic.physionet.org/about/mimic/ (accessed on 2 July 2020).
- Physionet.org, ‘eICU Collaborative Research Database’. Available online: https://eicu-crd.mit.edu/about/eicu/ (accessed on 19 October 2020).
- Villar, J.; Ambrós, A.; Soler, J.A.; Martínez, D.; Ferrando, C.; Solano, R.; Mosteiro, F.; Blanco, J.; Martín-Rodríguez, C.; Fernández, M.D.M.; et al. Age, PaO2/FIO2, and Plateau Pressure Score: A Proposal for a Simple Outcome Score in Patients With the Acute Respiratory Distress Syndrome. Crit. Care Med. 2016, 44, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Wang, S.; Liu, R.; Wang, H.; Zheng, J.; Yu, K. Risk factors for outcomes of acute respiratory distress syndrome patients: A retrospective study. J. Thorac. Dis. 2019, 11, 673–685. [Google Scholar] [CrossRef]
- Le, S.; Pellegrini, E.; Green-Saxena, A.; Summers, C.; Hoffman, J.; Calvert, J.; Das, R. Supervised machine learning for the early prediction of acute respiratory distress syndrome (ARDS). J. Crit. Care 2020, 60, 96–102. [Google Scholar] [CrossRef]
- Gong, M.N.; Schenk, L.; Gajic, O.; Mirhaji, P.; Sloan, J.; Dong, Y.; Festic, E.; Herasevich, V. Early intervention of patients at risk for acute respiratory failure and prolonged mechanical ventilation with a checklist aimed at the prevention of organ failure: Protocol for a pragmatic stepped-wedged cluster trial of PROOFCheck. BMJ Open 2016, 6. [Google Scholar] [CrossRef]
- Dehua, W.; Yang, Z.; Yi, Z. LightGBM: An effective miRNA classification method in breast cancer patients. In Proceedings of the 2017 International Conference on Computational Biology and Bioinformatics (ICCBB 2017), Newark, NJ, USA, 18–20 October 2017; pp. 7–11. [Google Scholar] [CrossRef]
- Boulesteix, A.-L.; Janitza, S.; Kruppa, J.; König, I.R. Overview of random forest methodology and practical guidance with emphasis on computational biology and bioinformatics. Wiley Interdiscip. Rev. Data Min. Knowl. Discov. 2012, 2, 493–507. [Google Scholar] [CrossRef]
- Chen, T.; Carlos, G. XGBoost: A Scalable Tree Boosting System. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining (KDD ′16), San Francisco, CA, USA, 13–17 August 2016; pp. 785–794. [Google Scholar] [CrossRef]
- Hagan, R.; Gillan, C.J.; Spence, I.; McAuley, D.; Shyamsundar, M. Comparing regression and neural network techniques for personalized predictive analytics to promote lung protective ventilation in Intensive Care Units. Comput. Biol. Med. 2020, 126. [Google Scholar] [CrossRef]
- Marco, L. Intensive care resource allocation: When difficult choices have to be made. BJMP 2013, 6, 4–6. [Google Scholar]
- Seneff, M.G.; Zimmerman, J.E.; Knaus, W.A.; Wagner, D.P.; Draper, E.A. Predicting the Duration of Mechanical Ventilation. The importance of disease and patient characteristics. Chest 1996, 110, 469–479. [Google Scholar] [CrossRef]
- Marshall, Y.; Hayley, B.; Chao-Ping, W.; Michelle, N. Increased Economic Costs Associated with Acute Respiratory Distress Syndrome in Mechanically Ventilated Patients in the Intensive Care Unit. Am. J. Respir. Crit. Care Med. 2017, 195, A7579. [Google Scholar]
- Sheikhalishahi, S.; Balaraman, V.; Osmani, V. Benchmarking machine learning models on multi-centre eICU critical care dataset. PLoS ONE 2020, 15, e0235424. [Google Scholar] [CrossRef] [PubMed]
| 24-h | 48-h | 72-h | |
|---|---|---|---|
| A. MIMIC-III ARDS Patients | 2466 (100%) | 1445 (58.6%) | 1278 (51.8%) |
| B. Means and 95% CI Age | 62.2 (61.5, 62.8] | 60.8 (59.9, 61.6) | 60.9 (60.0, 61.8) |
| PEEP | 7.6 (7.5, 7.7) | 9.1 (8.9, 9.4) | 8.9 (8.8, 9.2) |
| FiO2 | 0.66 (0.65, 0.67) | 0.54 (0.53, 0.55) | 0.51 (0.49, 0.51) |
| PaO2 | 114.5 (112.8, 116.2) | 97.6 (96.3, 98.9) | 95.4 (94.1, 96.6) |
| PaCO2 | 43.4 (42.9, 43.9) | 42.3 (41.8, 42.9) | 42.9 (42.4, 43.6) |
| PaO2/FiO2 | 184.3 (181.9, 186.6) | 170.9 (167.7, 174.2) | 179.1 (175.7, 182.5) |
| C. eICU ARDS Patients | 5153 (100%) | 2981 (57.8%) | 2326 (45.1%) |
| D. Means and 95% CI Age | 63.4 (62.9, 63.8] | 63.4 (62.8, 63.9) | 62.9 (62.4, 63.6) |
| PEEP | 6.6 (6.6, 6.7) | 7.1 (7.0, 7.2) | 7.3 (7.1, 7.4) |
| FiO2 | 0.63 (0.63, 0.64) | 0.53 (0.52, 0.54) | 0.52 (0.51, 0.53) |
| PaO2 | 104.1 (102.9, 105.2) | 89.1 (88.1, 90.1) | 86.4 (85.3, 87.4) |
| PaCO2 | 43.5 (43.2, 43.9) | 41.3 (40.9, 41.7) | 41.8 (41.4, 42.2) |
| PaO2/FiO2 | 160.2 (158.3, 162.1) | 175.2 (172.9, 177.5) | 174.5(171.8, 177.2) |
| ICU Day (n) | Database | MV Duration Median Days (IQR Days) |
|---|---|---|
| Day 1 (2466) | MIMIC-III | 6.5 (4.4–9.8) |
| Day 2 (1445) | 6.8 (4.7–10.5) | |
| Day 3 (1278) | 6.9 (4.7–10.6) | |
| Day 1 (5153) | eICU | 5.0 (3.0–9.0) |
| Day 2 (2981) | 6.0 (4.0–10.0) | |
| Day 3 (2326) | 6.0 (4.0–10.0) |
| Scenario I: Predicting MV Duration in ARDS Using Data in the 1st ICU Day | |
|---|---|
| Algorithm | RMSE, mean±SD |
| XGBoost | 6.81 ± 1.18 |
| RF | 6.79 ± 1.22 |
| LightGBM | 6.41 ± 1.55 |
| * Scenario II: Predicting MV duration in ARDS using data in the 2nd ICU day | |
| Algorithm | RMSE, mean±SD |
| XGBoost | 6.53 ± 0.96 |
| RF | 6.55 ± 1.16 |
| * LightGBM | 6.10 ± 0.72 |
| Scenario III: Predicting MV duration in ARDS using data in the 1st & 2nd ICU days | |
| Algorithm | RMSE, mean±SD |
| XGBoost | 6.57 ± 1.08 |
| RF | 6.60 ± 1.01 |
| LightGBM | 6.35 ± 0.69 |
| Scenario IV: Predicting MV duration in ARDS using the data in the 3rd ICU day | |
| Algorithm | RMSE, mean±SD |
| XGBoost | 6.14 ± 0.85 |
| RF | 6.19 ± 0.66 |
| LightGBM | 5.92 ± 0.47 |
| Predictive Scenario | RMSE, Mean ± SD |
|---|---|
| Scenario I | 6.08 ± 0.72 |
| * Scenario II | 5.87 ± 0.67 |
| Scenario III | 5.93 ± 0.44 |
| Scenario IV | 5.71 ± 0.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sayed, M.; Riaño, D.; Villar, J. Predicting Duration of Mechanical Ventilation in Acute Respiratory Distress Syndrome Using Supervised Machine Learning. J. Clin. Med. 2021, 10, 3824. https://doi.org/10.3390/jcm10173824
Sayed M, Riaño D, Villar J. Predicting Duration of Mechanical Ventilation in Acute Respiratory Distress Syndrome Using Supervised Machine Learning. Journal of Clinical Medicine. 2021; 10(17):3824. https://doi.org/10.3390/jcm10173824
Chicago/Turabian StyleSayed, Mohammed, David Riaño, and Jesús Villar. 2021. "Predicting Duration of Mechanical Ventilation in Acute Respiratory Distress Syndrome Using Supervised Machine Learning" Journal of Clinical Medicine 10, no. 17: 3824. https://doi.org/10.3390/jcm10173824
APA StyleSayed, M., Riaño, D., & Villar, J. (2021). Predicting Duration of Mechanical Ventilation in Acute Respiratory Distress Syndrome Using Supervised Machine Learning. Journal of Clinical Medicine, 10(17), 3824. https://doi.org/10.3390/jcm10173824







