Post-Irradiation Hyperamylasemia Is a Prognostic Marker for Allogeneic Hematopoietic Stem Cell Transplantation Outcomes in Pediatric Population: A Retrospective Single-Centre Cohort Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Inclusion and Exclusion Criteria
2.3. Study Predictors
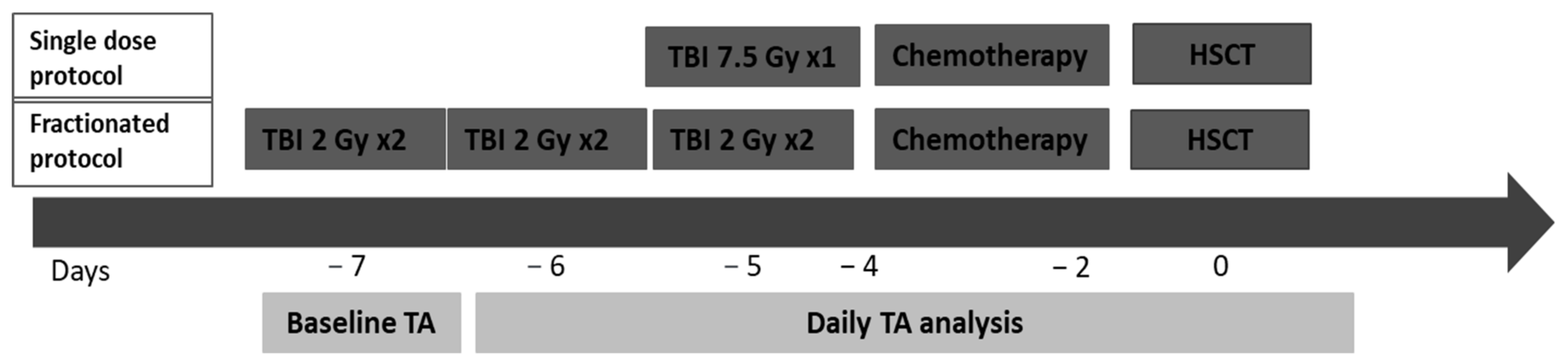
2.4. HSCT Procedure and TBI Treatment
2.5. Total Amylase and Pancreatic α-Amylase Values Assessment
2.6. Assessing TBI-Related Inflammatory Status
2.7. Follow-Up
2.8. Endpoints
2.9. Statistics
3. Results
3.1. Patients
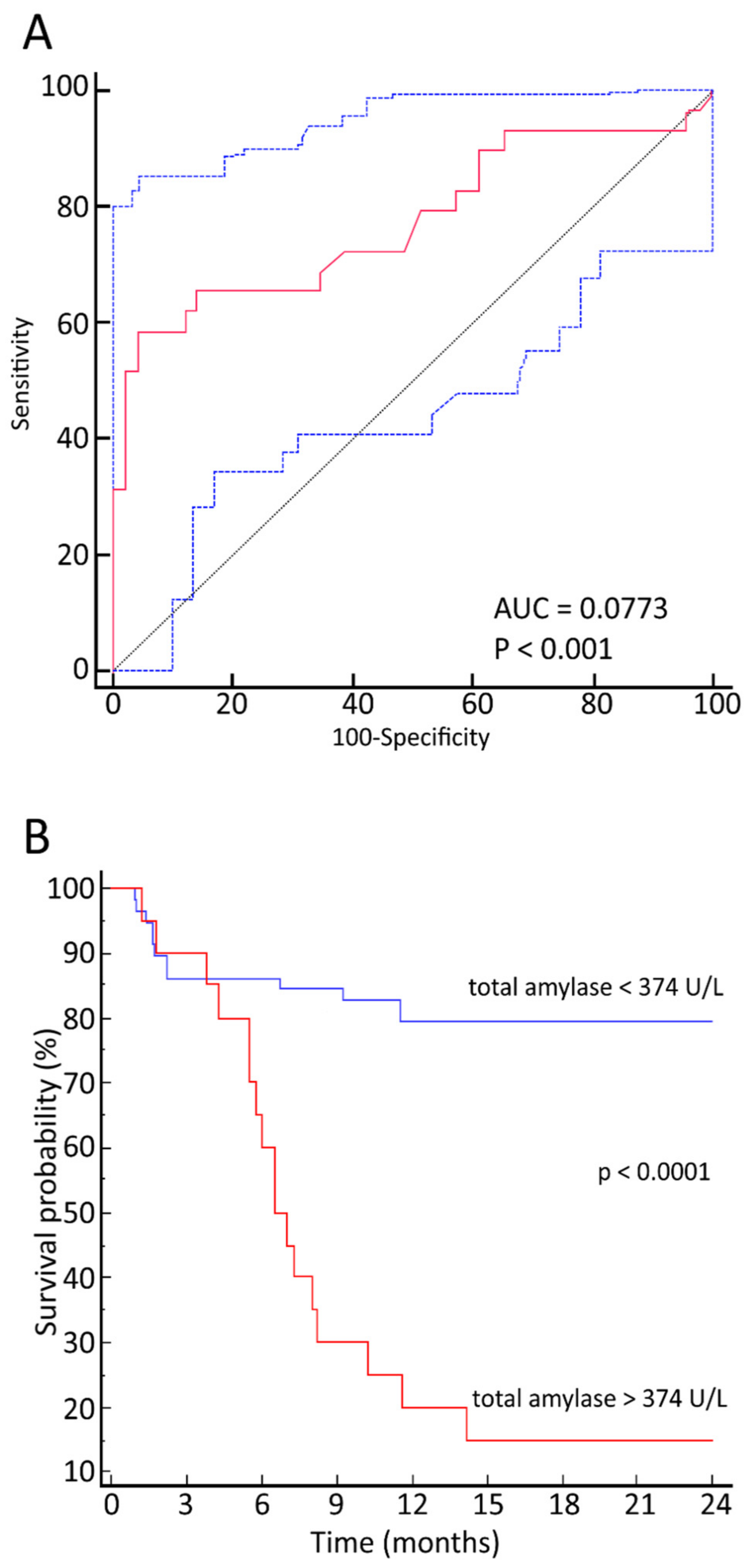
3.2. Relationship between Maximum TA Values and OS
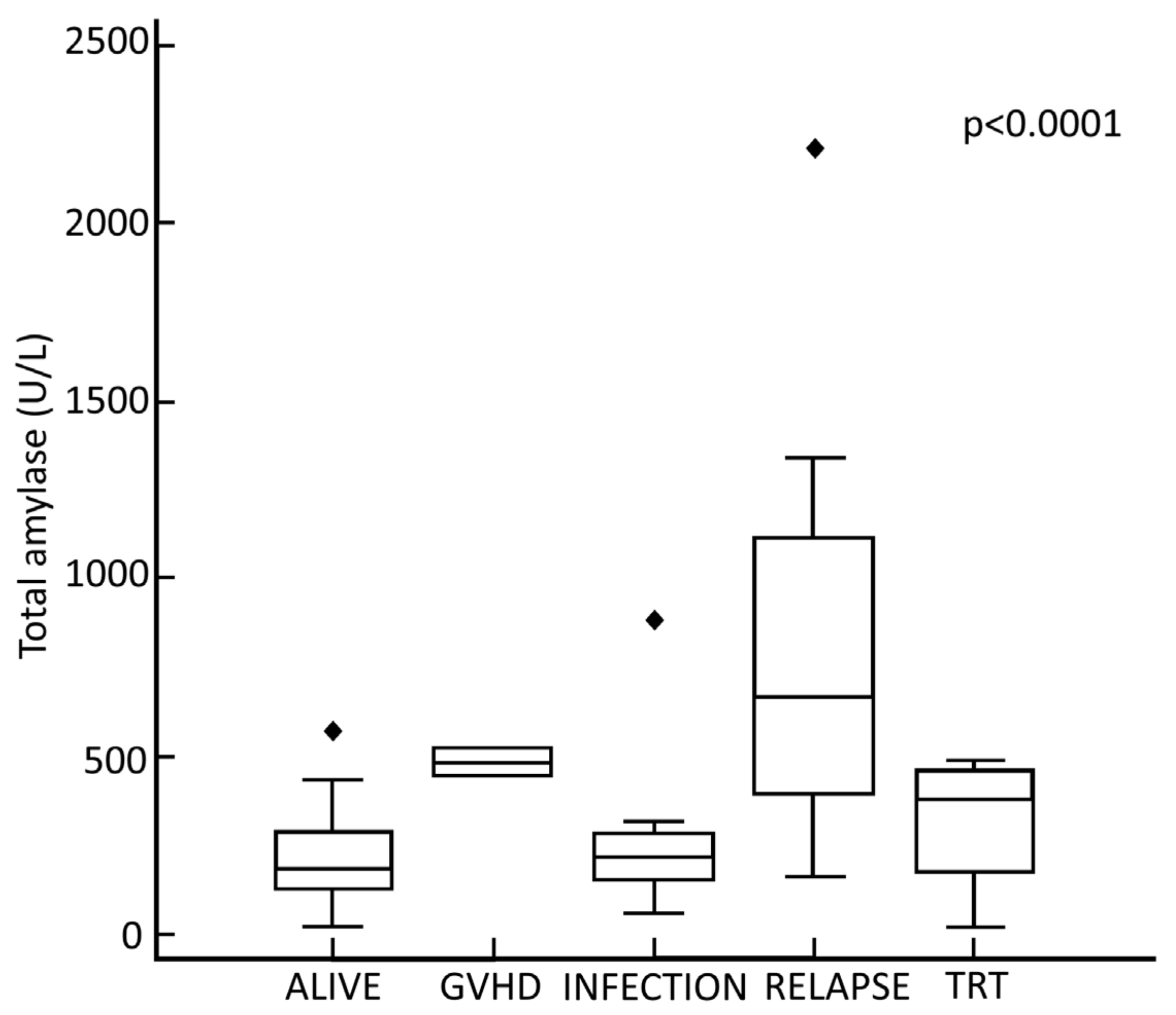
3.3. Relationship between Maximum TA Values, Status of Disease at Transplant and Causes of Death
3.4. Relationship between Maximum TA Values and Early Transplant-Related Complications
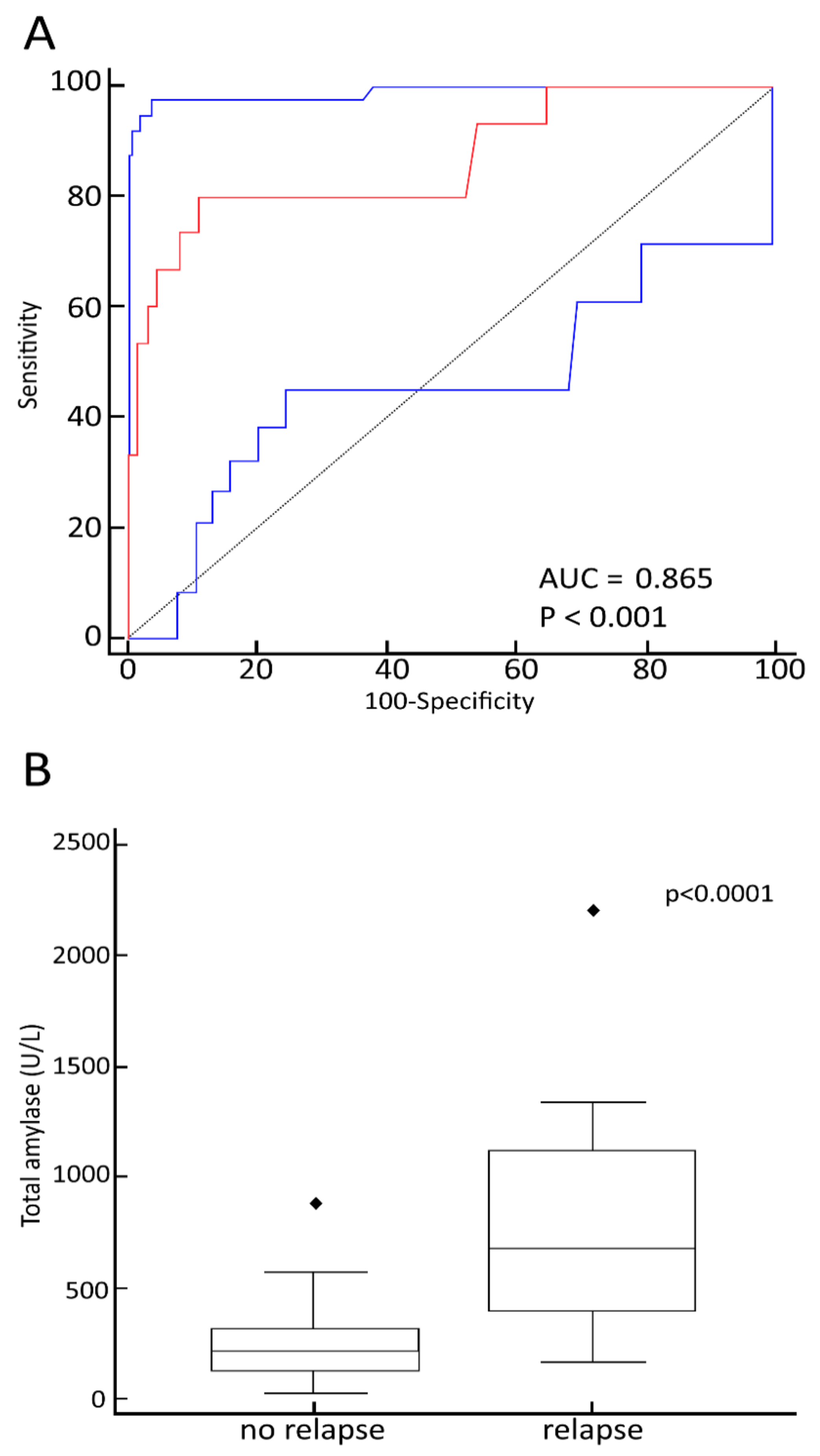
3.5. Relationship between Maximum TA Values and Late Transplant-Related Complications
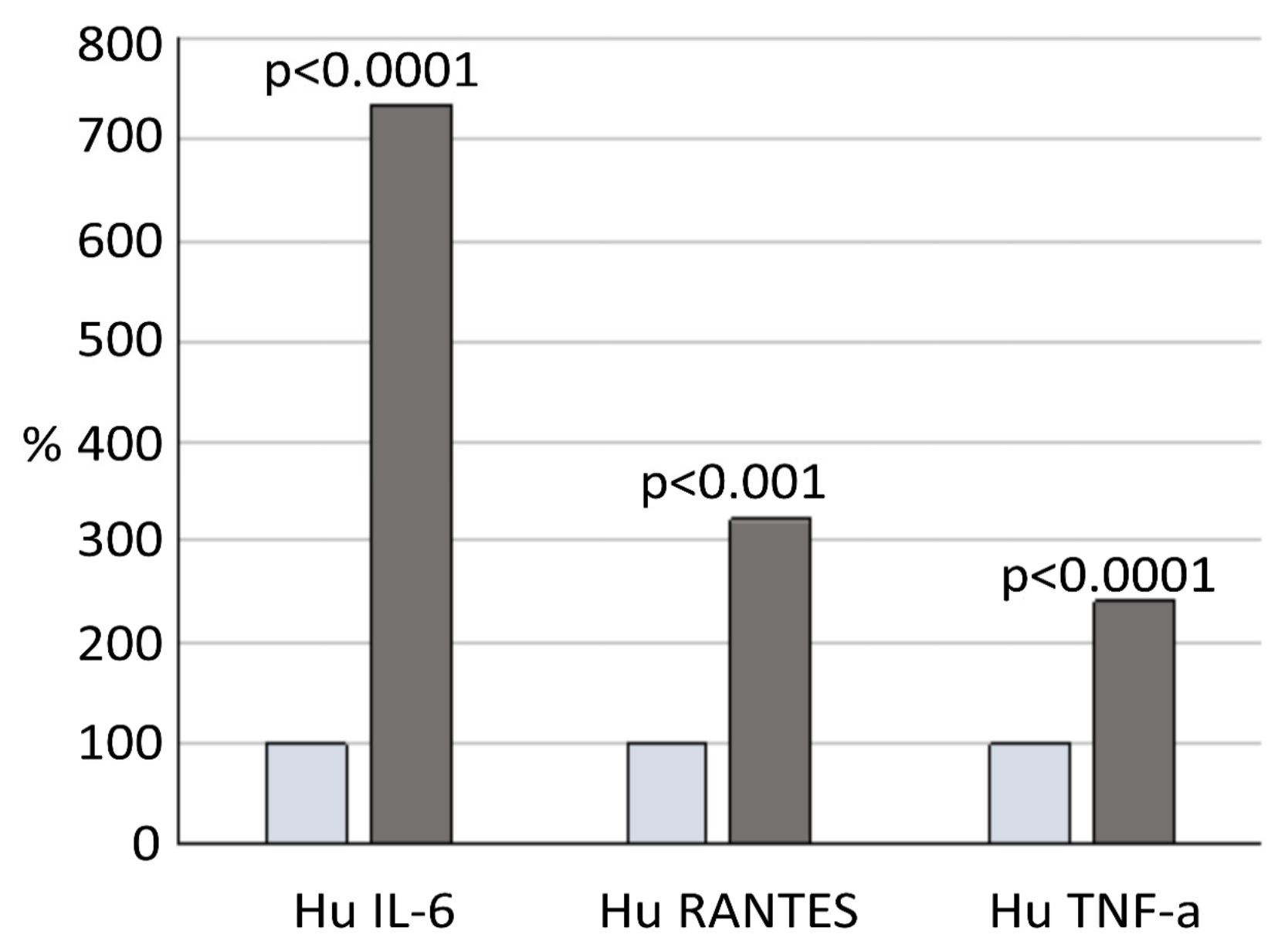
3.6. Relationship between Maximum TA Values and Inflammation Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barnes, D.W.H.; Ford, C.E.; Ilbery, P.L.T.; Koller, P.C.; Loutit, J.F. Tissue transplantation in the radiation chimera. J. Cell. Physiol. 1957, 50, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Appelbaum, F.R. Hematopoietic-cell transplantation at 50. N. Engl. J. Med. 2007, 357, 1472–1475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linsenmeier, C.; Thoennessen, D.; Negretti, L.; Bourquin, J.P.; Streller, T.; Lütolf, U.M.; Oertel, S. Total body irradiation (TBI) in pediatric patients. A single-center experience after 30 years of low-dose rate irradiation. Strahlenther. Onkol. 2010, 186, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Paix, A.; Antoni, D.; Waissi, W.; Ledoux, M.P.; Bilger, K.; Fornecker, L.; Noel, G. Total body irradiation in allogeneic bone marrow transplantation conditioning regimens: A review. Crit. Rev. Oncol. Hematol. 2018, 123, 138–148. [Google Scholar] [CrossRef]
- Peters, L.J.; Withers, H.R.; Cundiff, J.H.; Dicke, K.A. Radiobiological considerations in the use of total-body irradiation for bone-marrow transplantation. Radiology 1979, 131, 243–247. [Google Scholar] [CrossRef]
- Giebel, S.; Miszczyk, L.; Slosarek, K.; Moukhtari, L.; Ciceri, F.; Esteve, J.; Gorin, N.C.; Labopin, M.; Nagler, A.; Schmid, C.; et al. Extreme heterogeneity of myeloablative total body irradiation techniques in clinical practice: A survey of the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Cancer 2014, 120, 2760–2765. [Google Scholar] [CrossRef]
- Cosset, J.M.; Girinsky, T.; Malaise, E.; Chaillet, M.P.; Dutreix, J. Clinical basis for TBI fractionation. Radiother. Oncol. 1990, 18, 60–67. [Google Scholar] [CrossRef]
- Deeg, H.J.; Sullivan, K.M.; Buckner, C.D.; Storb, R.; Appelbaum, F.R.; Clift, R.A.; Doney, K.; Sanders, J.E.; Witherspoon, R.P.; Thomas, E.D. Marrow transplantation for acute nonlymphoblastic leukemia in first remission: Toxicity and long-term follow-up of patients conditioned with single dose or fractionated total body irradiation. Bone Marrow. Transpl. 1986, 1, 151–157. [Google Scholar]
- Gerstein, J.; Meyer, A.; Sykora, K.W.; Frühauf, J.; Karstens, J.H.; Bremer, M. Long-term renal toxicity in children following fractionated total-body irradiation (TBI) before allogeneic stem cell transplantation (SCT). Strahlenther. Onkol. 2009, 185, 751–755. [Google Scholar] [CrossRef]
- Ree, A.H.; Redalen, K.R. Personalized radiotherapy: Concepts, biomarkers and trial design. Br. J. Radiol. 2015, 88, 20150009. [Google Scholar] [CrossRef] [Green Version]
- Paulino, A.C. Treatment strategies to reduce radiotherapy late effects in children. J. Radiat. Oncol. 2013, 2, 121–128. [Google Scholar] [CrossRef] [Green Version]
- Krasin, M.J.; Constine, L.S.; Friedman, D.L.; Marks, L.B. Radiation-related treatment effects across the age spectrum: Differences and similarities or what the old and young can learn from each other. Semin. Radiat. Oncol. 2010, 20, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Kong, F.M.; Ao, X.; Wang, L.; Lawrence, T.S. The use of blood biomarkers to predict radiation lung toxicity: A potential strategy to individualize thoracic radiation therapy. Cancer Control. 2008, 15, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Brattström, C.; Tollemar, J.; Ringdén, O.; Bergström, K.; Tydén, G. Isoamylase levels in bone marrow transplant patients are affected by total body irradiation and not by graft-versus-host disease. Transpl. Int. 1991, 4, 96–98. [Google Scholar] [CrossRef] [PubMed]
- Junglee, D.; Katrak, A.; Mohiuddin, J.; Blacklock, H.; Prentice, H.G.; Dandona, P. Salivary amylase and pancreatic enzymes in serum after total body irradiation. Clin. Chem. 1986, 32, 609–610. [Google Scholar] [CrossRef] [PubMed]
- Chaillet, M.P.; Cosset, J.M.; Socie, G.; Pico, J.L.; Grimaud, E.; Dubray, B.; Alapetite, C.; Girinsky, T. Prospective study of the clinical symptoms of therapeutic whole body irradiation. Health Phys. 1993, 64, 370–374. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE); Version 4.0; National Cancer Institute: Bethesda, MD, USA, 2009. [Google Scholar]
- Maximova, N.; Schillani, G.; Simeone, R.; Maestro, A.; Zanon, D. Comparison of Efficacy and Safety of Caspofungin Versus Micafungin in Pediatric Allogeneic Stem Cell Transplant Recipients: A Retrospective Analysis. Adv. Ther. 2017, 34, 1184–1199. [Google Scholar] [CrossRef] [PubMed]
- Maximova, N.; Granzotto, M.; Barbieri, F.; Marcuzzi, A.; Tommasini, A.; Monasta, L.; Simeone, R.; Zanon, D.; Sala, R. Monocyte-predominant engraftment, cytokine levels and early transplant-related complications in pediatric hematopoietic stem cell recipients. Cancer Med. 2019, 8, 890–901. [Google Scholar] [CrossRef] [Green Version]
- Styczyński, J.; Tridello, G.; Koster, L.; Iacobelli, S.; van Biezen, A.; van der Werf, S.; Mikulska, M.; Gil, L.; Cordonnier, C.; Ljungman, P.; et al. Infectious Diseases Working Party EBMT. Death after hematopoietic stem cell transplantation: Changes over calendar year time, infections and associated factors. Bone Marrow Transpl. 2019, 55, 126–136. [Google Scholar] [CrossRef] [Green Version]
- Gratwohl, A.; Stern, M.; Brand, R.; Apperley, J.; Baldomero, H.; de Witte, T.; Dini, G.; Rocha, V.; Passweg, J.; Sureda, A.; et al. Risk score for outcome after allogeneic hematopoietic stem cell transplantation. Cancer 2009, 115, 4715–4726. [Google Scholar] [CrossRef]
- De Ruysscher, D.; Niedermann, G.; Burnet, N.G.; Siva, S.; Lee, A.W.M.; Hegi-Johnson, F. Radiotherapy toxicity. Nat. Rev. Dis. Primers 2019, 5, 13, Erratum in 2019, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Pedraza Muriel, V. Hypofractionation in radiotherapy. Clin. Transl. Oncol. 2007, 9, 21–27. [Google Scholar] [CrossRef]
- Konings, A.W.; Coppes, R.P.; Vissink, A. On the mechanism of salivary gland radiosensitivity. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 1187–1194, Erratum in 2006, 64, 330. [Google Scholar] [CrossRef]
- De Felice, F.; Tombolini, M.; Musella, A.; Marampon, F.; Tombolini, V.; Musio, D. Radiation therapy and serum salivary amylase in head and neck cancer. Oncotarget 2017, 8, 90496–90500. [Google Scholar] [CrossRef] [Green Version]
- Dubray, B.; Girinski, T.; Thames, H.D.; Becciolini, A.; Porciani, S.; Hennequin, C.; Socié, G.; Bonnay, M.; Cosset, J.M. Post-irradiation hyperamylasemia as a biological dosimeter. Radiother. Oncol. 1992, 24, 21–26. [Google Scholar] [CrossRef]
- Rajaraman, P.; Hauptmann, M.; Bouffler, S.; Wojcik, A. Human individual radiation sensitivity and prospects for prediction. Ann. ICRP 2018, 47, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Barnett, G.C.; West, C.M.; Dunning, A.M.; Elliott, R.M.; Coles, C.E.; Pharoah, P.D.; Burnet, N.G. Normal tissue reactions to radiotherapy: Towards tailoring treatment dose by genotype. Nat. Rev. Cancer 2009, 9, 134–142. [Google Scholar] [CrossRef] [Green Version]
- Palumbo, E.; Piotto, C.; Calura, E.; Fasanaro, E.; Groff, E.; Busato, F.; El Khouzai, B.; Rigo, M.; Baggio, L.; Romualdi, C.; et al. Individual Radiosensitivity in Oncological Patients: Linking Adverse Normal Tissue Reactions and Genetic Features. Front. Oncol. 2019, 9, 987. [Google Scholar] [CrossRef]
- Peters, C.; Dalle, J.H.; Locatelli, F.; Poetschger, U.; Sedlacek, P.; Buechner, J.; Shaw, P.J.; Staciuk, R.; Ifversen, M.; Pichler, H.; et al. Total Body Irradiation or Chemotherapy Conditioning in Childhood ALL: A Multinational, Randomized, Noninferiority Phase III Study. J. Clin. Oncol. 2021, 39, 295–307. [Google Scholar] [CrossRef]
- Acar, M.; Kocherlakota, K.S.; Murphy, M.M.; Peyer, J.G.; Oguro, H.; Inra, C.N.; Jaiyeola, C.; Zhao, Z.; Luby-Phelps, K.; Morrison, S.J. Deep imaging of bone marrow shows non-dividing stem cells are mainly perisinusoidal. Nature 2015, 526, 126–130. [Google Scholar] [CrossRef] [Green Version]
- Wright, L.E.; Buijs, J.T.; Kim, H.S.; Coats, L.E.; Scheidler, A.M.; John, S.K.; She, Y.; Murthy, S.; Ma, N.; Chin-Sinex, H.J.; et al. Single-Limb Irradiation Induces Local and Systemic Bone Loss in a Murine Model. J. Bone Miner. Res. 2015, 30, 1268–1279. [Google Scholar] [CrossRef] [Green Version]
- Abbuehl, J.P.; Tatarova, Z.; Held, W.; Huelsken, J. Long-Term Engraftment of Primary Bone Marrow Stromal Cells Repairs Niche Damage and Improves Hematopoietic Stem Cell Transplantation. Cell Stem Cell 2017, 21, 241–255.e6. [Google Scholar] [CrossRef]
- Sweeney, C.; Vyas, P. The Graft-Versus-Leukemia Effect in AML. Front. Oncol. 2019, 9, 1217. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Bhatia, R. Influence of Bone Marrow Microenvironment on Leukemic Stem Cells: Breaking Up an Intimate Relationship. Adv. Cancer Res. 2015, 127, 227–252. [Google Scholar] [PubMed]
- Duan, C.W.; Shi, J.; Chen, J.; Wang, B.; Yu, Y.H.; Qin, X.; Zhou, X.C.; Cai, Y.J.; Li, Z.Q.; Zhang, F.; et al. Leukemia propagating cells rebuild an evolving niche in response to therapy. Cancer Cell. 2014, 25, 778–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Rooij, B.; Polak, R.; van den Berk, L.C.J.; Stalpers, F.; Pieters, R.; den Boer, M.L. Acute lymphoblastic leukemia cells create a leukemic niche without affecting the CXCR4/CXCL12 axis. Haematologica 2017, 102, e389–e393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, M.; Mishra, S.K.; Kumar, B.S.; Khushu, S.; Rana, P. Early detection of whole body radiation induced microstructural and neuroinflammatory changes in hippocampus: A diffusion tensor imaging and gene expression study. J. Neurosci. Res. 2017, 95, 1067–1078. [Google Scholar] [CrossRef]
- Lorimore, S.A.; Coates, P.J.; Scobie, G.E.; Miline, G.; Wright, E.G. Inflammation type responses after exposure to ionizing radiation in vivo: A mechanism for radiation-induced bystander effects? Oncogene 2001, 20, 7085–7095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redlich, K.; Smolen, J.S. Inflammatory bone loss: Pathogenesis and therapeutic intervention. Nat. Rev. Drug Discov. 2012, 11, 234–250. [Google Scholar] [CrossRef]
- Appay, V.; Rowland-Jones, S.L. RANTES: A versatile and controversial chemokine. Trends Immunol. 2001, 22, 83–87. [Google Scholar] [CrossRef]
- Mohan, S.; Baylink, D.J. Bone growth factors. Clin. Orthop. Relat. Res. 1991, 263, 30–48. [Google Scholar] [CrossRef]
- Weilbaecher, K.N.; Guise, T.A. Cancer to bone: A fatal attraction. Nat. Rev. Cancer 2011, 11, 411–425. [Google Scholar] [CrossRef] [PubMed]
| Pre-Transplant Baseline Charatteristics | Whole Cohort |
|---|---|
| Number of patients (%) | 78 (100) |
| Sex (%): | |
| Males | 49 (62.8) |
| Females | 29 (37.2) |
| Age at transplant, years (mean (SD) | 10.4 (4.7) |
| Disease stage at transplant, number (%): * | |
| Early | 21 (26.9) |
| Late | 57 (73.1) |
| Donor type, number (%): | |
| HLA-matched related | 26 (33.3) |
| HLA-matched unrelated | 33 (42.3) |
| Haploidentical | 19 (24.4) |
| TBI protocol, number (%): | |
| 12 Gy | 57 (73.1) |
| 7.5 Gy | 21 (26.9) |
| TBI-associated chemotherapy, number (%): | |
| Thiotepa + cyclophosphamide ± ATG | 64 (82.1) |
| Cyclophosphamide ± ATG | 8 (10.2) |
| Fludarabine + thiotepa ± ATG | 6 (7.7) |
| Dose-rate, cGy/min (mean (±SD): | |
| 12 Gy protocol | 14.0 (2.0) |
| 7.5 Gy protocol | 18.7 (1.7) |
| Variation in entrance dose, % (mean (±SD): | |
| 12 Gy protocol | 1.5 (1.0) |
| 7.5 Gy protocol | −0.9 (1.9) |
| Baseline serum amylase value, U/L (mean (±SD): | |
| Total | 35.2 (11.4) |
| Pancreatic | 13.6 (7.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldo, F.; Simeone, R.; Marcuzzi, A.; Grasso, A.G.; Vidimari, R.; Ciriello, F.; Zanon, D.; Maestro, A.; Barbi, E.; Maximova, N. Post-Irradiation Hyperamylasemia Is a Prognostic Marker for Allogeneic Hematopoietic Stem Cell Transplantation Outcomes in Pediatric Population: A Retrospective Single-Centre Cohort Analysis. J. Clin. Med. 2021, 10, 3834. https://doi.org/10.3390/jcm10173834
Baldo F, Simeone R, Marcuzzi A, Grasso AG, Vidimari R, Ciriello F, Zanon D, Maestro A, Barbi E, Maximova N. Post-Irradiation Hyperamylasemia Is a Prognostic Marker for Allogeneic Hematopoietic Stem Cell Transplantation Outcomes in Pediatric Population: A Retrospective Single-Centre Cohort Analysis. Journal of Clinical Medicine. 2021; 10(17):3834. https://doi.org/10.3390/jcm10173834
Chicago/Turabian StyleBaldo, Francesco, Roberto Simeone, Annalisa Marcuzzi, Antonio Giacomo Grasso, Rossella Vidimari, Francesca Ciriello, Davide Zanon, Alessandra Maestro, Egidio Barbi, and Natalia Maximova. 2021. "Post-Irradiation Hyperamylasemia Is a Prognostic Marker for Allogeneic Hematopoietic Stem Cell Transplantation Outcomes in Pediatric Population: A Retrospective Single-Centre Cohort Analysis" Journal of Clinical Medicine 10, no. 17: 3834. https://doi.org/10.3390/jcm10173834
APA StyleBaldo, F., Simeone, R., Marcuzzi, A., Grasso, A. G., Vidimari, R., Ciriello, F., Zanon, D., Maestro, A., Barbi, E., & Maximova, N. (2021). Post-Irradiation Hyperamylasemia Is a Prognostic Marker for Allogeneic Hematopoietic Stem Cell Transplantation Outcomes in Pediatric Population: A Retrospective Single-Centre Cohort Analysis. Journal of Clinical Medicine, 10(17), 3834. https://doi.org/10.3390/jcm10173834








