Relationship between Medium-Term Changes in Intraocular Lens Position and Refraction after Cataract Surgery with Two Different Models of Monofocal Lenses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Ocular Examination and Follow-Up
2.3. Surgical Technique
2.4. Intraocular Lenses
2.5. Optical Calculations
2.6. Statistical Analysis
3. Results
3.1. Postoperative Differences between IOL Groups
3.2. Changes in ACD and ELP over Time in Each Group
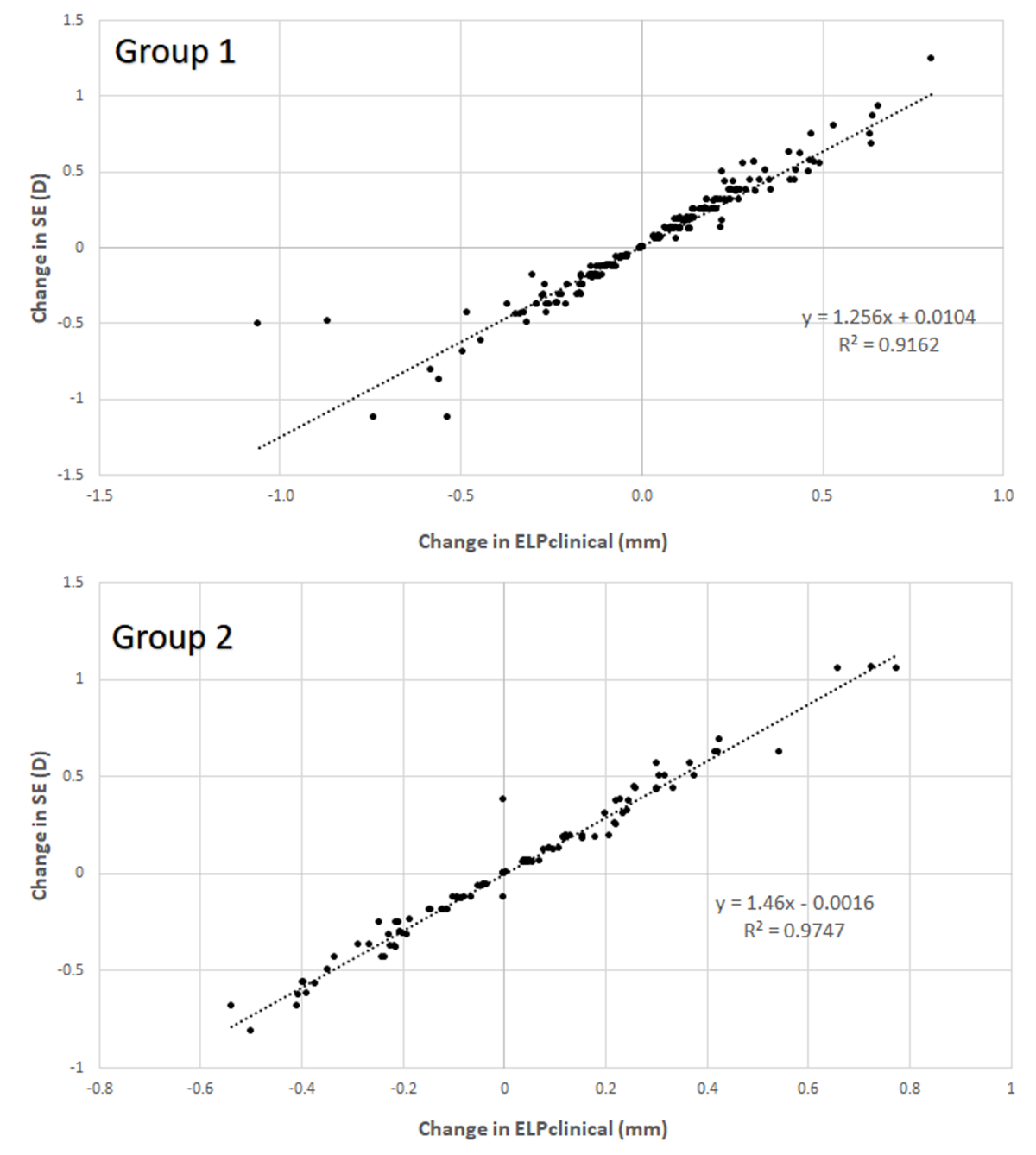
3.3. Correlation between Anatomical and Refractive Changes during the Follow-Up in Each Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prokofyeva, E.; Wegener, A.; Zrenner, E. Cataract prevalence and prevention in Europe: A literature review. Acta Ophthalmol. 2013, 91, 395–405. [Google Scholar] [CrossRef]
- Behndig, A.; Montan, P.; Stenevi, U.; Kugelberg, M.; Lundström, M. One million cataract surgeries: Swedish National Cataract Register 1992–2009. J. Cataract Refract. Surg. 2011, 37, 1539–1545. [Google Scholar] [CrossRef] [PubMed]
- Behndig, A.; Montan, P.; Stenevi, U.; Kugelberg, M.; Zetterström, C.; Lundström, M. Aiming for emmetropia after cataract surgery: Swedish National Cataract Register Study. J. Cataract Refract. Surg. 2012, 38, 1181–1186. [Google Scholar] [CrossRef]
- Fukumitsu, H.; Camps, V.J.; Miraflores, S.; Piñero, D.P. Could anatomical changes occurring with cataract surgery have a clinically significant effect on effective intraocular lens position? Int. Ophthalmol. 2021, 41, 1895–1907. [Google Scholar] [CrossRef]
- Altan, C.; Bayraktar, S.; Altan, T.; Eren, H.; Yilmaz, O.F. Anterior chamber depth, iridocorneal angle width, and intraocular pressure changes after uneventful phacoemulsification in eyes without glaucoma and with open iridocorneal angles. J. Cataract Refract. Surg. 2004, 30, 832–838. [Google Scholar] [CrossRef]
- Kucumen, R.B.; Yenerel, N.M.; Gorgun, E.; Kulacoglu, D.N.; Dinc, U.A.; Alimgil, M.L. Anterior segment optical coherence tomography measurement of anterior chamber depth and angle changes after phacoemulsification and intraocular lens implantation. J. Cataract Refract. Surg. 2008, 34, 1694–1698. [Google Scholar] [CrossRef]
- Uçakhan, O.O.; Ozkan, M.; Kanpolat, A. Anterior chamber parameters measured by the Pentacam CES after uneventful phacoemulsification in normotensive eyes. Acta Ophthalmol. 2009, 87, 544–548. [Google Scholar] [CrossRef]
- Olsen, T. Sources of error in intraocular lens power calculation. J. Cataract Refract. Surg. 1992, 18, 125–129. [Google Scholar] [CrossRef]
- Erickson, P. Effects of intraocular lens position errors on postoperative refractive error. J. Cataract Refract. Surg. 1990, 16, 305–311. [Google Scholar] [CrossRef]
- Hirnschall, N.; Buehren, T.; Trost, M.; Findl, O. Pilot evaluation of refractive prediction errors associated with a new method for ray-tracing-based intraocular lens power calculation. J. Cataract Refract. Surg. 2019, 45, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Hirnschall, N.; Farrokhi, S.; Amir-Asgari, S.; Hienert, S.; Findl, O. Intraoperative optical coherence tomography measurements of aphakic eyes to predict postoperative position of 2 intraocular lens designs. J. Cataract Refract. Surg. 2018, 44, 1310–1316. [Google Scholar] [CrossRef]
- Norrby, S.; Bergman, R.; Hirnschall, N.; Nishi, Y.; Findl, O. Prediction of the true IOL position. Br. J. Ophthalmol. 2017, 101, 1440–1446. [Google Scholar] [CrossRef] [PubMed]
- Hirnschall, N.; Amir-Asgari, S.; Maedel, S.; Findl, O. Predicting the postoperative intraocular lens position using continuous intraoperative optical coherence tomography measurements. Invest. Ophthalmol. Vis. Sci. 2013, 54, 5196–5203. [Google Scholar] [CrossRef] [PubMed]
- Koeppl, C.; Findl, O.; Kriechbaum, K.; Sacu, S.; Drexler, W. Change in IOL position and capsular bag size with an angulated intraocular lens early after cataract surgery. J. Cataract Refract. Surg. 2005, 31, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Findl, O.; Struhal, W.; Dorffner, G.; Drexler, W. Analysis of nonlinear systems to estimate intraocular lens position after cataract surgery. J. Cataract Refract. Surg. 2004, 30, 863–866. [Google Scholar] [CrossRef]
- Petternel, V.; Menapace, R.; Findl, O.; Kiss, B.; Wirtitsch, M.; Rainer, G.; Drexler, W. Effect of optic edge design and haptic angulation on postoperative intraocular lens position change. J. Cataract Refract. Surg. 2004, 30, 52–57. [Google Scholar] [CrossRef]
- Muzyka-Woźniak, M.; Ogar, A. Anterior chamber depth and iris and lens position before and after phacoemulsification in eyes with a short or long axial length. J. Cataract Refract. Surg. 2016, 42, 563–568. [Google Scholar] [CrossRef]
- Wirtitsch, M.G.; Findl, O.; Menapace, R.; Kriechbaum, K.; Koeppl, C.; Buehl, W.; Drexler, W. Effect of haptic design on change in axial lens position after cataract surgery. J. Cataract Refract. Surg. 2004, 30, 45–51. [Google Scholar] [CrossRef]
- Koeppl, C.; Findl, O.; Kriechbaum, K.; Buehl, W.; Wirtitsch, M.; Menapace, R.; Drexler, W. Postoperative change in effective lens position of a 3-piece acrylic intraocular lens. J. Cataract Refract. Surg. 2003, 29, 1974–1979. [Google Scholar] [CrossRef]
- Klijn, S.; Sicam, V.A.D.P.; Reus, N.J. Long-term changes in intraocular lens position and corneal curvature after cataract surgery and their effect on refraction. J. Cataract Refract. Surg. 2016, 42, 35–43. [Google Scholar] [CrossRef]
- Fukumitsu, H.; Camps, V.J.; Piñero, D.P. Intrasession repeatability of biometric measurements obtained with a low-coherence interferometry system in pseudophakic eyes. Curr. Eye Res. 2020, 45, 221–226. [Google Scholar] [CrossRef]
- Retzlaff, J.A.; Sanders, D.R.; Kraff, M.C. Development of the SRK/T intraocular lens implant power calculation formula. J. Cataract Refract. Surg. 1990, 16, 333–340. [Google Scholar] [CrossRef]
- Ramji, H.; Moore, J.; Moore, C.B.; Shah, S. Can the accuracy of multifocal intraocular lens power calculation be improved to make patients spectacle free? Cont. Lens Anterior Eye 2016, 39, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Hoffer, K.J. IOL Power, 1st ed.; Slack Inc.: Thorofare, NJ, USA, 2011. [Google Scholar]
- Shammas, H.J. Intraocular Lens Power Calculations, 1st ed.; Slack Inc.: Thorofare, NJ, USA, 2003. [Google Scholar]
- Sahin, A.; Hamrah, P. Clinically relevant biometry. Curr. Opin. Ophthalmol. 2012, 23, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Sheard, R. Optimising biometry for best outcomes in cataract surgery. Eye Lond. 2014, 28, 118–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinero, D.P. Technologies for anatomical and geometric characterization of the corneal structure and anterior segment: A review. Semin. Ophthalmol. 2015, 30, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Olsen, T. Calculation of intraocular lens power: A review. Acta Ophthalmol. Scand. 2007, 85, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Norrby, S. Sources of error in intraocular lens power calculation. J. Cataract Refract. Surg. 2008, 34, 368–376. [Google Scholar] [CrossRef]
- Eom, Y.; Kang, S.Y.; Song, J.S.; Kim, H.M. Comparison of the actual amount of axial movement of 3 aspheric intraocular lenses using anterior segment optical coherence tomography. J. Cataract Refract. Surg. 2013, 39, 1528–1533. [Google Scholar] [CrossRef]
- Schild, A.M.; Rosentreter, A.; Hellmich, M.; Lappas, A.; Dinslage, S.; Dietlein, T.S. Effect of a capsular tension ring on refractive outcomes in eyes with high myopia. J. Cataract Refract. Surg. 2010, 36, 2087–2093. [Google Scholar] [CrossRef] [PubMed]
| Mean (SD) Range | Group 1 (247 Eyes) | Group 2 (104 Eyes) | p-Value |
|---|---|---|---|
| Age (years) | 73.5 (6.4) 51.0 to 86.0 | 73.1 (8.4) 59.0 to 94.0 | 0.691 |
| Gender (male/female) | 92/155 | 49/55 | 0.096 |
| Corneal endothelial density (cell/mm2) | 2302.5 (411.9) 736.0 to 3543.0 | 2362.9 (366.3) 1587.0 to 3461.0 | 0.571 |
| Central corneal thickness (μm) | 541.5 (32.3) 445.0 to 628.0 | 544.9 (39.4) 474.0 to 629.0 | 0.465 |
| IOP (mm Hg) | 16.8 (3.5) 10.0 to 29.0 | 16.3 (3.6) 9.0 to 25.0 | 0.218 |
| Sphere (D) | 0.02 (3.43) −17.50 to 6.75 | 0.26 (2.62) −8.25 to 4.75 | 0.521 |
| Cylinder (D) | −1.44 (1.05) −8.75 to −0.25 | −1.35 (1.01) −5.75 to 0.00 | 0.423 |
| SE (D) | −0.70 (3.53) −17.88 to 5.88 | −0.41 (2.61) −8.75 to 3.88 | 0.464 |
| Axial length (mm) | 23.30 (1.13) 20.59 to 27.65 | 23.23 (0.90) 21.40 to 25.66 | 0.558 |
| ACD (mm) | 3.12 (0.36) 2.24 to 3.99 | 3.22 (0.37) 2.09 to 3.96 | 0.018 |
| Lens thickness (mm) | 4.67 (0.40) 3.66 to 5.79 | 4.61 (0.36) 3.69 to 5.35 | 0.183 |
| KM (D) | 44.12 (1.57) 40.17 to 47.58 | 44.09 (1.30) 41.91 to 47.10 | 0.873 |
| WTW (mm) | 11.55 (0.36) 10.64 to 12.69 | 11.58 (0.39) 10.77 to 12.63 | 0.463 |
| IOL power (D) | 21.52 (3.13) 9.50 to 29.50 | 22.05 (2.35) 15.50 to 27.50 | 0.120 |
| Predicted postoperative SE (D) | −0.16 (0.18) −0.82 to 0.15 | −0.20 (0.16) −0.62 to 0.12 | 0.056 |
| Mean (SD) Range | 1 Month Postoperative | 6 Months Postoperative | ||||
|---|---|---|---|---|---|---|
| Group 1 | Group 2 | p-Value | Group 1 | Group 2 | p-Value | |
| Sphere (D) | 0.64 (0.66) −2.00 to 2.50 | 0.30 (0.74) −1.75 to 2.50 | <0.001 | 0.70 (0.67) −1.75 to 2.87 | 0.32 (0.73) −1.75 to 2.25 | <0.001 |
| Cylinder (D) | −1.22 (0.71) −3.50 to 0.00 | −1.26 (0.84) −4.50 to 0.00 | 0.674 | −1.23 (0.75) −3.75 to 0.00 | −1.22 (0.90) −4.62 to 0.00 | 0.949 |
| SE (D) | 0.03 (0.57) −2.62 to 1.69 | −0.33 (0.63) −2.50 to 0.88 | <0.001 | 0.08 (0.57) −2.50 to 1.56 | −0.30 (0.63) −2.56 to 1.62 | <0.001 |
| Axial length (mm) | 23.22 (1.13) 20.55 to 27.52 | 23.14 (0.91) 21.34 to 25.48 | 0.560 | 23.22 (1.13) 20.54 to 27.50 | 23.13 (0.91) 21.32 to 25.48 | 0.422 |
| ACD (mm) | 4.61 (0.42) 3.20 to 6.23 | 5.36 (0.53) 3.07 to 6.33 | <0.001 | 4.57 (0.37) 3.66 to 6.16 | 5.45 (0.73) 3.07 to 8.86 | <0.001 |
| ELPSRK-T (mm) | Group 1 5.35 (0.48) 4.55 to 8.36 | Group 2 5.30 (0.31) 4.77 to 6.41 | 0.268 | |||
| ELPclinical (mm) | 5.66 (0.59) 4.23 to 9.65 | 5.51 (0.52) 4.12 to 6.63 | 0.025 | 5.69 (0.56) 4.46 to 8.59 | 5.53 (0.50) 4.21 to 6.68 | 0.010 |
| Mean (SD) Range | Group 1 (247 Eyes) | Group 2 (104 Eyes) | p-Value |
|---|---|---|---|
| Change axial length (mm) | −0.003 (0.040) −0.18 to 0.16 | −0.007 (0.11) −0.95 to 0.21 | 0.611 |
| Change ACD (mm) | −0.05 (0.30) −0.94 to 1.60 | 0.10 (0.63) −1.05 to 3.50 | 0.065 |
| Change ELPclinical (mm) | 0.04 (0.24) −1.06 to 0.80 | 0.02 (0.26) −0.54 to 0.78 | 0.634 |
| Change SE (D) | 0.06 (0.31) −1.12 to 1.24 | 0.03 (0.38) −0.82 to 1.06 | 0.516 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukumitsu, H.; Camps, V.J.; Miraflores, S.; Piñero, D.P. Relationship between Medium-Term Changes in Intraocular Lens Position and Refraction after Cataract Surgery with Two Different Models of Monofocal Lenses. J. Clin. Med. 2021, 10, 3856. https://doi.org/10.3390/jcm10173856
Fukumitsu H, Camps VJ, Miraflores S, Piñero DP. Relationship between Medium-Term Changes in Intraocular Lens Position and Refraction after Cataract Surgery with Two Different Models of Monofocal Lenses. Journal of Clinical Medicine. 2021; 10(17):3856. https://doi.org/10.3390/jcm10173856
Chicago/Turabian StyleFukumitsu, Hideki, Vicent J. Camps, Sara Miraflores, and David P. Piñero. 2021. "Relationship between Medium-Term Changes in Intraocular Lens Position and Refraction after Cataract Surgery with Two Different Models of Monofocal Lenses" Journal of Clinical Medicine 10, no. 17: 3856. https://doi.org/10.3390/jcm10173856






