Hemodynamics Prior to Valve Replacement for Severe Aortic Stenosis and Pulmonary Hypertension during Long-Term Follow-Up
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Cardiac Catheterization
2.3. Definition of Pulmonary Hypertension
2.4. Echocardiography and Follow-Up
2.5. Statistical Analysis
3. Results
3.1. Study Population
3.2. Baseline Cardiac Catheterization
3.3. Aortic Valve Replacement
3.4. Post-AVR PH Status
3.5. Pre-AVR Clinical Characteristics of Patients with versus without Post-AVR PH
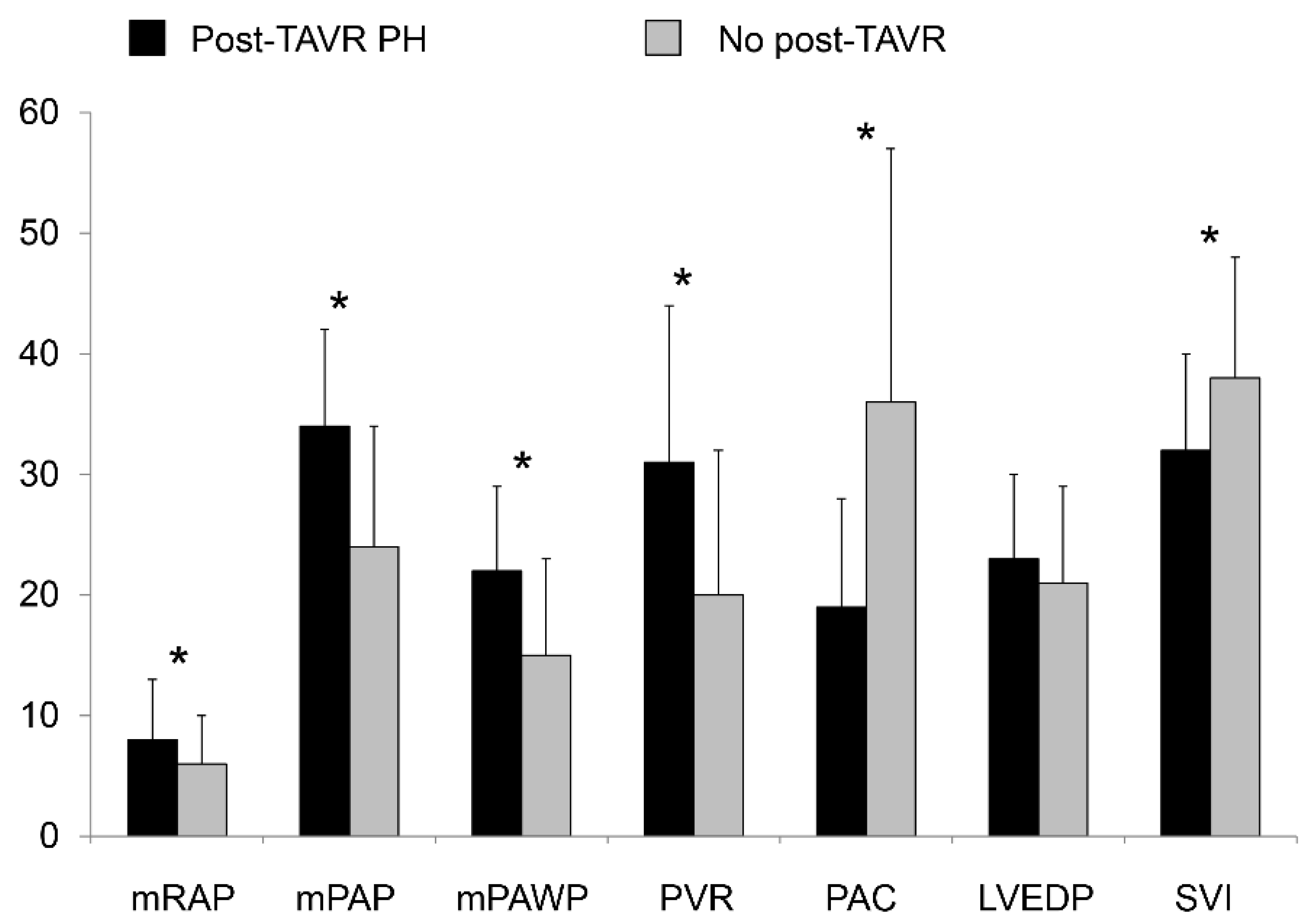
3.6. Pre-AVR Invasive Hemodynamics of Patients with versus without Post-AVR PH
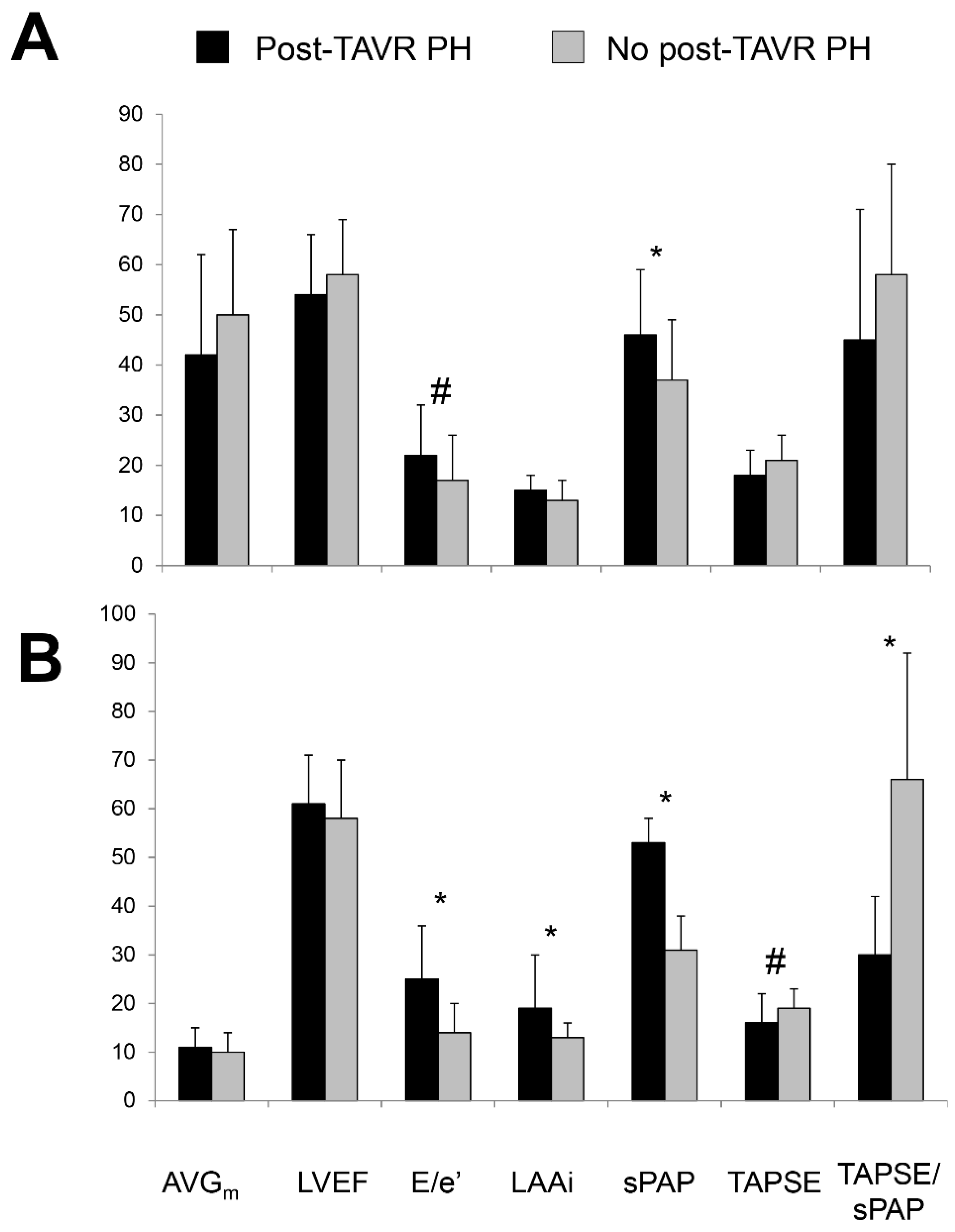
3.7. Clinical Status and Echocardiography at Follow-Up
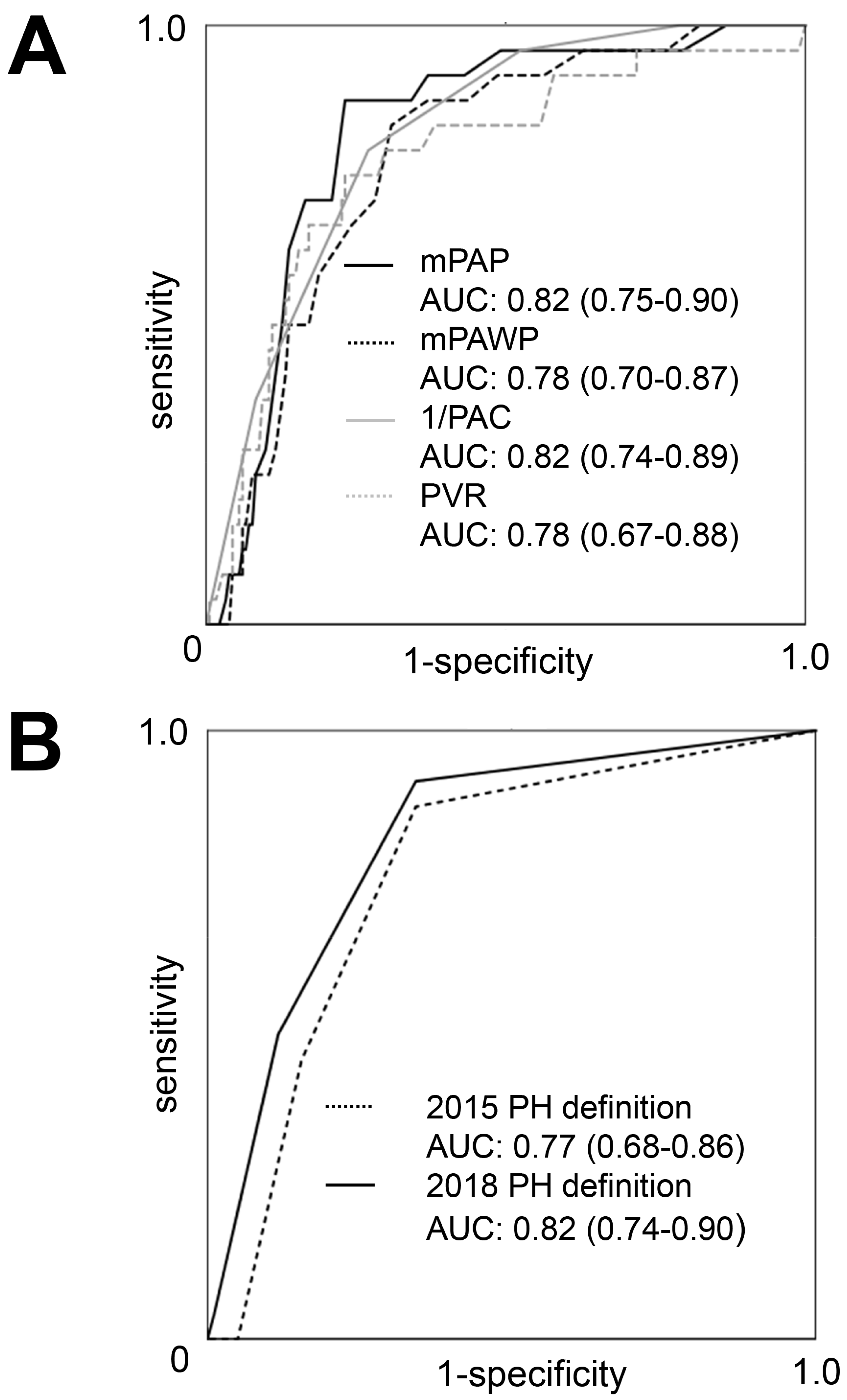
3.8. Prediction of Post-AVR PH
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maeder, M.T.; Weber, L.; Rickli, H. Pulmonary hypertension in aortic valve stenosis. Trends Cardiovasc. Med. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Sinning, J.M.; Hammerstingl, C.; Chin, D.; Ghanem, A.; Schueler, R.; Sedaghat, A.; Bence, J.; Spyt, T.; Werner, N.; Kovac, J.; et al. Decrease of pulmonary hypertension impacts on prognosis after transcatheter aortic valve replacement. EuroIntervention 2014, 9, 1042–1049. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, C.J.; Wenaweser, P.; Ceylan, O.; Rat-Wirtzler, J.; Stortecky, S.; Heg, D.; Spitzer, E.; Zanchin, T.; Praz, F.; Tüller, D.; et al. Effect of Pulmonary Hypertension Hemodynamic Presentation on Clinical Outcomes in Patients with Severe Symptomatic Aortic Valve Stenosis Undergoing Transcatheter Aortic Valve Implantation: Insights from the New Proposed Pulmonary Hypertension Classification. Circ. Cardiovasc. Interv. 2015, 8, e002358. [Google Scholar] [PubMed] [Green Version]
- Masri, A.; Abdelkarim, I.; Sharbaugh, M.S.; Althouse, A.D.; Xu, J.; Han, W.; Chan, S.Y.; Katz, W.E.; Crock, F.W.; Harinstein, M.E.; et al. Outcomes of persistent pulmonary hypertension following transcatheter aortic valve replacement. Heart 2018, 104, 821–827. [Google Scholar] [CrossRef]
- Schewel, J.; Schmidt, T.; Kuck, K.H.; Frerker, C.; Schewel, D. Impact of Pulmonary Hypertension Hemodynamic Status on Long-Term Outcome after Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2019, 12, 2155–2168. [Google Scholar] [CrossRef] [PubMed]
- Maeder, M.T.; Weber, L.; Buser, M.; Gerhard, M.; Haager, P.K.; Maisano, F.; Rickli, H. Pulmonary Hypertension in Aortic and Mitral Valve Disease. Front. Cardiovasc. Med. 2018, 5, 40. [Google Scholar] [CrossRef] [Green Version]
- Genereux, P.; Pibarot, P.; Redfors, B.; Mack, M.J.; Makkar, R.R.; Jaber, W.A.; Svensson, L.G.; Kapadia, S.; Tuzcu, E.M.; Thourani, V.H.; et al. Staging classification of aortic stenosis based on the extent of cardiac damage. Eur. Heart J. 2017, 38, 3351–3358. [Google Scholar] [CrossRef] [Green Version]
- Tastet, L.; Genereux, P.; Bernard, J.; Pibarot, P. The Role of Extra-valvular Cardiac Damage Staging in Aortic Valve Disease Management. Can. J. Cardiol. 2021, 37, 1004–1015. [Google Scholar] [CrossRef] [PubMed]
- Eleid, M.F.; Padang, R.; Pislaru, S.V.; Greason, K.L.; Crestanello, J.; Nkomo, V.T.; Pellikka, P.A.; Jentzer, J.C.; Gulati, R.; Sandhu, G.S.; et al. Effect of Transcatheter Aortic Valve Replacement on Right Ventricular-Pulmonary Artery Coupling. JACC Cardiovasc. Interv. 2019, 12, 2145–2154. [Google Scholar] [CrossRef]
- Villari, B.; Vassalli, G.; Monrad, E.S.; Chiariello, M.; Turina, M.; Hess, O.M. Normalization of diastolic dysfunction in aortic stenosis late after valve replacement. Circulation 1995, 91, 2353–2358. [Google Scholar] [CrossRef]
- Weber, L.; Rickli, H.; Haager, P.K.; Joerg, L.; Weilenmann, D.; Brenner, R.; Taramasso, M.; Baier, P.; Maisano, F.; Maeder, M.T. Haemodynamic mechanisms and long-term prognostic impact of pulmonary hypertension in patients with severe aortic stenosis undergoing valve replacement. Eur. J. Heart Fail. 2019, 21, 172–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeder, M.T.; Weber, L.; Ammann, P.; Buser, M.; Ehl, N.F.; Gerhard, M.; Brenner, R.; Haager, P.K.; Maisano, F.; Rickli, H. Relationship between B-type natriuretic peptide and invasive haemodynamics in patients with severe aortic valve stenosis. ESC Heart Fail. 2020, 7, 577–587. [Google Scholar] [CrossRef] [Green Version]
- Maeder, M.; Weber, L.; Weilenmann, D.; Haager, P.K.; Joerg, L.; Taramasso, M.; Buser, M.; Ehl, N.F.; Maisano, F.; Rickli, H. Invasive Hemodynamic Staging Classification of Cardiac Damage in Patients with Aortic Stenosis Undergoing Valve Replacement. Can. J. Cardiol. 2020, 36, 1667–1674. [Google Scholar] [CrossRef] [PubMed]
- Weber, L.; Rickli, H.; Ammann, P.; Taramasso, M.; Brenner, R.; Ehl, N.F.; Buser, M.; Haager, P.K.; Maisano, F.; Maeder, M.T. Hemodynamic profile of patients with severe aortic valve stenosis and atrial fibrillation versus sinus rhythm. Int. J. Cardiol. 2020, 311, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Maeder, M.T.; Weber, L.; Weilenmann, D.; Chronis, J.; Joerg, L.; Pohle, S.; Haager, P.K.; Brutsche, M.; Neumann, T.; Schoch, O.D.; et al. Impact of the new pulmonary hypertension definition on long-term mortality in patients with severe aortic stenosis undergoing valve replacement. Clin. Cardiol. 2021. [Google Scholar] [CrossRef]
- Maeder, M.T.; Weber, L.; Weilenmann, D.; Haager, P.K.; Joerg, L.; Rohner, F.; Ammann, P.; Chronis, J.; Rigger, J.; Rickli, H. Impact of a volume challenge on haemodynamics and prognosis in patients with severe aortic stenosis. ESC Heart Fail. 2021, 8, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Tschumper, M.; Weber, L.; Rickli, H.; Seidl, S.; Brenner, R.; Buser, M.; Ehl, N.F.; Jäger-Rhomberg, F.; Ammann, P.; Maeder, M.T. Corrected QT Interval in Severe Aortic Stenosis: Clinical and Hemodynamic Correlates and Prognostic Impact. Am. J. Med. 2021, 134, 267–277. [Google Scholar] [CrossRef]
- Wright, S.P.; Moayedi, Y.; Foroutan, F.; Agarwal, S.; Paradero, G.; Alba, A.C.; Baumwol, J.; Mak, S. Diastolic Pressure Difference to Classify Pulmonary Hypertension in the Assessment of Heart Transplant Candidates. Circ. Heart Fail. 2017, 10, e004077. [Google Scholar] [CrossRef]
- Houston, B.A.; Tedford, R.J. What We Talk about When We Talk about the Wedge Pressure. Circ. Heart Fail. 2017, 10, e004450. [Google Scholar] [CrossRef] [PubMed]
- Reddy, Y.N.; El-Sabbagh, A.; Nishimura, R.A. Comparing Pulmonary Arterial Wedge Pressure and Left Ventricular End Diastolic Pressure for Assessment of Left-Sided Filling Pressures. JAMA Cardiol. 2018, 3, 453–454. [Google Scholar] [CrossRef]
- Galie, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Heart J. 2016, 37, 67–119. [Google Scholar] [PubMed]
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef]
- Vachiery, J.L.; Tedford, R.J.; Rosenkranz, S.; Palazzini, M.; Lang, I.; Guazzi, M.; Coghlan, G.; Chazova, I.; De Marco, T. Pulmonary hypertension due to left heart disease. Eur. Respir. J. 2019, 53, 1801897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, I.M. Diastolic Pulmonary Artery to Pulmonary Capillary Wedge Pressure Difference: A Predictor of Outcome after Left Ventricular Assist Device Implantation. J. Am. Heart Assoc. 2020, 9, e016110. [Google Scholar] [CrossRef]
- Gerges, M.; Gerges, C.; Lang, I.M. How to define pulmonary hypertension due to left heart disease. Eur. Respir. J. 2016, 48, 553–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guazzi, M.; Bandera, F.; Pelissero, G.; Castelvecchio, S.; Menicanti, L.; Ghio, S.; Temporelli, P.L.; Arena, R. Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: An index of right ventricular contractile function and prognosis. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H1373-81. [Google Scholar] [CrossRef]
- Sultan, I.; Fukui, M.; Bianco, V.; Brown, J.A.; Kliner, D.E.; Hickey, G.; Thoma, F.W.; Lee, J.S.; Schindler, J.T.; Kilic, A.; et al. Impact of Combined Pre and Postcapillary Pulmonary Hypertension on Survival after Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2020, 131, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Baumann, P.; Manthey, J.; Hoffmann, M.; Schuler, G.; Mehmel, H.C.; Schmitz, W.; Kübler, W. The effect of aortic valve replacement on survival. Circulation 1982, 66, 1105–1110. [Google Scholar] [CrossRef] [Green Version]
- Medvedofsky, D.; Klempfner, R.; Fefer, P.; Chernomordik, F.; Hamdan, A.; Hay, I.; Goldenberg, I.; Raanani, E.; Guetta, V.; Segev, A. The significance of pulmonary arterial hypertension pre- and post-transfemoral aortic valve implantation for severe aortic stenosis. J. Cardiol. 2015, 65, 337–342. [Google Scholar] [CrossRef] [Green Version]
- Strachinaru, M.; Ren, B.; van Dalen, B.M.; Van Mieghem, N.; De Jaegere, P.P.; van Gils, L.; Galema, T.W.; Geleijnse, M.L. Determinants of changes in pulmonary artery pressure in patients with severe aortic stenosis treated by transcatheter aortic valve implantation. Acta Cardiol. 2021, 76, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Fayyaz, A.U.; Edwards, W.D.; Maleszewski, J.J.; Konik, E.A.; DuBrock, H.M.; Borlaug, B.A.; Frantz, R.P.; Jenkins, S.M.; Redfield, M.M. Global Pulmonary Vascular Remodeling in Pulmonary Hypertension Associated with Heart Failure and Preserved or Reduced Ejection Fraction. Circulation 2018, 137, 1796–1810. [Google Scholar] [CrossRef]
- Zimpfer, D.; Zrunek, P.; Roethy, W.; Czerny, M.; Schima, H.; Huber, L.; Grimm, M.; Rajek, A.; Wolner, E.; Wieselthaler, G. Left ventricular assist devices decrease fixed pulmonary hypertension in cardiac transplant candidates. J. Thorac Cardiovasc. Surg. 2007, 133, 689–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bing, R.; Cavalcante, J.L.; Everett, R.J.; Clavel, M.A.; Newby, D.E.; Dweck, M.R. Imaging and Impact of Myocardial Fibrosis in Aortic Stenosis. JACC Cardiovasc. Imaging 2019, 12, 283–296. [Google Scholar] [CrossRef]
- Dupont, M.; Mullens, W.; Skouri, H.N.; Abrahams, Z.; Wu, Y.; Taylor, D.O.; Starling, R.C.; Tang, W.W. Prognostic role of pulmonary arterial capacitance in advanced heart failure. Circ. Heart Fail. 2012, 5, 778–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tedford, R.J.; Hassoun, P.M.; Mathai, S.C.; Girgis, R.E.; Russell, S.D.; Thiemann, D.R.; Cingolani, O.H.; Mudd, J.O.; Borlaug, B.A.; Redfield, M.M.; et al. Pulmonary capillary wedge pressure augments right ventricular pulsatile loading. Circulation 2012, 125, 289–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bermejo, J.; Yotti, R.; Garcia-Orta, R.; Sanchez-Fernandez, P.L.; Castano, M.; Segovia-Cubero, J.; Escribano-Subías, P.; San Román, J.A.; Borrás, X.; Alonso-Gómez, A.; et al. Sildenafil for improving outcomes in patients with corrected valvular heart disease and persistent pulmonary hypertension: A multicenter, double-blind, randomized clinical trial. Eur. Heart J. 2018, 39, 1255–1264. [Google Scholar] [CrossRef] [Green Version]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Munoz, D.R.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef] [PubMed]
- Gertz, Z.M.; McCauley, B.D.; Raina, A.; O’Donnell, W.; Shellenberger, C.; Willhide, J.; Forfia, P.R.; Herrmann, H.C. Estimation of oxygen consumption in elderly patients with aortic stenosis. Catheter. Cardiovasc. Interv. 2014, 83, E128–E133. [Google Scholar] [CrossRef]
| All Patients (n = 205) | Post-AVR PH (n = 24) | No Post-AVR PH (n = 181) | p Value | |
|---|---|---|---|---|
| Age (years) | 75 ± 10 | 81 ± 6 | 74 ± 10 | 0.001 |
| Gender (male) | 102 (50%) | 6 (25%) | 96 (53%) | 0.01 |
| Body mass index (kg/m2) | 27.3 ± 4.8 | 27.6 ± 4.5 | 27.2 ± 4.9 | 0.69 |
| Body surface area (m2) | 1.84 ± 0.22 | 1.78 ± 0.20 | 1.85 ± 0.23 | 0.14 |
| eGFR (mL/min/1.73 m2) | 72 ± 27 | 60 ± 19 | 73 ± 28 | 0.03 |
| Hemoglobin (g/L) | 134 ± 17 | 127 ± 16 | 135 ± 17 | 0.03 |
| Diabetes | 29 (14%) | 6 (25%) | 23 (13%) | 0.10 |
| Stroke | 10 (5%) | 1 (4%) | 9 (5%) | 0.86 |
| Chronic obstructive lung disease | 18 (9%) | 2 (8%) | 16 (9%) | 0.93 |
| FEV1 (% predicted) | 89 ± 21 | 80 ± 19 | 90 ± 21 | 0.03 |
| Heart rhythm | 0.58 | |||
| Sinus rhythm | 178 (87%) | 19 (79%) | 159 (88%) | |
| Atrial fibrillation | 19 (9%) | 4 (17%) | 15 (8%) | |
| pacemaker | 8 (4%) | 1 (4%) | 7 (4%) | |
| Heart rate (bpm) | 70 ± 13 | 73 ± 16 | 69 ± 12 | 0.14 |
| Medication | ||||
| Oral anticoagulation | 35 (17%) | 9 (38%) | 26 (14%) | 0.05 |
| Aspirin | 112 (55%) | 14 (58%) | 98 (54%) | 0.70 |
| Loop diuretics | 92 (45%) | 19 (79%) | 73 (40%) | <0.001 |
| Betablocker | 88 (43%) | 13 (54%) | 75 (41%) | 0.24 |
| ACEI/ARB | 99 (48%) | 10 (42%) | 89 (49%) | 0.49 |
| Digoxin | 17 (8%) | 4 (17%) | 13 (7%) | 0.11 |
| Spironolactone | 10 (5%) | 4 (17%) | 6 (3%) | 0.004 |
| Symptoms | ||||
| Dyspnea NYHA class | 0.008 | |||
| I | 36 (18%) | 3 (12%) | 33 (18%) | |
| II | 109 (53%) | 9 (38%) | 100 (55%) | |
| III | 51 (25%) | 8 (33%) | 43 (24%) | |
| IV | 9 (4%) | 4 (17%) | 5 (3%) | |
| B-type natriuretic peptide (ng/L) | 180 (75–448) | 445 (256–779) | 146 (59–333) | <0.001 |
| Mode of AVR | <0.001 | |||
| Surgical AVR | 143 (70%) | 7 (29%) | 136 (75%) | |
| Transcatheter AVR | 62 (30%) | 17 (71%) | 45 (25%) |
| All Patients (n = 205) | Post-AVR PH (n = 24) | No Post-AVR PH (n = 181) | p Value | |
|---|---|---|---|---|
| Echocardiography | ||||
| Left ventricular end-diastolic diameter (mm) | 46 ± 8 | 44 ± 8 | 47 ± 8 | 0.18 |
| Indexed left ventricular end-diastolic diameter (mm/m2) | 26 ± 4 | 25 ± 4 | 26 ± 5 | 0.68 |
| Left ventricular ejection fraction (%) | 58 ± 11 | 54 ± 12 | 58 ± 11 | 0.05 |
| E/e’ | 17.3 ± 9.1 | 21.7 ± 9.7 | 16.5 ± 8.9 | 0.04 |
| Left atrial area (cm2) | 25 ± 6 | 26 ± 6 | 24 ± 6 | 0.29 |
| Indexed left atrial area (cm2/m2) | 13 ± 3 | 15 ± 3 | 13 ± 4 | 0.08 |
| TAPSE (mm) | 21 ± 5 | 18 ± 5 | 21 ± 5 | 0.08 |
| Estimated sPAP (mmHg) | 39 ± 12 | 46 ± 13 | 37 ± 12 | 0.006 |
| TAPSE/sPAP (mm/mmHg) | 0.56 ± 0.23 | 0.45 ± 0.26 | 0.58 ± 0.22 | 0.08 |
| Mean aortic valve gradient (mmHg) | 49 ± 18 | 42 ± 20 | 50 ± 17 | 0.03 |
| Aortic valve area (cm2) | 0.77 ± 0.22 | 0.76 ± 0.19 | 0.77 ± 0.23 | 0.75 |
| Indexed aortic valve area (cm2/m2) | 0.42 ± 0.12 | 0.43 ± 0.11 | 0.42 ± 0.12 | 0.69 |
| Aortic regurgitation (at least moderate) | 24 (12%) | 1 (4%) | 23 (13%) | 0.21 |
| Mitral regurgitation | 0.08 | |||
| no | 100 (49%) | 6 (25%) | 94 (52%) | |
| mild | 87 (42%) | 15 (63%) | 72 (39%) | |
| moderate | 16 (8%) | 3 (12%) | 13 (8%) | |
| severe | 2 (1%) | 0 | 2 (1%) | |
| Coronary artery disease | 1.0 | |||
| No coronary artery disease | 123 (60%) | 11 (46%) | 112 (62%) | |
| 1-vessel disease | 35 (17%) | 3 (12%) | 32 (18%) | |
| 2-vessel disease | 23 (11%) | 6 (25%) | 17 (9%) | |
| 3-vessel disease | 24 (12%) | 4 (17%) | 20 (11%) | |
| Invasive hemodynamics | ||||
| Mean right atrial pressure (mmHg) | 6 ± 4 | 8 ± 5 | 6 ± 4 | 0.009 |
| Right ventricular end-diastolic pressure (mmHg) | 8 ± 4 | 10 ± 5 | 7 ± 4 | 0.004 |
| sPAP (mmHg) | 39 ± 15 | 52 ± 11 | 37 ± 14 | <0.001 |
| dPAP (mmHg) | 15 ± 8 | 21 ± 7 | 14 ± 8 | <0.001 |
| mPAP (mmHg) | 25 ± 10 | 34 ± 8 | 24 ± 10 | <0.001 |
| mPAWP (mmHg) | 16 ± 8 | 22 ± 7 | 15 ± 8 | <0.001 |
| Transpulmonary gradient (mmHg) | 9 ± 4 | 12 ± 4 | 9 ± 4 | 0.002 |
| Pulmonary vascular resistance (Wood units) | 2.1 ± 1.3 | 3.1 ± 1.3 | 2.0 ± 1.2 | <0.001 |
| Diastolic pressure gradient (mmHg) | −1 (−3–1) | −2 (−5–3) | 0 (−3–1) | 0.35 |
| Pulmonary artery capacitance (mL/mmHg) | 3.4 ± 2.0 | 1.9 ± 0.9 | 3.6 ± 2.1 | <0.001 |
| Left ventricular end-diastolic pressure (mmHg) | 21 ± 8 (n = 146) | 23 ± 7 (n = 17) | 21 ± 8 (n = 129) | 0.41 |
| Systolic aortic pressure (mmHg) | 148 ± 26 | 161 ± 28 | 146 ± 25 | 0.007 |
| Diastolic aortic pressure (mmHg) | 68 ± 12 | 71 ± 13 | 68 ± 11 | 0.21 |
| Mean aortic pressure (mmHg) | 100 ± 15 | 106 ± 16 | 99 ± 14 | 0.03 |
| Systemic vascular resistance (Wood units) | 21.0 ± 5.7 | 25.0 ± 5.6 | 20.5 ± 5.5 | <0.001 |
| Arterial oxygen saturation (%) | 95 (94–97) | 94 (92–96) | 95 (94–97) | 0.03 |
| Mixed venous oxygen saturation (%) | 69 (64–73) | 64 (58–7) | 70 (65–73) | <0.001 |
| Cardiac output (L/min) | 4.6 ± 1.0 | 3.9 ± 0.7 | 4.7 ± 1.0 | <0.001 |
| Cardiac index (L/min/m2) | 2.5 ± 0.5 | 2.2 ± 0.4 | 2.6 ± 0.5 | 0.003 |
| Stroke volume index (mL/m2) | 37 ± 10 | 32 ± 8 | 38 ± 10 | 0.003 |
| PH classification 2015 | <0.001 | |||
| no PH | 122 (60%) | 3 (12%) | 119 (66%) | |
| isolated postcapillary PH | 44 (21%) | 10 (42%) | 34 (19%) | |
| combined pre- and postcapillary PH | 30 (15%) | 11 (46%) | 19 (10%) | |
| precapillary PH | 9 (4%) | 0 | 9 (5%) | |
| PH classification 2018 | <0.001 | |||
| no PH | 121 (59%) | 2 (8%) | 119 (66%) | |
| isolated postcapillary PH | 51 (25%) | 10 (42%) | 41 (23%) | |
| combined pre- and postcapillary PH | 30 (15%) | 11 (46%) | 19 (10%) | |
| precapillary PH | 3 (1%) | 1 (4%) | 2 (1%) |
| All Patients (n = 205) | Post-AVR PH (n = 24) | No Post-AVR PH (n = 181) | p Value | |
|---|---|---|---|---|
| Symptoms | ||||
| Dyspnea NYHA class | <0.001 | |||
| I | 130 (64%) | 11 (46%) | 119 (66%) | |
| II | 60 (29%) | 7 (29%) | 53 (29%) | |
| III | 13 (6%) | 4 (17%) | 9 (5%) | |
| IV | 2 (1%) | 2 (8%) | 0 | |
| Echocardiography | ||||
| Left ventricular end-diastolic diameter (mm) | 47 ± 7 | 47 ± 8 | 46 ± 7 | 0.68 |
| Indexed left ventricular end-diastolic diameter (mm/m2) | 26 ± 4 | 27 ± 5 | 25 ± 4 | 0.20 |
| Left atrial area (cm2) | 25 ± 9 | 36 ± 20 | 24 ± 7 | <0.001 |
| Indexed left atrial area (cm2/m2) | 14 ± 5 | 19 ± 11 | 13 ± 3 | <0.001 |
| E/e’ | 15.5 ± 7.8 | 25.2 ± 10.6 | 14.0 ± 6.1 | <0.001 |
| Left ventricular ejection fraction (%) | 61 ± 10 | 58 ± 12 | 61 ± 9 | 0.15 |
| Mean aortic valve gradient (mmHg) | 11 ± 4 | 10 ± 4 | 11 ± 4 | 0.13 |
| Mitral regurgitation | 0.002 | |||
| no | 98 (48%) | 6 (25%) | 92 (51%) | |
| mild | 93 (45%) | 13 (54%) | 80 (44%) | |
| moderate | 13 (6%) | 4 (17%) | 9 (5%) | |
| severe | 1 (1%) | 1 (4%) | 0 | |
| sPAP (mmHg) | 33 ± 10 | 53 ± 5 | 31 ± 7 | <0.001 |
| TAPSE (mm) | 18 ± 4 | 16 ± 6 | 19 ± 4 | 0.03 |
| TAPSE/sPAP (mm/mmHg) | 0.61 ± 0.27 | 0.30 ± 0.12 | 0.66 ± 0.26 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weber, L.; Rickli, H.; Haager, P.K.; Joerg, L.; Weilenmann, D.; Chronis, J.; Rigger, J.; Buser, M.; Ehl, N.F.; Maeder, M.T. Hemodynamics Prior to Valve Replacement for Severe Aortic Stenosis and Pulmonary Hypertension during Long-Term Follow-Up. J. Clin. Med. 2021, 10, 3878. https://doi.org/10.3390/jcm10173878
Weber L, Rickli H, Haager PK, Joerg L, Weilenmann D, Chronis J, Rigger J, Buser M, Ehl NF, Maeder MT. Hemodynamics Prior to Valve Replacement for Severe Aortic Stenosis and Pulmonary Hypertension during Long-Term Follow-Up. Journal of Clinical Medicine. 2021; 10(17):3878. https://doi.org/10.3390/jcm10173878
Chicago/Turabian StyleWeber, Lukas, Hans Rickli, Philipp K. Haager, Lucas Joerg, Daniel Weilenmann, Joannis Chronis, Johannes Rigger, Marc Buser, Niklas F. Ehl, and Micha T. Maeder. 2021. "Hemodynamics Prior to Valve Replacement for Severe Aortic Stenosis and Pulmonary Hypertension during Long-Term Follow-Up" Journal of Clinical Medicine 10, no. 17: 3878. https://doi.org/10.3390/jcm10173878
APA StyleWeber, L., Rickli, H., Haager, P. K., Joerg, L., Weilenmann, D., Chronis, J., Rigger, J., Buser, M., Ehl, N. F., & Maeder, M. T. (2021). Hemodynamics Prior to Valve Replacement for Severe Aortic Stenosis and Pulmonary Hypertension during Long-Term Follow-Up. Journal of Clinical Medicine, 10(17), 3878. https://doi.org/10.3390/jcm10173878





