Drug–Drug Interaction between Tacrolimus and Vonoprazan in Kidney Transplant Recipients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Drug Administrations
2.2. Determination of Blood Tacrolimus Concentration
2.3. Genomic DNA Extraction and Genotyping
2.4. Statistical Analysis
3. Results
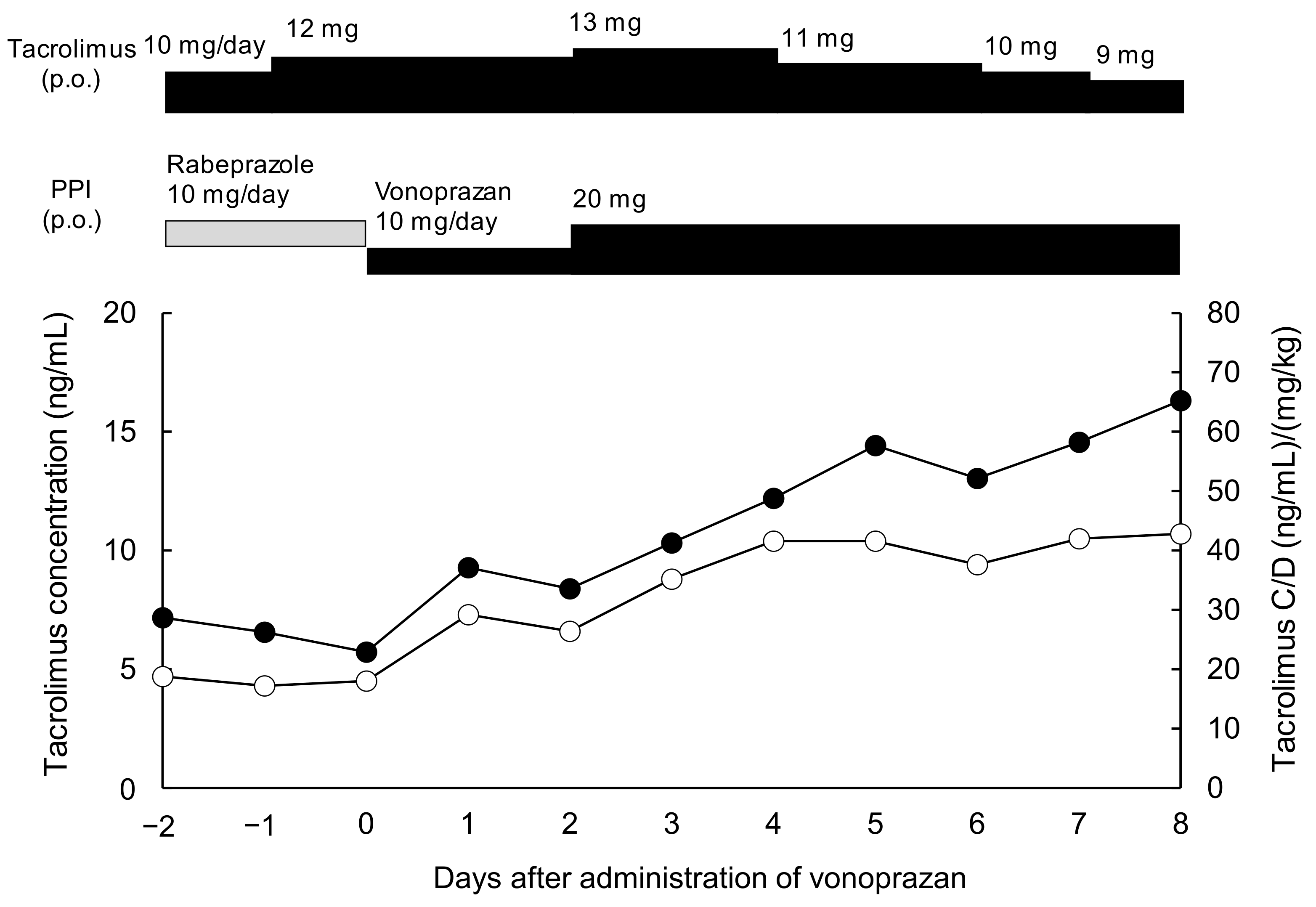
3.1. Case Presentation
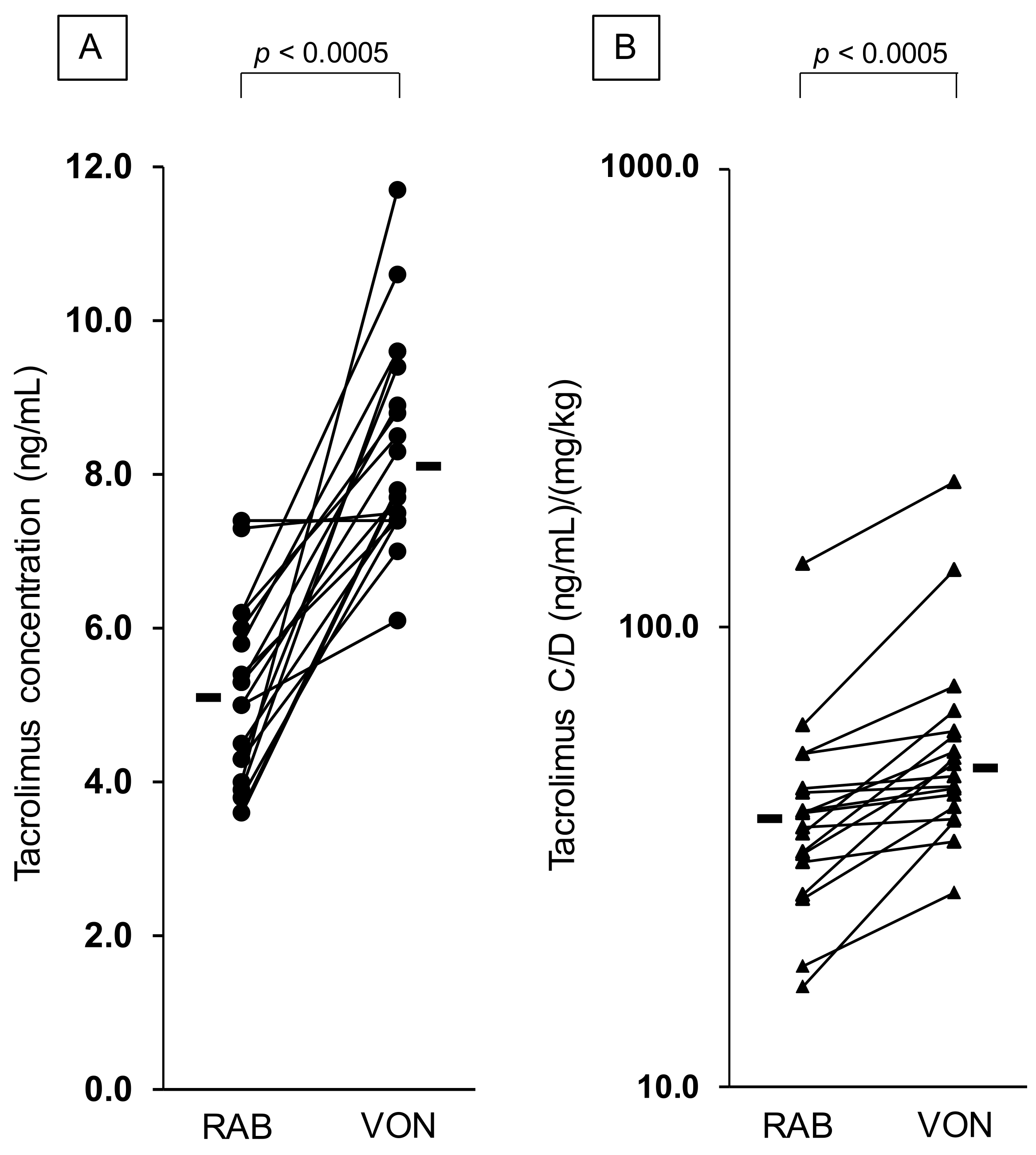
3.2. Effect of Vonoprazan Coadministration on the C/D Ratio of Tacrolimus
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Halloran, P.F. Immunosuppressive drugs for kidney transplantation. N. Engl. J. Med. 2004, 351, 2715–2729. [Google Scholar] [CrossRef] [Green Version]
- Wallemacq, P.; Armstrong, V.W.; Brunet, M.; Haufroid, V.; Holt, D.W.; Johnston, A.; Kuypers, D.; Le Meur, Y.; Marquet, P.; Oellerich, M.; et al. Opportunities to optimize tacrolimus therapy in solid organ transplantation: Report of the European consensus conference. Ther. Drug Monit. 2009, 31, 139–152. [Google Scholar] [CrossRef] [Green Version]
- Yasuhara, M.; Hashida, T.; Toraguchi, M.; Hashimoto, Y.; Kimura, M.; Inui, K.; Hori, R.; Inomata, Y.; Tanaka, K.; Yamaoka, Y. Pharmacokinetics and pharmacodynamics of FK 506 in pediatric patients receiving living-related donor liver transplantations. Transpl. Proc. 1995, 27, 1108–1110. [Google Scholar]
- Kershner, R.P.; Fitzsimmons, W.E. Relationship of FK506 whole blood concentrations and efficacy and toxicity after liver and kidney transplantation. Transplantation 1996, 62, 920–926. [Google Scholar] [CrossRef]
- Langer, R.M.; Hené, R.; Vitko, S.; Christiaans, M.; Tedesco-Silva, H., Jr.; Ciechanowski, K.; Cassuto, E.; Rostaing, L.; Vilatoba, M.; Machein, U.; et al. Everolimus plus early tacrolimus minimization: A phase III, randomized, open-label, multicentre trial in renal transplantation. Transpl. Int. 2012, 25, 592–602. [Google Scholar] [CrossRef]
- Park, S.; Kim, Y.S.; Lee, J.; Huh, W.; Yang, C.W.; Kim, Y.L.; Kim, Y.H.; Kim, J.K.; Oh, C.K.; Park, S.K. Reduced Tacrolimus Trough Level Is Reflected by Estimated Glomerular Filtration Rate (eGFR) Changes in Stable Renal Transplantation Recipients: Results of the OPTIMUM Phase 3 Randomized Controlled Study. Ann. Transpl. 2018, 23, 401–411. [Google Scholar] [CrossRef]
- Tönshoff, B.; Ettenger, R.; Dello Strologo, L.; Marks, S.D.; Pape, L.; Tedesco-Silva, H., Jr.; Bjerre, A.; Christian, M.; Meier, M.; Martzloff, E.D.; et al. Early conversion of pediatric kidney transplant patients to everolimus with reduced tacrolimus and steroid elimination: Results of a randomized trial. Am. J. Transpl. 2019, 19, 811–822. [Google Scholar] [CrossRef]
- Trofe-Clark, J.; Brennan, D.C.; West-Thielke, P.; Milone, M.C.; Lim, M.A.; Neubauer, R.; Nigro, V.; Bloom, R.D. Results of ASERTAA, a Randomized Prospective Crossover Pharmacogenetic Study of Immediate-Release Versus Extended-Release Tacrolimus in African American Kidney Transplant Recipients. Am. J. Kidney Dis. 2018, 71, 315–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiraga, T.; Niwa, T.; Teramura, Y.; Kagayama, A.; Tsutsui, M.; Ohno, Y.; Iwasaki, K. Oxidative Metabolism of Tacrolimus and its Metabolite by Human Cytochrome P450 3A Subfamily. Xenobio. Metabol. Dispos. 1999, 14, 277–285. [Google Scholar] [CrossRef] [Green Version]
- Niwa, T.; Yamamoto, S.; Saito, M.; Shiraga, T.; Takagi, A. Effect of cyclosporine and tacrolimus on cytochrome p450 activities in human liver microsomes. Yakugaku Zasshi 2007, 127, 209–216. [Google Scholar] [CrossRef] [Green Version]
- Logan, A.J.; Morris-Stiff, G.J.; Bowrey, D.J.; Jurewicz, W.A. Upper gastrointestinal complications after renal transplantation: A 3-yr sequential study. Clin. Transpl. 2002, 16, 163–167. [Google Scholar] [CrossRef]
- Homma, M.; Itagaki, F.; Yuzawa, K.; Fukao, K.; Kohda, Y. Effects of lansoprazole and rabeprazole on tacrolimus blood concentration: Case of a renal transplant recipient with CYP2C19 gene mutation. Transplantation 2002, 73, 303–304. [Google Scholar] [CrossRef]
- Yasuda, S.; Horai, Y.; Tomono, Y.; Nakai, H.; Yamato, C.; Manabe, K.; Kobayashi, K.; Chiba, K.; Ishizaki, T. Comparison of the kinetic disposition and metabolism of E3810, a new proton pump inhibitor, and omeprazole in relation to S-mephenytoin 4′-hydroxylation status. Clin. Pharmacol. Ther. 1995, 58, 143–154. [Google Scholar] [CrossRef]
- Ishizaki, T.; Horai, Y. Review article: Cytochrome P450 and the metabolism of proton pump inhibitors—Emphasis on rabeprazole. Aliment. Pharmacol. Ther. 1999, 13, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Miura, M.; Satoh, S.; Tada, H.; Habuchi, T.; Suzuki, T. Stereoselective metabolism of rabeprazole-thioether to rabeprazole by human liver microsomes. Eur. J. Clin. Pharmacol 2006, 62, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Miura, M.; Kagaya, H.; Tada, H.; Sagae, Y.; Satoh, S.; Habuchi, T.; Suzuki, T. Comparison of enantioselective disposition of rabeprazole versus lansoprazole in renal-transplant recipients who are CYP2C19 extensive metabolizers. Xenobiotica 2005, 35, 479–486. [Google Scholar] [CrossRef]
- Itagaki, F.; Homma, M.; Yuzawa, K.; Nishimura, M.; Naito, S.; Ueda, N.; Ohkohchi, N.; Kohda, Y. Effect of lansoprazole and rabeprazole on tacrolimus pharmacokinetics in healthy volunteers with CYP2C19 mutations. J. Pharm. Pharmacol. 2004, 56, 1055–1059. [Google Scholar] [CrossRef]
- Hori, Y.; Imanishi, A.; Matsukawa, J.; Tsukimi, Y.; Nishida, H.; Arikawa, Y.; Hirase, K.; Kajino, M.; Inatomi, N. 1-[5-(2-Fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine monofumarate (TAK-438), a novel and potent potassium-competitive acid blocker for the treatment of acid-related diseases. J. Pharmacol. Exp. Ther. 2010, 335, 231–238. [Google Scholar] [CrossRef] [Green Version]
- Sakurai, Y.; Nishimura, A.; Kennedy, G.; Hibberd, M.; Jenkins, R.; Okamoto, H.; Yoneyama, T.; Jenkins, H.; Ashida, K.; Irie, S.; et al. Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of Single Rising TAK-438 (Vonoprazan) Doses in Healthy Male Japanese/non-Japanese Subjects. Clin. Transl. Gastroenterol. 2015, 6, e94. [Google Scholar] [CrossRef] [PubMed]
- Ashida, K.; Sakurai, Y.; Hori, T.; Kudou, K.; Nishimura, A.; Hiramatsu, N.; Umegaki, E.; Iwakiri, K. Randomised clinical trial: Vonoprazan, a novel potassium-competitive acid blocker, vs. lansoprazole for the healing of erosive oesophagitis. Aliment. Pharmacol. Ther. 2016, 43, 240–251. [Google Scholar] [CrossRef]
- Murakami, K.; Sakurai, Y.; Shiino, M.; Funao, N.; Nishimura, A.; Asaka, M. Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: A phase III, randomised, double-blind study. Gut 2016, 65, 1439–1446. [Google Scholar] [CrossRef] [Green Version]
- Miwa, H.; Uedo, N.; Watari, J.; Mori, Y.; Sakurai, Y.; Takanami, Y.; Nishimura, A.; Tatsumi, T.; Sakaki, N. Randomised clinical trial: Efficacy and safety of vonoprazan vs. lansoprazole in patients with gastric or duodenal ulcers—Results from two phase 3, non-inferiority randomised controlled trials. Aliment. Pharmacol. Ther. 2017, 45, 240–252. [Google Scholar] [CrossRef]
- Yamasaki, H.; Kawaguchi, N.; Nonaka, M.; Takahashi, J.; Morohashi, A.; Hirabayashi, H.; Moriwaki, T.; Asahi, S. In vitro metabolism of TAK-438, vonoprazan fumarate, a novel potassium-competitive acid blocker. Xenobiotica 2017, 47, 1027–1034. [Google Scholar] [CrossRef]
- Chen, K.J.; Chen, C.H.; Cheng, C.H.; Wu, M.J.; Shu, K.H. Risk factors for peptic ulcer disease in renal transplant patient–11 years of experience from a single center. Clin. Nephrol. 2004, 62, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yano, I.; Fukuhara, Y.; Katsura, T.; Takahashi, T.; Ito, N.; Yamamoto, S.; Ogawa, O.; Inui, K. Distinct effects of omeprazole and rabeprazole on the tacrolimus blood concentration in a kidney transplant recipient. Drug Metab. Pharm. 2007, 22, 441–444. [Google Scholar] [CrossRef]
- Hosohata, K.; Masuda, S.; Ogura, Y.; Oike, F.; Takada, Y.; Katsura, T.; Uemoto, S.; Inui, K. Interaction between tacrolimus and lansoprazole, but not rabeprazole in living-donor liver transplant patients with defects of CYP2C19 and CYP3A5. Drug Metab. Pharm. 2008, 23, 134–138. [Google Scholar] [CrossRef]
- Mei, T.; Noguchi, H.; Suetsugu, K.; Hisadome, Y.; Kaku, K.; Okabe, Y.; Masuda, S.; Nakamura, M. Effects of Concomitant Administration of Vonoprazan Fumarate on the Tacrolimus Blood Concentration in Kidney Transplant Recipients. Biol. Pharm. Bull. 2020, 43, 1600–1603. [Google Scholar] [CrossRef]
- Jenkins, H.; Jenkins, R.; Patat, A. Effect of Multiple Oral Doses of the Potent CYP3A4 Inhibitor Clarithromycin on the Pharmacokinetics of a Single Oral Dose of Vonoprazan: A Phase I, Open-Label, Sequential Design Study. Clin. Drug Investig. 2017, 37, 311–316. [Google Scholar] [CrossRef]
- Birdwell, K.A.; Decker, B.; Barbarino, J.M.; Peterson, J.F.; Stein, C.M.; Sadee, W.; Wang, D.; Vinks, A.A.; He, Y.; Swen, J.J.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines for CYP3A5 Genotype and Tacrolimus Dosing. Clin. Pharmacol. Ther. 2015, 98, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Watari, S.; Araki, M.; Matsumoto, J.; Yoshinaga, K.; Sekito, T.; Maruyama, Y.; Mitsui, Y.; Sadahira, T.; Kubota, R.; Nishimura, S.; et al. Blood concentrations of tacrolimus upon conversion from rabeprazole to vonoprazan in renal transplant recipients: Correlation with cytochrome P450 gene polymorphisms. Drug Metab. Pharm. 2021, 40, 100407. [Google Scholar] [CrossRef]
- Saeki, T.; Ueda, K.; Tanigawara, Y.; Hori, R.; Komano, T. Human P-glycoprotein transports cyclosporin A and FK506. J. Biol. Chem. 1993, 268, 6077–6080. [Google Scholar] [CrossRef]
- Itagaki, F.; Homma, M.; Takara, K.; Ohnishi, N.; Yokoyama, T.; Sakaeda, T.; Yagami, T.; Kobayashi, H.; Okamura, N.; Kohda, Y. Effect of rabeprazole on MDR1-mediated transport of Rhodamine 123 in Caco-2 and Hvr100-6 cells. Biol. Pharm. Bull. 2004, 27, 1694–1696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Pharmaceuticals and Medical Devices Agency of Japan. Drug Approval Review for Vonoprazan Fumarate (in English). 2014. Available online: http://www.pmda.go.jp/:000211075.pdf (accessed on 1 August 2020).
- Food and Drug Administration. In Vitro Drug Interaction Studies-Cytochrome P450 Enzyme- and Transporter-Mediated Drug Interactions Guidance for Industry. Available online: https://www.fda.gov/media/134582/download2020 (accessed on 1 August 2020).
- European Medicines Agency. Guideline on the Investigation of Drug Interactions-Revision 1. 2012. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-investigation-drug-interactions-revision-1_en.pdf (accessed on 1 August 2020).
- Pharmaceuticals and Medical Devices Agency. Guideline on Drug Interaction for Drug Development and Appropriate Provision of Information. 2019. Available online: https://www.pmda.go.jp/files/000228122.pdf (accessed on 1 August 2020).
- Hebert, M.F.; Lam, A.Y. Diltiazem increases tacrolimus concentrations. Ann. Pharm. 1999, 33, 680–682. [Google Scholar] [CrossRef] [PubMed]
- Wlodarczyk, Z.; Squifflet, J.P.; Ostrowski, M.; Rigotti, P.; Stefoni, S.; Citterio, F.; Vanrenterghem, Y.; Krämer, B.K.; Abramowicz, D.; Oppenheimer, F.; et al. Pharmacokinetics for once- versus twice-daily tacrolimus formulations in de novo kidney transplantation: A randomized, open-label trial. Am. J. Transpl. 2009, 9, 2505–2513. [Google Scholar] [CrossRef]
| Proton-Pump Inhibitor | ||
|---|---|---|
| Rabeprazole | Vonoprazan | |
| Number of patients (male/female) | 18 (12/6) | |
| Age (years) | 45 (23–65) | |
| Body weight (kg) | 61.9 (45.5–88.8) | |
| Tacrolimus | ||
| Dose (mg/kg/day) | 0.14 (0.05–0.24) | 0.17 (0.04–0.31) |
| Concentration (ng/mL) | 5.2 (3.6–7.4) | 8.1 (6.1–11.7) |
| C/D ratio (ng/mL)/(mg/kg) | 38.1 (16.5–138.1) | 48.9 (26.6–207.2) |
| Laboratory data | ||
| Aspartate aminotransferase (IU/mL) | 16 (9–44) | 15 (6–31) |
| Alanine aminotransferase (IU/mL) | 28 (5–89) | 22 (5–104) |
| Blood urine nitrogen (mg/dL) | 24.2 (9.8–78.7) | 17.4 (7.5–45.4) |
| Serum creatinine (mg/dL) | 1.55 (0.62–4.31) | 1.21 (0.58–2.84) |
| Proton-pump inhibitor | ||
| Dose (mg/kg/day) | 0.16 (0.11–0.22) | 0.31 (0.11–0.44) |
| Administration period (days) | 9 (1–37) | 7 (5–13) |
| Tacrolimus Concentration (ng/mL) | Tacrolims C/D Ratio [(ng/mL)/(mg/kg/day)] | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genotype | n | Rabeprazole | Vonoprazan | ΔConcentration | Rabeprazole | Vonoprazan | ΔC/D Ratio | ||||||
| CYP2C19 | |||||||||||||
| EM | 5 | 4.0 | (3.6–6.2) | 8.5 | (7.4–11.7) | 3.6 | (2.3–7.7) | 25.6 | (16.5–53.5) | 40.7 | (26.6–74.4) | 15.1 | (8.2–21.5) |
| IM/PM | 13 | 5.3 | (3.9–7.4) | 7.7 | (6.1–10.6) | 3.0 | (0.0–5.7) | 39.5 | (26.2–138.1) | 50.4 | (34.3–207.2) | 6.3 | (1.2–73.3) |
| CYP3A5 | |||||||||||||
| *1 carrier | 5 | 5.3 | (3.6–7.4) | 8.5 | (7.0–11.7) | 2.8 | (0.0–7.7) | 36.9 | (16.5–138.1) | 45.1 | (26.6–207.2) | 14.6 | (1.2–69.1) |
| *1 noncarrier | 13 | 5.0 | (3.8–6.2) | 7.5 | (6.1–10.6) | 3.3 | (1.1–4.4) | 44.9 | (30.8–61.2) | 50.4 | (34.3–134.5) | 18.3 | (2.5–73.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, Y.; Yoshihashi, T.; Takahashi, K.; Furuya, K.; Ohkohchi, N.; Oda, T.; Homma, M. Drug–Drug Interaction between Tacrolimus and Vonoprazan in Kidney Transplant Recipients. J. Clin. Med. 2021, 10, 3964. https://doi.org/10.3390/jcm10173964
Suzuki Y, Yoshihashi T, Takahashi K, Furuya K, Ohkohchi N, Oda T, Homma M. Drug–Drug Interaction between Tacrolimus and Vonoprazan in Kidney Transplant Recipients. Journal of Clinical Medicine. 2021; 10(17):3964. https://doi.org/10.3390/jcm10173964
Chicago/Turabian StyleSuzuki, Yoshiharu, Takuya Yoshihashi, Kazuhiro Takahashi, Kinji Furuya, Nobuhiro Ohkohchi, Tatsuya Oda, and Masato Homma. 2021. "Drug–Drug Interaction between Tacrolimus and Vonoprazan in Kidney Transplant Recipients" Journal of Clinical Medicine 10, no. 17: 3964. https://doi.org/10.3390/jcm10173964
APA StyleSuzuki, Y., Yoshihashi, T., Takahashi, K., Furuya, K., Ohkohchi, N., Oda, T., & Homma, M. (2021). Drug–Drug Interaction between Tacrolimus and Vonoprazan in Kidney Transplant Recipients. Journal of Clinical Medicine, 10(17), 3964. https://doi.org/10.3390/jcm10173964






