Pain Control Affects the Radiographic Diagnosis of Segmental Instability in Patients with Degenerative Lumbar Spondylolisthesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Recruitment
2.2. Analgesia Drug Injection and Lumbar Flexion and Extension Radiography
2.3. Clinical Evaluation
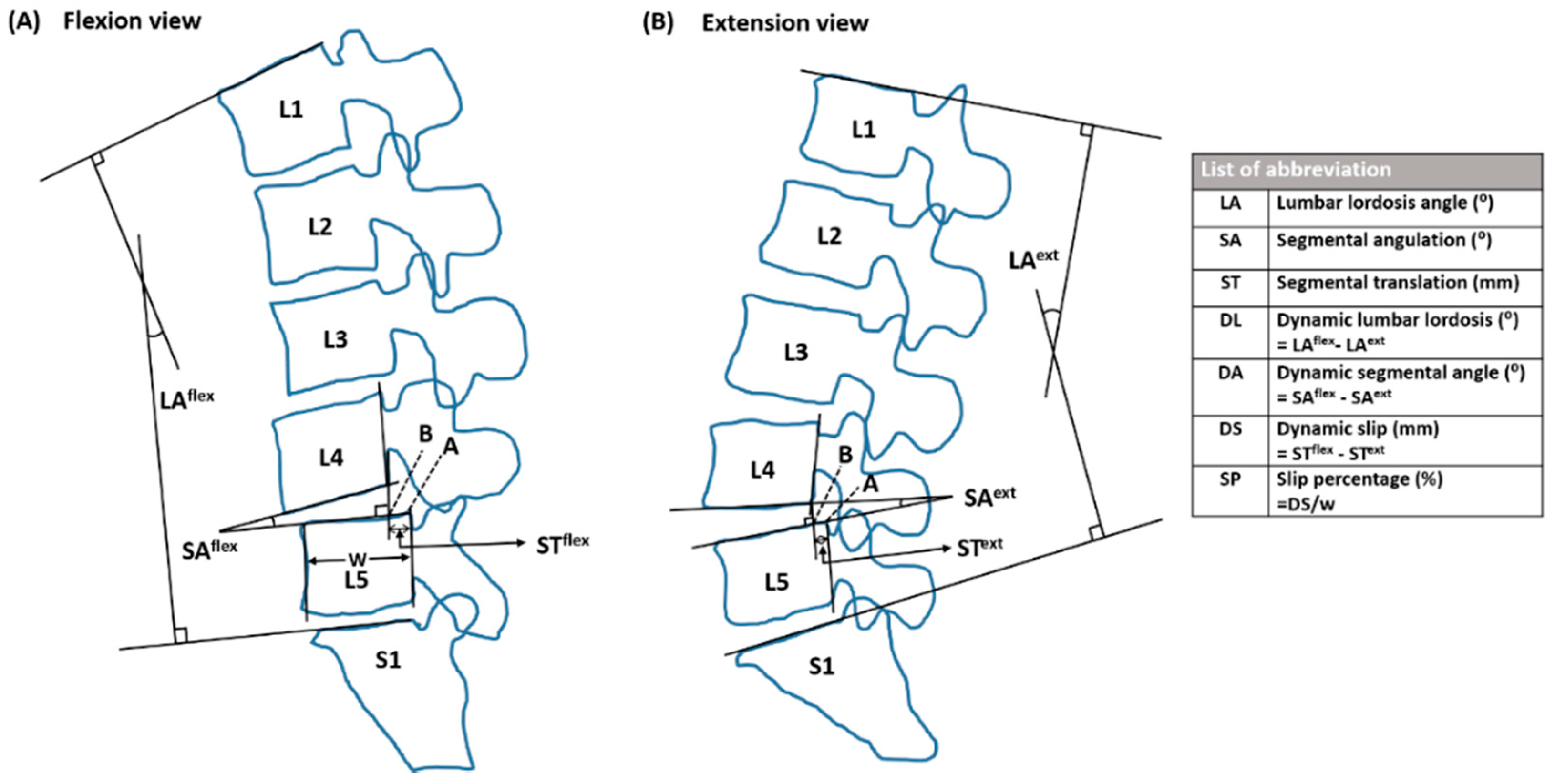
2.4. Radiographic Evaluation
2.5. Statistical Analysis
3. Results
3.1. Baseline Cohort Analyses
3.2. The Changes in Pain Intensity and Radiographic Parameters after Analgesia
3.3. The Detection of Instability Segments after Analgesia Relative to Prior Analgesia
3.4. The Difference in Motion Change in Moderate and Severe Pain Subgroups
3.5. Association between Pain Score and Radiographic Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, A.; March, L.; Zheng, X.; Zheng, X.; Huang, J.; Wang, X.; Zhao, J.; Blyth, F.M.; Smith, E.; Buchbinder, R.; et al. Global low back pain prevalence and years lived with disability from 1990 to 2017: Estimates from the Global Burden of Disease Study. Ann. Transl. Med. 2020, 8, 299. [Google Scholar]
- Chou, R. Low back pain (chronic). BMJ Clin. Evid. 2010, 2010, 1116. [Google Scholar]
- Stevans, J.M.; Delitto, A.; Khoja, S.S.; Patterson, C.G.; Smith, C.N.; Schneider, M.J.; Freburger, J.K.; Greco, C.M.; Freel, J.A.; Sowa, G.A.; et al. Risk Factors Associated with Transition from Acute to Chronic Low Back Pain in US Patients Seeking Primary Care. JAMA Netw. Open 2021, 4, e2037371. [Google Scholar] [CrossRef] [PubMed]
- Ishimoto, Y.; Yoshimura, N.; Muraki, S.; Yamada, H.; Nagata, K.; Hashizume, H.; Takiguchi, N.; Minmide, A.; Oka, H.; Tanaka, S.; et al. Association of Lumbar Spondylolisthesis with Low Back Pain and Symptomatic Lumbar Spinal Stenosis in a Population-based Cohort: The Wakayama Spine Study. Spine 2017, 42, E666–E671. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Kitahara, K.; Shimoda, H.; Ishii, K.; Ono, M.; Homma, T.; Watanabe, K. Lumbar degenerative spondylolisthesis is not always unstable: Clinicobiomechanical evidence. Spine 2014, 39, 2127–2135. [Google Scholar] [PubMed] [Green Version]
- Alfieri, A.; Gazzeri, R.; Prell, J.; Rollinghoff, M. The current management of lumbar spondylolisthesis. J. Neurosurg. Sci. 2013, 57, 103–113. [Google Scholar]
- Simmonds, A.M.; Rampersaud, Y.R.; Dvorak, M.F.; Dea, N.; Melnyk, A.D.; Fisher, C.G. Defining the inherent stability of degenerative spondylolisthesis: A systematic review. J. Neurosurg. Spine 2015, 23, 178–189. [Google Scholar]
- White, I.I.I.; Augustus, A.; Monohar, M. Clinical Biomechanics of the Spine, 2nd ed.; Lippincott: Philadelphia, PA, USA, 1990; pp. 23–45. [Google Scholar]
- Pieper, C.C.; Groetz, S.F.; Nadal, J.; Schild, H.H.; Niggemann, P.D. Radiographic evaluation of ventral instability in lumbar spondylolisthesis: Do we need extension radiographs in routine exams? Eur. Spine J. 2014, 23, 96–101. [Google Scholar]
- Lattig, F.; Fekete, T.F.; Kleinstuck, F.S.; Porchet, F.; Jeszenszky, D.; Mannion, A.F. Lumbar facet joint effusion on MRI as a sign of unstable degenerative spondylolisthesis: Should it influence the treatment decision? J. Spinal Disord. Tech. 2015, 28, 95–100. [Google Scholar]
- Hasegawa, K.; Kitahara, K.; Shimoda, H.; Hara, T. Facet joint opening in lumbar degenerative diseases indicating segmental instability. J. Neurosurg. Spine 2010, 12, 687–693. [Google Scholar] [PubMed] [Green Version]
- Petersen, T.; Laslett, M.; Juhl, C. Clinical classification in low back pain: Best-evidence diagnostic rules based on systematic reviews. BMC Musculoskelet. Disord. 2017, 18, 188. [Google Scholar]
- Camara, J.R.; Keen, J.R.; Asgarzadie, F. Functional radiography in examination of spondylolisthesis. AJR Am. J. Roentgenol. 2015, 204, W461–W469. [Google Scholar] [CrossRef]
- Matz, P.G.; Meagher, R.J.; Lamer, T.; Tontz, W.L., Jr.; Annaswamy, T.M.; Cassidy, R.C.; Cho, C.H.; Dougherty, P.; Easa, J.E.; Enix, D.E.; et al. Guideline summary review: An evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spondylolisthesis. Spine J. 2016, 16, 439–448. [Google Scholar]
- Hu, S.S.; Tribus, C.B.; Diab, M.; Ghanayem, A.J. Spondylolisthesis and spondylolysis. J. Bone Jt. Surg. 2008, 57, 431–445. [Google Scholar]
- Jarzem, P.F.; Harvey, E.J.; Arcaro, N.; Kaczorowski, J. Transcutaneous Electrical Nerve Stimulation [TENS] for Short-Term Treatment of Low Back Pain–Randomized Double Blind Crossover Study of Sham versus Conventional TENS. J. Musculoskelet. Pain 2005, 13, 11–17. [Google Scholar]
- Lilius, G.; Laasonen, E.M.; Myllynen, P.; Harilainen, A.; Gronlund, G. Lumbar facet joint syndrome. A randomised clinical trial. J. Bone Jt. Surg. Br. 1989, 71, 681–684. [Google Scholar]
- Williams, J.M.; Haq, I.; Lee, R.Y. The effect of pain relief on dynamic changes in lumbar curvature. Man. Ther. 2013, 18, 149–154. [Google Scholar] [PubMed]
- Koslosky, E.; Gendelberg, D. Classification in brief: The Meyerding classification system of spondylolisthesis. Clin. Orthop. Relat. Res. 2020, 478, 1125–1130. [Google Scholar]
- Lassen, K.; Epstein-Stiles, M.; Olsson, G.L. Ketorolac: A new parenteral nonsteroidal anti-inflammatory drug for postoperative pain management. J. Post Anesth. Nurs. 1992, 7, 238–242. [Google Scholar] [PubMed]
- Lang, Z.; Li, J.S.; Yang, F.; Yu, Y.; Khan, K.; Jenis, L.G.; Cha, T.D.; Kang, J.D.; Li, G. Reoperation of decompression alone or decompression plus fusion surgeries for degenerative lumbar diseases: A systematic review. Eur. Spine J. 2019, 28, 1371–1385. [Google Scholar]
- Rienmuller, A.C.; Krieg, S.M.; Schmidt, F.A.; Meyer, E.L.; Meyer, B. Reoperation rates and risk factors for revision 4 years after dynamic stabilization of the lumbar spine. Spine J. 2019, 19, 113–120. [Google Scholar] [PubMed]
- Irmola, T.M.; Hakkinen, A.; Jarvenpaa, S.; Marttinen, I.; Vihtonen, K.; Neva, M. Reoperation Rates Following Instrumented Lumbar Spine Fusion. Spine 2018, 43, 295–301. [Google Scholar] [PubMed]
- Ghogawala, Z.; Dziura, J.; Butler, W.E.; Dai, F.; Terrin, N.; Magge, S.N.; Coumans, K.V.C.E.; Harrington, J.F.; Amin-Hanjani, S.; Schwartz, J.S.; et al. Laminectomy plus Fusion versus Laminectomy Alone for Lumbar Spondylolisthesis. N. Engl. J. Med. 2016, 374, 1424–1434. [Google Scholar]
- Blumenthal, C.; Curran, J.; Benzel, E.C.; Potter, R.; Magge, S.N.; Harrington, J.F.; Coumans, J.V.; Ghogawala, Z. Radiographic predictors of delayed instability following decompression without fusion for degenerative grade I lumbar spondylolisthesis. J. Neurosurg. Spine 2013, 18, 340–346. [Google Scholar]
- Sadler, S.G.; Spink, M.J.; Ho, A.; De Jonge, X.J.; Chuter, V.H. Restriction in lateral bending range of motion, lumbar lordosis, and hamstring flexibility predicts the development of low back pain: A systematic review of prospective cohort studies. BMC Musculoskelet. Disord. 2017, 18, 179. [Google Scholar]
- Hu, Y.; Siu, S.H.; Mak, J.N.; Luk, K.D. Lumbar muscle electromyographic dynamic topography during flexion-extension. J. Electromyogr. Kinesiol. 2010, 20, 246–255. [Google Scholar] [PubMed]
- Sanchez-Zuriaga, D.; Lopez-Pascual, J.; Garrido-Jaen, D.; Garcia-Mas, M.A. A comparison of lumbopelvic motion patterns and erector spinae behavior between asymptomatic subjects and patients with recurrent low back pain during pain-free periods. J. Manip. Physiol. Ther. 2015, 38, 130–137. [Google Scholar]
- Li, Y.; Zhang, X.; Dai, J.; Wang, J.; Wu, H.; Liu, J.; Chen, J.; Zhu, Y.; Zhao, F. Changes in the Flexion-Relaxation Response after Percutaneous Endoscopic Lumbar Discectomy in Patients with Disc Herniation. World Neurosurg. 2019, 125, e1042–e1049. [Google Scholar] [CrossRef] [PubMed]
- Mellin, G. Chronic low back pain in men 54–63 years of age. Correlations of physical measurements with the degree of trouble and progress after treatment. Spine 1986, 11, 421–426. [Google Scholar] [PubMed]
- Filippiadis, D.K.; Kelekis, A. A review of percutaneous techniques for low back pain and neuralgia: Current trends in epidural infiltrations, intervertebral disk and facet joint therapies. Br. J. Radiol. 2016, 89, 20150357. [Google Scholar]



| N = 100 | Mean | SD | Range |
|---|---|---|---|
| Age (years) | 53.9 (median: 57) | 11.9 | 28–69 |
| Sex (F/M) | 73/27 | ||
| Height (cm) | 162.8 | 8.4 | 155–181 |
| Body weight (kg) | 67.6 | 7.9 | 52–87 |
| BMI (kg/m2) | 27.5 | 5.2 | 18.7–33.6 |
| VAS score (mm) | |||
| Baseline | 66.9 | 0.97 | 40–90 |
| Final | 32.2 | 13.1 | 10–70 |
| Reduction in VAS | 34.7 | 15.5 | 10–70 |
| Measured Parameters | Pre-Analgesia | Post-Analgesia | Difference ◇ | Effect Size | p-Value |
|---|---|---|---|---|---|
| Segmental translation (mm) | |||||
| Flexion | −2.73 ± 3.49 | −4.24 ± 3.94 | −1.51 ± 1.34 | −1.13 | <0.0001 |
| Extension | −0.79 ± 3.75 | 0.10 ± 4.06 | 0.89 ± 1.33 | 0.67 | <0.0001 |
| Dynamic slip (DS) | 1.94 ± 1.34 | 4.36 ± 1.56 | 2.4 ± 1.36 | 1.76 | <0.0001 |
| Segmental angulation (°) | |||||
| Flexion | −1.36 ± 4.86 | −3.64 ± 5.08 | −2.27 ± 3.7 | −0.61 | <0.0001 |
| Extension | 9.52 ± 4.12 | 12.10 ± 4.46 | 2.57 ± 2.89 | 0.89 | <0.0001 |
| Dynamic segmental angle (DA) | 10.89 ± 4.30 | 15.74 ± 4.72 | 4.85 ± 4.59 | 1.06 | <0.0001 |
| Lumbar lordosis angle (°) | |||||
| Flexion | 9.06 ± 14.21 | 7.57 ± 12.55 | −1.49 ± 8.07 | −0.18 | 0.007 |
| Extension | 47.70 ± 11.81 | 50.18 ± 11.23 | 2.48 ± 0.55 | 4.51 | <0.0001 |
| Dynamic lumbar lordosis (DL) | 39.03 ± 13.61 | 42.61 ± 12.94 | 3.57 ± 8.07 | 0.44 | <0.0001 |
| Slip percentage (SP) (%) | 4.95 ± 3.41 | 11.11 ± 4.00 | 6.23 ± 3.46 | 1.80 | <0.0001 |
| T0 | Change T1–T0 | p Value | SRM | AMD (95% CI) T1 | p Value * | |
|---|---|---|---|---|---|---|
| Segmental Translation (mm) | ||||||
| Flexion | ||||||
| M group | −2.61 ± 3.45 | 1.41 ± 1.37 | 0.41 | 1.03 | −0.18 (−0.61, 0.26) | 0.42 |
| S group | −2.82 ± 3.53 | 1.60 ± 1.31 | 1.21 | |||
| Extension | ||||||
| M group | −0.79 ± 3.73 | 0.90 ± 1.46 | 0.92 | 0.62 | 0.02 (−0.42, 0.45) | 0.94 |
| S group | −0.78± 3.79 | 0.88 ± 1.23 | 0.72 | |||
| Dynamic slip | ||||||
| M group | 1.82 ± 1.30 | 2.32 ± 1.42 | 0.47 | 1.63 | −0.16 (−0.61, 0.28) | 0.47 |
| S group | 2.03 ± 1.36 | 2.48 ± 1.33 | 1.87 | |||
| Slip percentage (%) | ||||||
| M group | 4.62 ± 3.34 | 6.03 ± 3.55 | 0.52 | 1.70 | −0.37 (−1.50, 0.77) | 0.52 |
| S group | 5.21 ± 3.46 | 6.40 ± 3.42 | 1.87 | |||
| Segmental angulation (°) | ||||||
| Flexion | ||||||
| M group | −1.01 ± 4.34 | 1.68 ± 3.15 | 0.07 | 0.53 | −1.10 (−2.30, 0.11) | 0.07 |
| S group | −1.65 ± 5.24 | 2.76 ± 4.09 | 0.67 | |||
| Extension | ||||||
| M group | 10.27 ± 4.07 @ | 2.30 ± 2.99 | 0.30 | 0.77 | −0.51 (−1.43, 0.43) | 0.29 |
| S group | 8.92 ± 4.08 @ | 2.79 ± 2.80 | 0.99 | |||
| Dynamic segmental angle (°) | ||||||
| M group | 11.28 ± 3.93 | 3.98 ± 4.15 | 0.03 | 0.96 | −1.60 (−3.06, −0.14) | 0.03 |
| S group | 10.56 ± 4.55 | 5.66 ± 4.82 | 1.18 | |||
| Lumbar lordosis angle (°) | ||||||
| Flexion | ||||||
| M group | 9.47 ± 11.93 | −0.03 ± 7.07 | 0.08 | −0.004 | −2.81 (−6.00, 0.38) | 0.08 |
| S group | 8.70 ± 15.99 | 2.79 ± 8.69 | 0.32 | |||
| Extension | ||||||
| M group | 49.00 ± 11.27 | 2.23 ± 5.55 | 0.68 | 0.40 | −0.47 (−2.69, 1.74) | 0.67 |
| S group | 46.58 ± 12.24 | 2.69 ± 5.53 | 0.49 | |||
| Dynamic lumbar lordosis (°) | ||||||
| M group | 39.52 ± 12.04 | 2.20 ± 6.89 | 0.12 | 0.32 | −2.54 (−5.75, 0.66) | 0.12 |
| S group | 38.62 ± 14.90 | 4.74 ± 8.85 | 0.54 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, S.-H.; Lin, S.-Y.; Shen, P.-C.; Tu, H.-P.; Huang, H.-T.; Shih, C.-L.; Lu, C.-C. Pain Control Affects the Radiographic Diagnosis of Segmental Instability in Patients with Degenerative Lumbar Spondylolisthesis. J. Clin. Med. 2021, 10, 3984. https://doi.org/10.3390/jcm10173984
Chou S-H, Lin S-Y, Shen P-C, Tu H-P, Huang H-T, Shih C-L, Lu C-C. Pain Control Affects the Radiographic Diagnosis of Segmental Instability in Patients with Degenerative Lumbar Spondylolisthesis. Journal of Clinical Medicine. 2021; 10(17):3984. https://doi.org/10.3390/jcm10173984
Chicago/Turabian StyleChou, Shih-Hsiang, Sung-Yen Lin, Po-Chih Shen, Hung-Pin Tu, Hsuan-Ti Huang, Chia-Lung Shih, and Cheng-Chang Lu. 2021. "Pain Control Affects the Radiographic Diagnosis of Segmental Instability in Patients with Degenerative Lumbar Spondylolisthesis" Journal of Clinical Medicine 10, no. 17: 3984. https://doi.org/10.3390/jcm10173984
APA StyleChou, S.-H., Lin, S.-Y., Shen, P.-C., Tu, H.-P., Huang, H.-T., Shih, C.-L., & Lu, C.-C. (2021). Pain Control Affects the Radiographic Diagnosis of Segmental Instability in Patients with Degenerative Lumbar Spondylolisthesis. Journal of Clinical Medicine, 10(17), 3984. https://doi.org/10.3390/jcm10173984






