Neutrophil-to-Lymphocyte, Platelet-to-Lymphocyte Ratios, and Systemic Immune-Inflammation Index as Potential Biomarkers of Chronic Inflammation in Patients with Newly Diagnosed Acromegaly: A Single-Centre Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Statement of Ethics
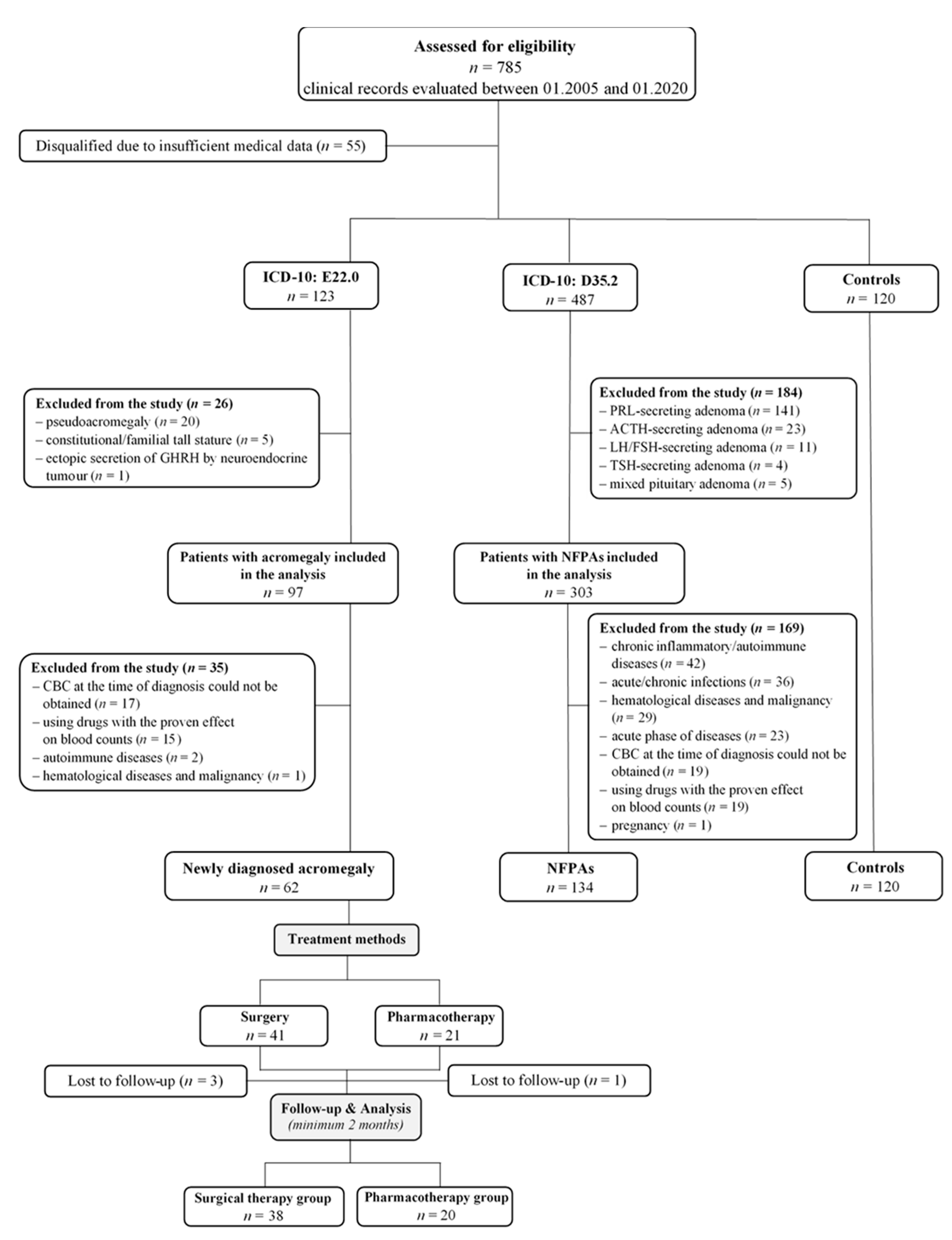
2.2. Study Design and Patients
2.3. Clinical, Radiological and Laboratory Assessments
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Study and Control Groups
3.2. Hematological Parameters Reflecting Inflammatory Process: Comparison with Traditional Markers of Acromegaly
3.3. Correlation between Selected Clinical, Radiological and Laboratory Data in Acromegaly and NFPAs
3.4. Changes in Systemic Inflammatory Parameters during Follow-up in Patients with Acromegaly
4. Discussion
5. Limitations of the Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| CBC | complete blood count |
| GH | growth hormone |
| IGF-1 | insulin-like growth factor 1 |
| LMR | lymphocyte-to-monocyte ratio |
| LYM | lymphocyte |
| MD | maximum diameter |
| MONO | monocyte |
| MPV | mean platelet value |
| MPV/PLT | mean platelet volume-to-platelet ratio |
| MRI | magnetic resonance imaging |
| NEU | neutrophil |
| NFPA | non-functioning pituitary adenoma |
| NLR | neutrophil-to-lymphocyte ratio |
| PLR | platelet-to-lymphocyte ratio |
| SII | systemic immune-inflammation index |
| SSA | somatostatin analogue |
| WBC | white blood cell |
References
- Aldallal, S. Acromegaly: A challenging condition to diagnose. Int. J. Gen. Med. 2018, 11, 337–343. [Google Scholar] [CrossRef] [Green Version]
- Dal, J.; Feldt-Rasmussen, U.; Andersen, M.; Kristensen, L.; Laurberg, P.; Pedersen, L.; Dekkers, O.M.; Sørensen, H.T.; Jørgensen, J.O.L. Acromegaly incidence, prevalence, complications and long-term prognosis: A nationwide cohort study. Eur. J. Endocrinol. 2016, 175, 181–190. [Google Scholar] [CrossRef] [Green Version]
- Bolanowski, M.; Ruchała, M.; Zgliczyński, W.; Kos-Kudła, B.; Hubalewska-Dydejczyk, A.; Lewiński, A. Diagnostics and treatment of acromegaly—updated recommendations of the Polish Society of Endocrinology. Endokrynol. Pol. 2019, 70, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Akirov, A.; Masri-Iraqi, H.; Dotan, I.; Shimon, I. The Biochemical Diagnosis of Acromegaly. J. Clin. Med. 2021, 10, 1147. [Google Scholar] [CrossRef]
- Wolters, T.L.C.; Netea, M.G.; Riksen, N.P.; Hermus, A.R.; Netea-Maier, R.T. Acromegaly, inflammation and cardiovascular disease: A review. Rev. Endocr. Metab. Disord. 2020, 21, 547–568. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Wolters, T.L.C.; van der Heijden, C.D.C.C.; van Leeuwen, N.; Hijmans-Kersten, B.T.P.; Netea, M.G.; Smit, J.W.A.; Thijssen, D.H.J.; Hermus, A.R.; Riksen, N.P.; Netea-Maier, R.T. Persistent inflammation and endothelial dysfunction in patients with treated acromegaly. Endocr. Connect. 2019, 8, 1553–1567. [Google Scholar] [CrossRef] [Green Version]
- Wolters, T.L.C.; Netea, M.G.; Hermus, A.R.; Smit, J.W.A.; Netea-Maier, R.T. IGF1 potentiates the pro-inflammatory response in human peripheral blood mononuclear cells via MAPK. J. Mol. Endocrinol. 2017, 59, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Rapaport, R.G.; Bozzola, M. Role of B-cells in growth hormone-immune interactions. Acta Paediatr. Suppl. 1997, 423, 82–83. [Google Scholar] [CrossRef] [PubMed]
- Colao, A.; Ferone, D.; Marzullo, P.; Lombardi, G. Acromegaly and immune function. In NeuroImmune Biology; Matera, L., Rapaport, R., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2002; Volume 2, pp. 247–257. [Google Scholar]
- Qiao, S.; Gao, W.; Guo, S. Neutrophil–Lymphocyte Ratio (NLR) for Predicting Clinical Outcomes in Patients with Coronary Artery Disease and Type 2 Diabetes Mellitus: A Propensity Score Matching Analysis. Ther. Clin. Risk. Manag. 2020, 16, 437–443. [Google Scholar] [CrossRef]
- Pergialiotis, V.; Trakakis, E.; Parthenis, C.; Hatziagelaki, E.; Chrelias, C.; Thomakos, N.; Papantoniou, N. Correlation of platelet to lymphocyte and neutrophil to lymphocyte ratio with hormonal and metabolic parameters in women with PCOS. Horm. Mol. Biol. Clin. Investig. 2018, 34, 20170073. [Google Scholar] [CrossRef]
- Szydełko, J.; Litwińczuk, M.; Szydełko, M.; Matyjaszek-Matuszek, B. Neutrophil-to-Lymphocyte, Monocyte-to-Lymphocyte and Platelet-to-Lymphocyte Ratios in Relation to Clinical Parameters and Smoking Status in Patients with Graves’ Orbitopathy-Novel Insight into Old Tests. J. Clin. Med. 2020, 9, 3111. [Google Scholar] [CrossRef] [PubMed]
- Bugada, D.; Allegri, M.; Lavand’Homme, P.; De Kock, M.; Fanelli, G. Inflammation-Based Scores: A New Method for Patient-Targeted Strategies and Improved Perioperative Outcome in Cancer Patients. BioMed Res. Int. 2014, 2014, 142425. [Google Scholar] [CrossRef] [PubMed]
- Tolle, F.; Umansky, V.; Utikal, J.; Kreis, S.; Br, S. Neutrophils in Tumorigenesis: Missing Targets for Successful Next Generation Cancer Therapies? Int. J. Mol. Sci. 2021, 22, 6744. [Google Scholar] [CrossRef]
- Shaul, M.E.; Fridlender, Z.G. Cancer-related circulating and tumor-associated neutrophils—Subtypes, sources and function. FEBS J. 2018, 285, 4316–4342. [Google Scholar] [CrossRef]
- Yang, R.; Chang, Q.; Meng, X.; Gao, N.; Wang, W. Prognostic value of Systemic immune-inflammation index in cancer: A meta-analysis. J. Cancer 2018, 9, 3295–3302. [Google Scholar] [CrossRef]
- Zhang, Z.; Xia, F.; Wang, W.; Huang, Y.; Li, X. The systemic immune-inflammation index-based model is an effective biomarker on predicting central lymph node metastasis in clinically nodal-negative papillary thyroid carcinoma. Gland Surg. 2021, 10, 1368–1373. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.O.; Keles, F.O. The relationship between neutrophil/lymphocyte ratio and clinical and radiological findings in patients with nonfunctional adrenal incidentaloma. Bratisl. Lek. List. 2021, 122, 493–496. [Google Scholar] [CrossRef]
- Gaitanidis, A.; Patel, D.; Nilubol, N.; Tirosh, A.; Sadowski, S.; Kebebew, E. Markers of Systemic Inflammatory Response are Prognostic Factors in Patients with Pancreatic Neuroendocrine Tumors (PNETs): A Prospective Analysis. Ann. Surg. Oncol. 2018, 25, 122–130. [Google Scholar] [CrossRef]
- Offi, C.; Romano, R.M.; Cangiano, A.; Candela, G.; Docimo, G. Clinical significance of neutrophil-to-lymphocyte ratio, lymphocyte-to-monocyte ratio, platelet-to-lymphocyte ratio and prognostic nutritional index in low-risk differentiated thyroid carcinoma. Acta Otorhinolaryngol. Ital. 2021, 41, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Yokota, M.; Katoh, H.; Nishimiya, H.; Kikuchi, M.; Kosaka, Y.; Sengoku, N.; Watanabe, M.; Yamashita, K. Lymphocyte-Monocyte Ratio Significantly Predicts Recurrence in Papillary Thyroid Cancer. J. Surg. Res. 2020, 246, 535–543. [Google Scholar] [CrossRef]
- Oba, T.; Maeno, K.; Amitani, M.; Shimizu, T.; Ohno, K.; Ono, M.; Ito, T.; Kanai, T.; Uehara, T.; Ito, K.I. Prognostic significance of neutrophil-to-lymphocyte ratio for long-term outcomes in patients with poorly differentiated thyroid cancer. Endocr. J. 2021. [Google Scholar] [CrossRef] [PubMed]
- Gaitanidis, A.; Wiseman, D.; El Lakis, M.; Nilubol, N.; Kebebew, E.; Patel, D. Preoperative systemic inflammatory markers are prognostic indicators in recurrent adrenocortical carcinoma. J. Surg. Oncol. 2019, 120, 1450–1455. [Google Scholar] [CrossRef] [PubMed]
- de Jong, M.C.; Mihai, R.; Khan, S. Neutrophil-to-Lymphocyte Ratio (NLR) and Platelet-to-Lymphocyte Ratio (PLR) as Possible Prognostic Markers for Patients Undergoing Resection of Adrenocortical Carcinoma. World J. Surg. 2021, 45, 754–764. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J.; Shen, C.; Zhu, S.; Gao, Y.; Zhang, J. Neutrophil-Lymphocyte Ratio as an Initial Screening Biomarker for Differential Diagnosis of Cushing’s Syndrome from Nonfunctional Adenoma in Patients with an Adrenal Mass. BioMed Res. Int. 2021, 2021, 6635594. [Google Scholar] [CrossRef] [PubMed]
- Pozza, A.; Pauletti, B.; Scarpa, M.; Ruffolo, C.; Bassi, N.; Massani, M. Prognostic role of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in patients with midgut neuroendocrine tumors undergoing resective surgery. Int. J. Color. Dis. 2019, 34, 1849–1856. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; He, M.; Liu, Z.; Song, Y.; Wang, Y.; Liang, R.; Chen, H.; Xu, J. Impact of neutrophil–lymphocyte ratio on long-term outcome in patients with craniopharyngioma. Medicine 2018, 97, e12375. [Google Scholar] [CrossRef]
- Chen, M.; Zheng, S.H.; Yang, M.; Chen, Z.H.; Li, S.T. The diagnostic value of preoperative inflammatory markers in craniopharyngioma: A multicenter cohort study. J. Neurooncol. 2018, 138, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.F.; Li, M.; Li, J.H.; Zuo, M.R.; Yang, Y.; Liu, Y.H. The significance of preoperative hematological inflammatory markers in patients with meningiomas. Clin. Neurol. Neurosurg. 2019, 182, 1–4. [Google Scholar] [CrossRef]
- Kuranari, Y.; Tamura, R.; Tsuda, N.; Kosugi, K.; Morimoto, Y.; Yoshida, K.; Toda, M. Prognostic Significance of Preoperative Neutrophil-to-Lymphocyte Ratio in Patients With Meningiomas. Front. Oncol. 2020, 10, 592470. [Google Scholar] [CrossRef]
- Marques, P.; de Vries, F.; Dekkers, O.M.; van Furth, W.R.; Korbonits, M.; Biermasz, N.R.; Pereira, A.M. Pre-operative serum inflammation-based scores in patients with pituitary adenomas. Pituitary 2021, 24, 334–350. [Google Scholar] [CrossRef] [PubMed]
- Üçler, R.; Aslan, M.; Atmaca, M.; Alay, M.; Ademoǧlu, E.N.; Gülşen, I. Evaluation of blood neutrophil to lymphocyte and platelet to lymphocyte ratios according to plasma glucose status and serum insulin-like growth factor 1 levels in patients with acromegaly. Hum. Exp. Toxicol. 2016, 35, 608–612. [Google Scholar] [CrossRef]
- Arpaci, D.; Kuzu, F.; Unal, M.; Ilikhan, S.U.; Buyukuysal, M.C.; Bayraktaroglu, T. Assessment of Mean Platelet Volume and its Effect on Disease Control in Patients with Acromegaly. Clin. Lab. 2016, 62, 2167–2171. [Google Scholar] [CrossRef] [PubMed]
- Demirpence, M.; Yasar, H.Y.; Colak, A.; Akinci, B.; Yener, S.; Toprak, B.; Karademirci, I. Mean platelet volume and platelet function analysis in acromegalic patients before and after treatment. Acta Endocrinol. 2016, 12, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Ucler, R.; Aslan, M.; Atmaca, M.; Alay, M.; Ademoglu, E.N.; Candan, Z.; Gulsen, I. The effect of disease control on mean platelet volume and red blood cell distribution in patients with acromegaly. J. Clin. Exp. Med. 2015, 8, 6060–6066. [Google Scholar]
- Ünübol, M.; Güney, E.; Türe, M.; Eryilmaz, U. Mean platelet volume and arterial stiffness in patients with acromegaly. Anadolu. Kardiyol. Derg. 2014, 14, 456–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katznelson, L.; Laws, E.R.; Melmed, S.; Molitch, M.E.; Murad, M.H.; Utz, A.; Wass, J.A.H. Acromegaly: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2014, 99, 3933–3951. [Google Scholar] [CrossRef]
- Frara, S.; Maffezzoni, F.; Mazziotti, G.; Giustina, A. Current and Emerging Aspects of Diabetes Mellitus in Acromegaly. Trends Endocrinol. Metab. 2016, 27, 470–483. [Google Scholar] [CrossRef] [PubMed]
- Bernabeu, I.; Aller, J.; Álvarez-Escolá, C.; Fajardo-Montañana, C.; Gálvez-Moreno, Á.; Guillín-Amarelle, C.; Sesmilo, G. Criteria for diagnosis and postoperative control of acromegaly, and screening and management of its comorbidities: Expert consensus. Endocrinol. Diabetes Nutr. 2018, 65, 297–305. [Google Scholar] [CrossRef]
- Esposito, D.; Olsson, D.S.; Ragnarsson, O.; Buchfelder, M.; Skoglund, T.; Johannsson, G. Non-functioning pituitary adenomas: Indications for pituitary surgery and post-surgical management. Pituitary 2019, 22, 422–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, F.; Han, A.; Shi, F.; Kong, L.; Yu, J. The postoperative neutrophil-to-lymphocyte ratio and changes in this ratio predict survival after the complete resection of stage I non-small cell lung cancer. Onco Targets Ther. 2016, 9, 6529–6537. [Google Scholar] [CrossRef] [Green Version]
- Szor, D.J.; Dias, A.R.; Pereira, M.A.; Ramos, M.F.K.P.; Zilberstein, B.; Cecconello, I.; Ribeiro Júnior, U. Neutrophil-lymphocyte ratio change after curative gastrectomy for gastric cancer: A subgroup analysis. Einstein 2019, 18, eAO4860. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, S.; Pant, I.; Chaturvedi, S. Pituitary tumors: Changing paradigms in understanding, nomenclature and the newer basis of classification. Astrocyte 2018, 4, 240–250. [Google Scholar] [CrossRef]
- van Esdonk, M.J.; van Zutphen, E.J.M.; Roelfsema, F.; Pereira, A.M.; van der Graaf, P.H.; Biermasz, N.R.; Stevens, J.; Burggraaf, J. How are growth hormone and insulin-like growth factor-1 reported as markers for drug effectiveness in clinical acromegaly research? A comprehensive methodologic review. Pituitary 2018, 21, 310–322. [Google Scholar] [CrossRef] [Green Version]
- Fleseriu, M.; Hashim, I.A.; Karavitaki, N.; Melmed, S.; Murad, M.H.; Salvatori, R.; Samuels, M.H. Hormonal Replacement in Hypopituitarism in Adults: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2016, 101, 3888–3921. [Google Scholar] [CrossRef]
- Melmed, S.; Casanueva, F.F.; Hoffman, A.R.; Kleinberg, D.L.; Montori, V.M.; Schlechte, J.A.; Wass, J.A.H. Diagnosis and treatment of hyperprolactinemia: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 273–288. [Google Scholar] [CrossRef]
- AlMalki, M.H.; Ahmad, M.M.; Brema, I.; AlDahmani, K.M.; Pervez, N.; Al-Dandan, S.; AlObaid, A.; Beshyah, S.A. Contemporary Management of Clinically Non-functioning Pituitary Adenomas: A Clinical Review. Clin. Med. Insights Endocrinol. Diabetes 2020, 13, 1179551420932921. [Google Scholar] [CrossRef]
- Vilar, L.; Abucham, J.; Albuquerque, J.L.; Araujo, L.A.; Azevedo, M.F.; Boguszewski, C.L.; Casulari, L.A.; Cunha Neto, M.B.C.; Czepielewski, M.A.; Duarte, F.H.G.; et al. Controversial issues in the management of hyperprolactinemia and prolactinomas-An overview by the Neuroendocrinology Department of the Brazilian Society of Endocrinology and Metabolism. Arch. Endocrinol. Metab. 2018, 62, 236–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araszkiewicz, A.; Bandurska-Stankiewicz, E.; Borys, S.; Budzyński, A.; Cyganek, K.; Cypryk, K.; Czech, A.; Czupryniak, L.; Drzewoski, J.; Dzida, G.; et al. 2021 Guidelines on the management of patients with diabetes. A position of Diabetes Poland. Clin. Diabetol. 2021, 10, 1–113. [Google Scholar] [CrossRef]
- Fornari, M.C.; Scolnik, M.P.; Palacios, M.F.; Intebi, A.D.; Diez, R.A. Growth hormone inhibits normal B-cell differentiation and neutrophils’ chemotaxis in vitro. Int. J. Immunopharmacol. 1994, 16, 667–673. [Google Scholar] [CrossRef]
- Touskova, V.; Trachta, P.; Kavalkova, P.; Drapalova, J.; Haluzikova, D.; Mraz, M.; Lacinova, Z.; Marek, J.; Haluzik, M. Serum concentrations and tissue expression of components of insulin-like growth factor-axis in females with type 2 diabetes mellitus and obesity: The influence of very-low-calorie diet. Mol. Cell. Endocrinol. 2012, 361, 172–178. [Google Scholar] [CrossRef]
- Sohmiya, M.; Kanazawa, I.; Kato, Y. Effect of recombinant human GH on circulating granulocyte colony-stimulating factor and neutrophils in patients with adult GH deficiency. Eur. J. Endocrinol. 2005, 152, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Liang, S.; Sun, B.; Kang, J. The Progress of Immunotherapy in Refractory Pituitary Adenomas and Pituitary Carcinomas. Front. Endocrinol. 2020, 11, 608422. [Google Scholar] [CrossRef]
- Lupi, I.; Manetti, L.; Caturegli, P.; Menicagli, M.; Cosottini, M.; Iannelli, A.; Acerbi, G.; Bevilacqua, G.; Bogazzi, F.; Martino, E. Tumor infiltrating lymphocytes but not serum pituitary antibodies are associated with poor clinical outcome after surgery in patients with pituitary adenoma. J. Clin. Endocrinol. Metab. 2010, 95, 289–296. [Google Scholar] [CrossRef]
- Rachidi, S.; Li, H.; Wallace, K.; Li, Z.; Balch, C.; Lautenschlaeger, T. Preoperative platelet counts and postoperative outcomes in cancer surgery: A multicenter, retrospective cohort study. Platelets 2020, 31, 79–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manatakis, D.K.; Tseleni-Balafouta, S.; Balalis, D.; Soulou, V.N.; Korkolis, D.P.; Sakorafas, G.H.; Plataniotis, G.; Gontikakis, E. Association of Baseline Neutrophil-to-Lymphocyte Ratio with Clinicopathological Characteristics of Papillary Thyroid Carcinoma. Int. J. Endocrinol. 2017, 2017, 8471235. [Google Scholar] [CrossRef]
- Cheong, T.Y.; Hong, S.D.; Jung, K.W.; So, Y.K. The diagnostic predictive value of neutrophil-to-lymphocyte ratio in thyroid cancer adjusted for tumor size. PLoS ONE 2021, 16, e0251446. [Google Scholar] [CrossRef] [PubMed]
- Dagmura, H.; Daldal, E. Can Simple Parameters such as Neutrophil-to-Lymphocyte Ratio and Neutrophil Count Predict the Nature of Adrenal Masses? Clin. Lab. 2021, 67. [Google Scholar] [CrossRef]
- Asa, S.L.; Ezzat, S. An Update on Pituitary Neuroendocrine Tumors Leading to Acromegaly and Gigantism. J. Clin. Med. 2021, 10, 2254. [Google Scholar] [CrossRef]
- Wolters, T.L.C.; van der Heijden, C.D.C.C.; Pinzariu, O.; Hijmans-Kersten, B.T.P.; Jacobs, C.; Kaffa, C.; Hoischen, A.; Netea, M.G.; Smit, J.W.A.; Thijssen, D.H.J.; et al. The association between treatment and systemic inflammation in acromegaly. Growth Horm. IGF Res. 2021, 57–58, 101391. [Google Scholar] [CrossRef]


| Variables | Acromegaly | NFPAs | Controls | p-Value |
|---|---|---|---|---|
| (n = 62) | (n = 134) | (n = 120) | ||
| Age at diagnosis [years] | 55 (44–62) | 53 (36–64) | 43 (26–59) | 0.002 *,1 |
| Female, n (%) | 41 (66.1) | 90 (67.2) | 71 (59.2) | 0.383 2 |
| Male, n (%) | 21 (33.9) | 44 (32.8) | 49 (40.8) | |
| BMI [kg/m2] a | 30.3 (26.5–34.6) | 27.1 (24.1–31.4) | 25.1 (23.3–28.7) | <0.001 *,1 |
| normal body weight, n (%) | 10 (16.1) | 46 (34.3) | 60 (50.0) | <0.001 *,1 |
| overweight, n (%) | 21 (33.9) | 43 (32.1) | 42 (35.0) | 0.885 1 |
| obesity class I, n (%) | 18 (29.0) | 33 (24.6) | 15 (12.5) | 0.012 *,1 |
| obesity class II, n (%) | 7 (11.3) | 8 (6.0) | 3 (2.5) | 0.053 1 |
| obesity class III, n (%) | 6 (9.7) | 4 (3.0) | 0 (0) | 0.002 *,3 |
| Duration of symptoms prior to diagnosis [years] | 7 (2–10) | - | - | - |
| Random GH at diagnosis [ng/mL] | 8.10 (3.59–18.50) | 0.27 (0.10–0.74) | - | <0.001 *,3 |
| IGF-1 at diagnosis [ng/mL] | 647.8 (407.7–900.0) | 136.3 (98.7–190.0) | - | <0.001 *,3 |
| IGF-1 × ULN at diagnosis | 3.14 (2.08–4.47) | 0.63 (0.47–0.79) | - | <0.001 *,3 |
| Pituitary adenoma mass effects | ||||
| Visual field defects, n (%) | 8 (12.9) | 33 (24.6) | - | 0.110 3 |
| Headache, n (%) | 34 (54.8) | 80 (59.7) | - | 0.523 3 |
| Radiological features of pituitary adenomas | ||||
| Primary tumour MD [mm] | 14.0 (9.0–23.0) | 14.0 (5.8–23.0) | - | 0.241 3 |
| Microadenoma, n (%) | 15 (24.2) | 54 (40.3) | - | 0.029 *,3 |
| Macroadenoma, n (%) | 45 (72.6) | 76 (56.7) | - | 0.034 *,3 |
| Giant tumour, n (%) | 2 (3.2) | 4 (3.0) | - | ** |
| At least 1 feature of pituitary adenoma invasiveness, n (%) | 35 (56.5) | 69 (51.5) | - | 0.520 3 |
| No features of pituitary adenoma invasiveness, n (%) | 27 (43.5) | 65 (48.5) | - | 0.520 3 |
| Sphenoid sinus invasion, n (%) | 30 (48.4) | 31 (23.1) | - | 0.817 3 |
| Cavernous sinus invasion, n (%) | 20 (32.3) | 41 (30.6) | - | <0.001 *,3 |
| Compression of the optic chiasm, n (%) | 17 (27.4) | 55 (41.0) | - | 0.067 3 |
| Hormonal status of pituitary gland | ||||
| Secondary hypothyroidism, n (%) | 1 (1.6) | 24 (17.9) | - | 0.002 *,3 |
| Secondary adrenal insufficiency, n (%) | 2 (3.2) | 22 (16.4) | - | 0.009 *,3 |
| Hypogonadism hypogonadotropic/estrogen depletion, n (%) | 8 (12.9) | 28 (20.9) | - | 0.181 3 |
| Hyperprolactinemia due to pituitary stalk deviation, n (%) | 13 (21.0) | 21 (15.7) | - | 0.365 3 |
| GH deficiency, n (%) | - | 6 (4.5) | - | ** |
| At least 1 pituitary deficiency, n (%) | 17 (27.4) | 47 (35.1) | - | 0.176 3 |
| No pituitary deficiency, n (%) | 45 (72.6) | 87 (64.9) | - | 0.176 3 |
| Glucose homeostasis disorders | ||||
| Normoglycemia, n (%) | 19 (30.6) | 105 (78.4) | - | <0.001 *,3 |
| Pre-DM, n (%) | 30 (48.4) | 17 (12.7) | - | <0.001 *,3 |
| IFG, n (%) | 18 (60) | 10 (58.8) | - | <0.001 *,3 |
| IGT, n (%) | 5 (16.7) | 2 (11.8) | - | 0.002 *,3 |
| IFG+IGT, n (%) | 7 (23.3) | 5 (29.4) | - | 0.041 *,3 |
| T2DM/secondary form of DM, n (%) | 13 (21.0) | 12 (9.0) | - | 0.019 *,3 |
| FPG [mg/dL] | 105.5 (93.0–117.0) | 90.0 (83.0–97.0) | 90.5 (85.0–97.0) | <0.001 *,1 |
| HbA1c [%] | 6.2 (5.6–6.6) | 5.9 (5.5–6.3) | - | 0.210 3 |
| Cardiovascular complications | ||||
| Hypertension, n (%) | 49 (79.0) | 59 (44.0) | - | <0.001 *,3 |
| Coronary artery disease, n (%) | 10 (16.1) | 12 (9.0) | - | 0.141 3 |
| Cardiac arrhythmias, n (%) | 7 (11.3) | 10 (7.5) | - | 0.222 3 |
| Disturbances in TTE, n (%) | 33 (53.2) | ND *** | - | - |
| Other comorbidities | ||||
| Obstructive sleep apnea syndrome, n (%) | 15 (24.2) | 42 (31.3) | - | 0.307 3 |
| Benign neoplasms, n (%) b | 28 (45.2) | ND *** | - | - |
| Degenerative changes in joints and bones, n (%) | 26 (41.9) | 48 (35.8) | - | 0.414 3 |
| Carpal tunnel syndrome, n (%) | 13 (21.0) | 8 (6.0) | - | 0.002 *,3 |
| Variables | Group 1 Acromegaly (n = 62) | Group 2 NFPAs (n = 134) | Group 3 Controls (n = 120) | p-Value | p (Groups 1 & 2) | p (Groups 1 & 3) | p (Groups 2 & 3) |
|---|---|---|---|---|---|---|---|
| WBC [109/L] | 5.83 (4.80–6.98) | 5.46 (4.58–6.43) | 5.66 (5.19–6.68) | 0.043 * | NS | NS | 0.050 |
| NEU [109/L] | 3.24 (2.48–4.05) | 2.68 (2.15–3.18) | 2.76 (2.50–3.22) | <0.001 * | <0.001 * | 0.012 * | NS |
| LYM [109/L] | 1.69 (1.45–2.16) | 2.11 (1.73–2.53) | 2.24 (1.96–2.60) | <0.001 * | 0.001 * | <0.001 * | 0.023 * |
| NLR | 1.84 (1.36–2.61) | 1.29 (1.08–1.52) | 1.23 (1.10–1.41) | <0.001 * | <0.001 * | <0.001 * | NS |
| MONO [109/L] | 0.35 (0.27–0.47) | 0.37 (0.30–0.43) | 0.37 (0.32–0.46) | 0.097 | NS | NS | NS |
| LMR | 5.15 (3.76–6.55) | 5.93 (4.89–7.11) | 5.83 (4.90–7.09) | 0.039 * | NS | NS | NS |
| PLT [109/L] | 223.0 (204.0–289.0) | 228.3 (193.0–276.0) | 235.5 (194.5–276.5) | 0.811 | NS | NS | NS |
| PLR | 131.15 (112.30–177.57) | 109.96 (93.33–137.43) | 100.86 (84.55–123.17) | <0.001 * | 0.001 * | <0.001 * | NS |
| MPV [fL] | 7.6 (7.0–8.2) | 7.8 (7.3–8.5) | 7.7 (7.3–8.5) | 0.437 | NS | NS | NS |
| MPV/PLT | 0.034 (0.027–0.039) | 0.034 (0.027–0.041) | 0.032 (0.027–0.044) | 0.825 | NS | NS | NS |
| SII | 439.75 (325.73–599.79) | 301.07 (233.52–359.31) | 291.47 (227.94–354.24) | <0.001 * | <0.001 * | <0.001 * | NS |
| AUC | SE | 95% CI | Sensitivity | Specificity | Cut-Off | p-Value | |
|---|---|---|---|---|---|---|---|
| NEU | 0.68 | 0.04 | 0.60–0.77 | 0.42 | 0.91 | 3.60 | <0.001 * |
| LYM | 0.66 | 0.04 | 0.58–0.75 | 0.76 | 0.24 | 1.70 | <0.001 * |
| NLR | 0.78 | 0.04 | 0.70–0.86 | 0.55 | 0.99 | 1.79 | <0.001 * |
| LMR | 0.60 | 0.05 | 0.51–0.69 | 0.50 | 0.72 | 5.04 | 0.021 |
| PLR | 0.67 | 0.04 | 0.58–0.75 | 0.76 | 0.57 | 112.30 | <0.001 * |
| SII | 0.77 | 0.04 | 0.69–0.85 | 0.53 | 0.90 | 425.98 | <0.001 * |
| Random GH | 0.97 | 0.01 | 0.95–0.99 | 0.92 | 0.89 | 1.66 | <0.001 * |
| IGF-1 | 0.98 | 0.01 | 0.96–1.00 | 0.92 | 0.96 | 274.00 | <0.001 * |
| IGF-1 × ULN | 1.00 | 0.00 | 1.00–1.00 | 1.00 | 1.00 | 0.99 | <0.001 * |
| Variables | Cured with Surgery n = 23 | Uncontrolled with Surgery n = 15 | p-Value |
|---|---|---|---|
| WBC [109/L] | 5.57 (4.75–7.15) | 5.77 (5.14–7.42) | 0.637 |
| NEU [109/L] | 2.92 (2.41–4.05) | 3.34 (3.11–5.17) | 0.095 |
| LYM [109/L] | 1.87 (1.55–2.45) | 1.67 (1.44–1.92) | 0.153 |
| NLR | 1.50 (1.34–1.94) | 1.95 (1.51–3.16) | 0.044 * |
| MONO [109/L] | 0.31 (0.27–0.48) | 0.30 (0.24–0.50) | 0.637 |
| LMR | 5.52 (4.54–8.04) | 5.54 (3.76–6.55) | 0.746 |
| PLT [109/L] | 212.0 (203.0–289.0) | 236.0 (206.00–313.0) | 0.442 |
| PLR | 114.43 (85.64–167.60) | 140.74 (123.35–194.00) | 0.048 * |
| MPV [fL] | 7.5 (7.2–8.2) | 7.2 (7.0–8.5) | 0.459 |
| MPV/PLT | 0.03 (0.03–0.04) | 0.03 (0.02–0.04) | 0.187 |
| SII | 369.41 (271.91–473.54) | 513.53 (355.28–777.83) | 0.035 * |
| Random GH at diagnosis [ng/mL] | 6.36 (2.70–14.60) | 18.50 (5.46–67.45) | 0.003 * |
| IGF-1 at diagnosis [ng/mL] | 671.7 (576.0–878.0) | 872.9 (399.0–903.0) | 0.723 |
| IGF-1 × ULN at diagnosis | 3.43 (2.48–4.60) | 2.94 (2.35–4.63) | 0.860 |
| adenoma MD [mm] | 13 (8–22) | 25 (14–28) | 0.016 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szydełko, J.; Szydełko-Gorzkowicz, M.; Matyjaszek-Matuszek, B. Neutrophil-to-Lymphocyte, Platelet-to-Lymphocyte Ratios, and Systemic Immune-Inflammation Index as Potential Biomarkers of Chronic Inflammation in Patients with Newly Diagnosed Acromegaly: A Single-Centre Study. J. Clin. Med. 2021, 10, 3997. https://doi.org/10.3390/jcm10173997
Szydełko J, Szydełko-Gorzkowicz M, Matyjaszek-Matuszek B. Neutrophil-to-Lymphocyte, Platelet-to-Lymphocyte Ratios, and Systemic Immune-Inflammation Index as Potential Biomarkers of Chronic Inflammation in Patients with Newly Diagnosed Acromegaly: A Single-Centre Study. Journal of Clinical Medicine. 2021; 10(17):3997. https://doi.org/10.3390/jcm10173997
Chicago/Turabian StyleSzydełko, Joanna, Magdalena Szydełko-Gorzkowicz, and Beata Matyjaszek-Matuszek. 2021. "Neutrophil-to-Lymphocyte, Platelet-to-Lymphocyte Ratios, and Systemic Immune-Inflammation Index as Potential Biomarkers of Chronic Inflammation in Patients with Newly Diagnosed Acromegaly: A Single-Centre Study" Journal of Clinical Medicine 10, no. 17: 3997. https://doi.org/10.3390/jcm10173997
APA StyleSzydełko, J., Szydełko-Gorzkowicz, M., & Matyjaszek-Matuszek, B. (2021). Neutrophil-to-Lymphocyte, Platelet-to-Lymphocyte Ratios, and Systemic Immune-Inflammation Index as Potential Biomarkers of Chronic Inflammation in Patients with Newly Diagnosed Acromegaly: A Single-Centre Study. Journal of Clinical Medicine, 10(17), 3997. https://doi.org/10.3390/jcm10173997






