Adverse Features of Rectourethral Fistula Requiring Extirpative Surgery and Permanent Dual Diversion: Our Experience and Recommendations
Abstract
:1. Introduction
2. Methods
3. Statistical Analysis
4. Results
5. Discussion
5.1. Surgical Approaches
5.2. Use of Tissue Interposition
5.3. Impact of Radiation on Clinical Recovery, Pelvic/Rectal Pain, Urinary and Bowel Functions
5.4. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Choi, J.H.; Jeon, B.G.; Choi, S.G.; Han, E.C.; Ha, H.-K.; Oh, H.-K.; Choe, E.K.; Moon, S.H.; Ryoo, S.-B.; Park, K.J. Rectourethral fistula: Systematic review of and experiences with various surgical treatment methods. Ann. Coloproctology 2014, 30, 35–41. [Google Scholar] [CrossRef]
- Mundy, A.R.; Andrich, D.E. Posterior urethral complications of the treatment of prostate cancer. BJU Int. 2012, 110, 304–325. [Google Scholar] [CrossRef]
- Thomas, C.; Jones, J.; Jäger, W.; Hampel, C.; Thüroff, J.W.; Gillitzer, R. Incidence, clinical symptoms, and management of rectourethral fistulas after radical prostatectomy. J. Urol. 2010, 183, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Hanna, J.M.; Turley, R.; Castleberry, A.; Hopkins, T.; Peterson, A.C.; Mantyh, C.; Migaly, J. Surgical management of complex rectourethral fistulas in irradiated and nonirradiated patients. Dis. Colon Rectum 2014, 57, 1105–1112. [Google Scholar] [CrossRef]
- Beddy, D.; Poskus, T.; Umbreit, E.; Larson, D.W.; Elliott, D.S.; Dozois, E.J. Impact of radiotherapy on surgical repair and outcome in patients with rectourethral fistula. Colorectal Dis. 2013, 15, 1515–1520. [Google Scholar] [CrossRef]
- Eswara, J.R.; Raup, V.T.; Heningburg, A.M.; Brandes, S.B. Pelvic radiation is associated with urinary fistulae repair failure and need for permanent urinary diversion. Urology 2015, 85, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Bryan Volzke McAninch, J.W.; Breyer, B.N.; Glass, A.S.; Garcia-Aguilar, J. Transperineal management for postoperative and radiation rectourethral fistulas. J. Urol. 2013, 189, 966–971. [Google Scholar]
- Linder, B.J.; Umbreit, E.C.; Larson, D.; Dozois, E.J.; Thapa, P.; Elliott, D.S. Effect of prior radiotherapy and ablative therapy on surgical outcomes for the treatment of rectourethral fistulas. J. Urol. 2013, 190, 1287–1291. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, D.A.; Zinman, L.N.; Buckley, J.C.; Marcello, P.; Browne, B.M.; Vanni, A.J. Short-and long-term complications and outcomes of radiation and surgically induced rectourethral fistula repair with buccal mucosa graft and muscle interposition flap. Urology 2016, 98, 170–175. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of surgical complications. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Angulo, J.C.; Arance, I.; Apesteguy, Y.; Felicio, J.; Martins, M.; Martins, F.E. Urorectal fistula reapir using different approaches: Operative results and quality of life issues. Int. Braz. J. Urol. 2020, 47, 399–412. [Google Scholar] [CrossRef]
- Amato, A. Rectourethral Fistulas: A Review. Clin. Surg. 2016, 1, 1121. [Google Scholar]
- Mundy, A.R.; Andrich, A.E. Urorectal fistulae following the treatment of prostate cancer. BJUI 2011, 107, 1298–1303. [Google Scholar] [CrossRef]
- Lane, B.R.; Stein, D.E.; Remzi, F.H.; Strong, S.A.; Fazio, V.W.; Angermeier, K.W. Management of radiotherapy induced rectouretrhal fistula. J. Urol. 2006, 175, 1382–1388. [Google Scholar] [CrossRef]
- Vanni, A.J.; Buckley, J.C.; Zinman, L.N. Management of surgical and radiation induced recyourethral fistulas with an interposition muscle fap and selective buccal mucosa onlay graft. J. Urol. 2010, 184, 2400–2404. [Google Scholar] [CrossRef]
- Xiong, Y.; Huang, P.; Ren, Q.-G. Transanal pull-through procedure with delayed versus immediate coloanal anastomosis for anus-preserving curative resection of lower rectal cancer: A case-control study. Ann. Surg. 2018, 82, 533–539. [Google Scholar]
- Rouanne, M.; Vaessen, C.; Bitker, M.-O.; Chartier-Kastler, E.; Rouprêt, M. Outcome of a modified York Mason technique in men with iatrogenic urethrorectal fistula after radical prostatectomy. Dis. Colon Rectum 2011, 54, 1008–1013. [Google Scholar] [CrossRef]
- Hampson LAMuncey, W.; Sinanan, M.N.; Voelzke, B.B. Outcomes and quality of life among men after anal sphincter-sparing transperineal rectourethral fistula repair. Urology 2018, 121, 175–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, I.M.; Marx, A.C. Conservative therapy of rectourethral fistula: Five-year follow-up. Urology 1990, 35, 533–536. [Google Scholar] [CrossRef]
- Hanna, J.M.; Peterson, A.C.; Mantyh, C. Rectourethral fistulas in the cancer survivor. Curr. Opin. Urol. 2014, 24, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Dinges, S.; Deger, S.; Koswig, S.; Boehmer, D.; Schnorr, D.; Wiegel, T.; Loening, S.A.; Dietel, M.; Hinkelbein, W.; Budach, V. High-dose rate interstitial with external beam irradiation for localized prostate cancer –results of a prospective trial. Radiother. Oncol. 1998, 48, 197–202. [Google Scholar] [CrossRef]
- Moreira, S.G.; Seigne, J.D.; Ordorica, R.C.; Marcet, J.; Pow-Sang, J.M.; Lockhart, J.L. Devastating complications after brachytherapy in the treatment of prostate adenocarcinoma. BJU Int. 2004, 93, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Gelet, A.; Chapelon, J.Y.; Poissonnier, L.; Bouvier, R.; Rouvière, O.; Curiel, L.; Janier, M.; Vallancien, G. Local recurrence of prostate cancer after external beam radiotherapy: Early experience of salvage therapy using high-intensity focused ultrasonography. Urology 2004, 63, 625–629. [Google Scholar] [CrossRef]
- Ahmed, H.U.; Ishaq, A.; Zacharakis, E.; Shaw, G.; Illing, R.; Allen, C.; Kirkham, A.; Emberton, M. Rectal fistula after salvage high-intensity focused ultrasound for recurrent prostate cancer after combined brachytherapy and external beam radiotherapy. BJU Int. 2009, 101, 19–26. [Google Scholar]
- Davis, J.W.; Schellhammer, P.F. Prostatorectal fitula 14 years following brachytherapy for prostate cancer. J. Urol. 2001, 165, 189. [Google Scholar] [CrossRef] [PubMed]
- Chrouser, K.L.; Leibovich, B.C.; Sweat, S.D.; Larson, D.W.; Davis, B.; Tran, N.V.; Zincke, H.; Blute, M.L. Urinary fistulas following external radiation or permanent brachytherapy for the treatment of prostate cancer. J. Urol. 2005, 173, 1953–1957. [Google Scholar] [CrossRef] [PubMed]
- Hamstra, D.A.; Mariados, N.; Sylvester, J.; Shah, D.; Karsh, L.; Hudes, R.; Beyer, D.; Kurtzman, S.; Bogart, J.; Hsi, R.A.; et al. Continued benefit to rectal separation for prostate radiation therapy: Final results of a phase III trial. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 976–985. [Google Scholar] [CrossRef] [Green Version]
- Mariados, N.; Sylvester, J.; Shah, D.; Karsh, L.; Hudes, R.; Beyer, D.; Kurtzman, S.; Bogart, J.; Hsi, R.A.; Kos, M.; et al. Hydrogel spacer prospective multicenter randomized controlled pivotal trial: Dosimetric and clinical effects of perirectal spacer application in men undergoing prostate image guided intensity modulated radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 971–977. [Google Scholar] [CrossRef] [Green Version]
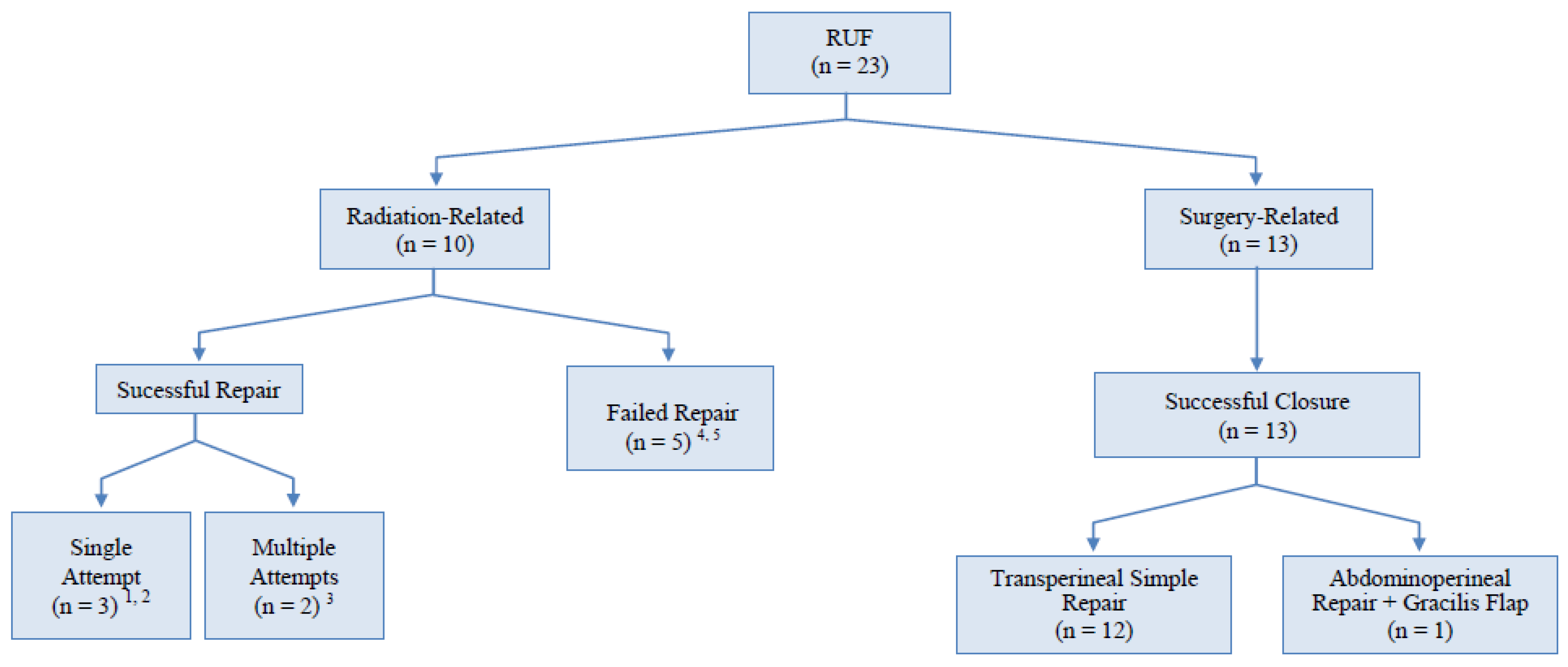
| N°pts | Age (y) | Etiology | Location/Type of Fistula | N° Previous Attempts | Surgical Approach | Ureteric Stenting | Tissue Graft Interposition | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Radiation/High Energy Ablation Group (n = 10) | ||||||||
| 1 | 62 | BT + EBRT | Prostatic urethra | 0 | Perineal | Yes | Gracilis muscle | Failure |
| 2 | 68 | BT+EBRT | Membranous and prostatic urethra | 0 | Perineal | No | Gracilis muscle | Success after 1 attempt |
| 3 | 59 | BT + EBRT | BN/LT | 2 | Abdomino perineal | No | Omentum | Success after 3 attempts |
| 4 | 78 | ARR + EBRT | BN/LT | 0 | Abdomino perineal | Yes | Gracilis muscle+ proctectomy | Success after 1 attempt |
| 5 | 66 | RRP + EBRT | BN/LT | 1 | Abdomino perineal | Yes | Omentum | Success after 2 attempts |
| 6 | 61 | HIFU + EBRT | Prostatic urethra | 0 | Perineal | No | None | Failure |
| 7 | 71 | Chemo + EBRT + TURBT | Trigone/BN | 0 | Perineal | Yes | None | Failure |
| 8 | 67 | BT + EBRT | Prostatic urethra | 0 | Abdomino perineal | No | Omentum | Success after 1 attempt |
| 9 | 69 | BT + TURP | Prostatic urethra | 1 | Abdomino perineal | No | None (Glubran®) | Failure |
| 10 | 60 | BT + TURP | Prostatic urethra | 2 | Abdomino perineal | No | None | Failure |
| Surgery Group (n = 13) | ||||||||
| 11 | 73 | ARR | BN/LT | 0 | Perineal | No | None | Success after 1 attempt |
| 12 | 75 | Lap RP | Giant fistula involving prostatic urethra and BN/LT | 2 | Abdomino perineal | No | Gracilis muscle | Success after 1 attempt |
| 13 | 64 | RRP | Prostatic urethra | 0 | Perineal | No | None | Success after 1 attempt |
| 14 | 63 | Lap RP | Prostatic urethra | 0 | Perineal | No | None | Success after 1 attempt |
| 15 | 59 | RRP | BN/LT | 0 | Perineal | No | None | Success after 1 attempt |
| 16 | 75 | RC + ileal neobladder | Neovesico-urethral anastomosis | 0 | Perineal | No | None | Success after 1 attempt |
| 17 | 57 | ARR | BN/LT | 0 | Perineal | Yes | None | Success after 1 attempt |
| 18 | 65 | RRP | BN | 0 | Perineal | No | None | Success after 1 attempt |
| 19 | 76 | PFUI + Lap RRP | Prostatic urethra | 0 | Perineal | No | None | Success after 1 attempt |
| 20 | 74 | RRP | Prostatic urethra | 0 | Perineal | No | None | Success after 1 attempt |
| 21 | 68 | TURP + RRP | Prostatic urethra | 0 | Perineal | No | None | Success after 1 attempt |
| 22 | 71 | Lap RRP | BN | 1 | Perineal | Yes | None | Success after 1 attempt |
| 23 | 79 | Lap RRP | Prostatic urethra | 0 | Perineal | No | None | Success after 1 attempt |
| Group 1 (n = 10) | Group 2 (n = 13) | p-Value | |
|---|---|---|---|
| Mean Age (years) (Min–Max) | 66.10 (59–78) | 69.15 (57–79) | 0.279 |
| Body Mass Index (kg/m2) (Min–Max) | 26.9 (18.9–31.2) | 26.29 (21.0–31.1) | 0.686 |
| Serum Albumin (g/dL) (Min–Max) | 3.81 (3.1–4.6) | 3.60 (2.8–4.32) | 0.284 |
| Smoking (pts/%) | 4/10 (40%) | 2/13 (15.4%) | 0.199 |
| Diabetes (pts/%) | 2/10 (20%) | 4/13 (30.8%) | 0.581 |
| Hypertension (pts/%) | 5/10 (50%) | 6/13 (46.2%) | 0.863 |
| Group 1 (Non-Surgical) | Group 2 (Surgical) | Total | |
|---|---|---|---|
| Surgical success after n° of attempts | |||
| 1 | 3 | 13 | 16/23 (70%) |
| 2 | 1 | 0 | 1/23 (4%) |
| 3 | 1 | 0 | 1/23 (4%) |
| Failures | 5 | 0 | 5/23 (22%) |
| Total | 10/23 (43%) | 13/23 (57%) | |
| Surgical approach | |||
| Perineal | 4/10 (40%) | 12/13 (92%) | 16/23 (70%) |
| Abdominoperineal | 6/10 (60%) | 1/13 (8%) | 7/23 (30%) |
| Interposition flap | |||
| Gracilis | 3 | 1 | 4/23 (17%) |
| Omentum | 3 | 0 | 3/23 (13%) |
| Total | 6/10 (60%) | 1/13 (8%) | |
| Dual permanent diversion | |||
| Required | 5 | 0 | 5/23 (22%) |
| Not Required | 5 | 13 | 18/23 (78%) |
| Total | 5/10 (50%) | 0/13 (0%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, F.E.; Felicio, J.; Oliveira, T.R.; Martins, N.; Oliveira, V.; Palmas, A. Adverse Features of Rectourethral Fistula Requiring Extirpative Surgery and Permanent Dual Diversion: Our Experience and Recommendations. J. Clin. Med. 2021, 10, 4014. https://doi.org/10.3390/jcm10174014
Martins FE, Felicio J, Oliveira TR, Martins N, Oliveira V, Palmas A. Adverse Features of Rectourethral Fistula Requiring Extirpative Surgery and Permanent Dual Diversion: Our Experience and Recommendations. Journal of Clinical Medicine. 2021; 10(17):4014. https://doi.org/10.3390/jcm10174014
Chicago/Turabian StyleMartins, Francisco E., João Felicio, Tiago Ribeiro Oliveira, Natália Martins, Vítor Oliveira, and Artur Palmas. 2021. "Adverse Features of Rectourethral Fistula Requiring Extirpative Surgery and Permanent Dual Diversion: Our Experience and Recommendations" Journal of Clinical Medicine 10, no. 17: 4014. https://doi.org/10.3390/jcm10174014
APA StyleMartins, F. E., Felicio, J., Oliveira, T. R., Martins, N., Oliveira, V., & Palmas, A. (2021). Adverse Features of Rectourethral Fistula Requiring Extirpative Surgery and Permanent Dual Diversion: Our Experience and Recommendations. Journal of Clinical Medicine, 10(17), 4014. https://doi.org/10.3390/jcm10174014







