Kidney Microcirculation as a Target for Innovative Therapies in AKI
Abstract
:1. Introduction
2. Renal Blood Circulation
3. Autoregulation of the Kidney Microcirculation
3.1. Myogenic Regulation
3.2. Tubuloglomerular and Connecting Tubule–Glomerular Feedback
3.3. Renin–Angiotensin–Aldosterone System (RAAS)
4. Sympathetic Regulation of the Renal Microcirculation
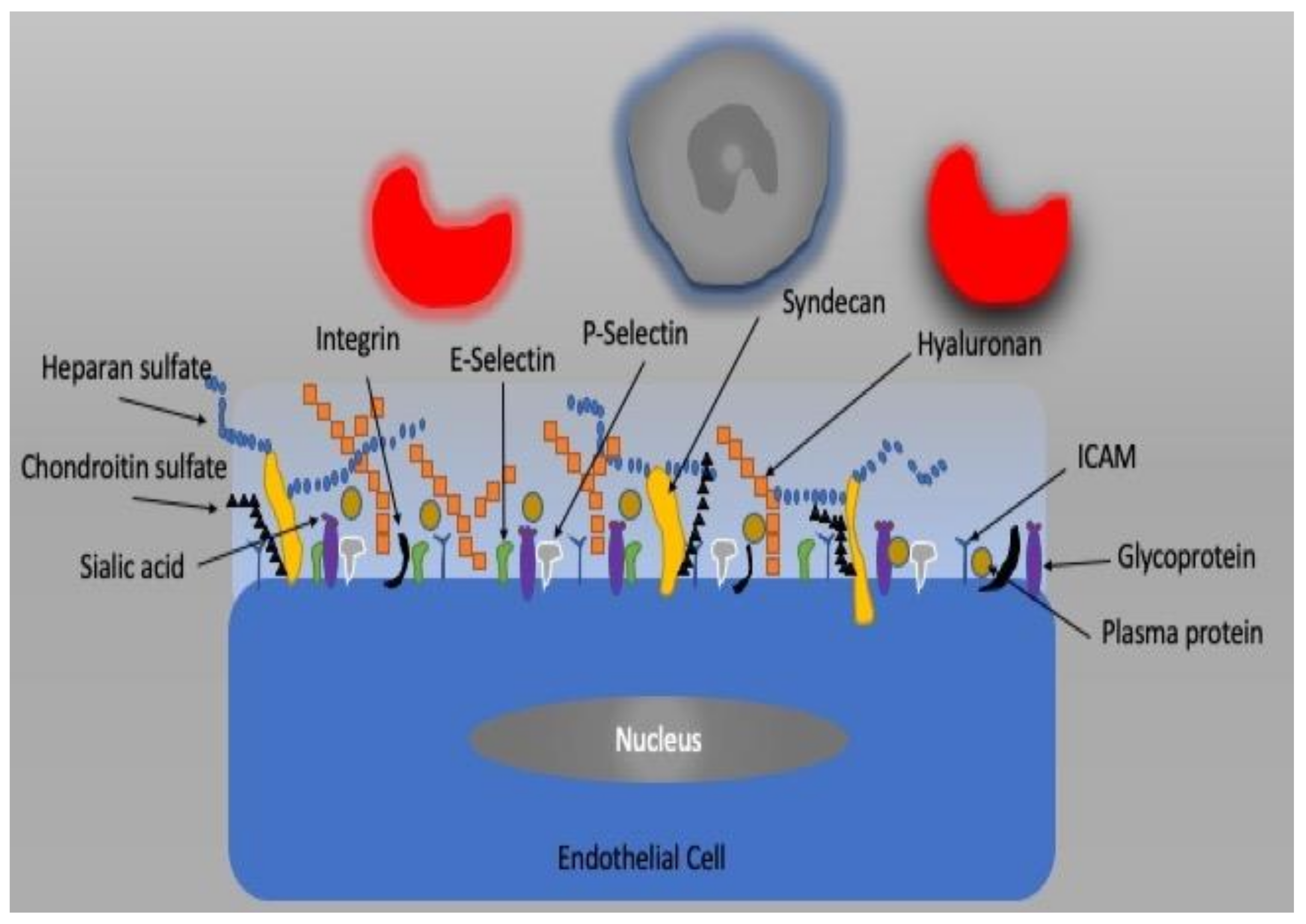
5. Vascular Endothelium and Glycocalyx
6. Arterial to Venous (A–V) Shunting
7. Microcirculatory Alterations during AKI
7.1. The Renal Microcirculation in Sepsis-Induced AKI
7.2. Renal Microcirculation in Heart Failure and Cardiogenic Shock-Induced AKI
7.3. Renal Microcirculation in I/R-Induced AKI
7.4. Renal Microcirculation in Hypovolemic/Hemorrhagic Shock-Induced AKI
7.5. Renal Microcirculation in Hemodilution-Induced AKI
7.6. Renal Microcirculation in Drug-Induced AKI
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pallone, T.L.; Edwards, A.; Mattson, D.L. Renal Medullary Circulation. Compr. Physiol. 2012, 2, 97–140. [Google Scholar]
- Evans, R.G.; Ince, C.; Joles, J.A.; Smith, D.W.; May, C.N.; O’Connor, P.M.; Gardiner, B.S. Haemodynamic Influences on Kidney Oxygenation: Clinical Implications of Integrative Physiology. Clin. Exp. Pharmacol. Physiol. 2013, 40, 106–122. [Google Scholar] [CrossRef]
- Lewington, A.J.P.; Cerdá, J.; Mehta, R.L. Raising Awareness of Acute Kidney Injury: A Global Perspective of a Silent Killer. Kidney Int. 2013, 84, 457–467. [Google Scholar] [CrossRef] [Green Version]
- Bellomo, R.; Kellum, J.A.; Ronco, C. Acute Kidney Injury. Lancet 2012, 380, 756–766. [Google Scholar] [CrossRef]
- Nash, K.; Hafeez, A.; Hou, S. Hospital-Acquired Renal Insufficiency. Am. J. Kidney Dis 2002, 39, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Thakar, C.V.; Christianson, A.; Freyberg, R.; Almenoff, P.; Render, M.L. Incidence and Outcomes of Acute Kidney Injury in Intensive Care Units: A Veterans Administration Study. Crit. Care Med. 2009, 37, 2552–2558. [Google Scholar] [CrossRef]
- Hoste, E.A.J.; Bagshaw, S.M.; Bellomo, R.; Cely, C.M.; Colman, R.; Cruz, D.N.; Edipidis, K.; Forni, L.G.; Gomersall, C.D.; Govil, D.; et al. Epidemiology of Acute Kidney Injury in Critically Ill Patients: The Multinational AKI-EPI Study. Intensive Care Med. 2015, 41, 1411–1423. [Google Scholar] [CrossRef] [PubMed]
- Dasta, J.F.; Kane-Gill, S.L.; Durtschi, A.J.; Pathak, D.S.; Kellum, J.A. Costs and Outcomes of Acute Kidney Injury (AKI) Following Cardiac Surgery. Nephrol. Dial. Transplant. 2008, 23, 1970–1974. [Google Scholar] [CrossRef] [Green Version]
- Rosner, M.H.; Okusa, M.D. Acute Kidney Injury Associated with Cardiac Surgery. Clin. J. Am. Soc. Nephrol. 2006, 1, 19–32. [Google Scholar] [CrossRef] [Green Version]
- Guerci, P.; Ergin, B.; Ince, C. The Macro- and Microcirculation of the Kidney. Best Pract. Res. Clin. Anaesthesiol. 2017, 31, 315–329. [Google Scholar] [CrossRef]
- Zafrani, L.; Ince, C. Microcirculation in Acute and Chronic Kidney Diseases. Am. J. Kidney Dis. 2015, 66, 1083–1094. [Google Scholar] [CrossRef] [PubMed]
- Pallone, T.L.; Silldorff, E.P.; Turner, M.R. Intrarenal Blood Flow: Microvascular Anatomy and the Regulation of Medullary Perfusion. Clin. Exp. Pharmacol. Physiol. 1998, 25, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Bankir, L.; Bouby, N.; Trinh-Trang-Tan, M.M. Heterogeneity of Nephron Anatomy. Kidney Int. Suppl. 1987, 20, S25–S39. [Google Scholar] [PubMed]
- Pallone, T.L.; Robertson, C.R.; Jamison, R.L. Renal Medullary Microcirculation. Physiol. Rev. 1990, 70, 885–920. [Google Scholar] [CrossRef]
- Michel, C.C. Renal Medullary Microcirculation: Architecture and Exchange. Microcirculation 1995, 2, 125–139. [Google Scholar] [CrossRef]
- Ren, Y.; Garvin, J.L.; Liu, R.; Carretero, O.A. Crosstalk between the Connecting Tubule and the Afferent Arteriole Regulates Renal Microcirculation. Kidney Int. 2007, 71, 1116–1121. [Google Scholar] [CrossRef] [Green Version]
- Carlström, M.; Wilcox, C.S.; Arendshorst, W.J. Renal Autoregulation in Health and Disease. Physiol. Rev. 2015, 95, 405–511. [Google Scholar] [CrossRef] [Green Version]
- Hilty, M.P.; Akin, S.; Boerma, C.; Donati, A.; Erdem, Ö.; Giaccaglia, P.; Guerci, P.; Milstein, D.M.; Montomoli, J.; Toraman, F.; et al. Automated Algorithm Analysis of Sublingual Microcirculation in an International Multicentral Database Identifies Alterations Associated With Disease and Mechanism of Resuscitation. Crit. Care Med. 2020, 48, e864–e875. [Google Scholar] [CrossRef]
- Guan, Z.; Osmond, D.A.; Inscho, E.W. P2X Receptors as Regulators of the Renal Microvasculature. Trends Pharmacol. Sci. 2007, 28, 646–652. [Google Scholar] [CrossRef]
- Hansen, P.B.; Schnermann, J. Vasoconstrictor and Vasodilator Effects of Adenosine in the Kidney. Am. J. Physiol. Renal Physiol. 2003, 285, F590–F599. [Google Scholar] [CrossRef] [Green Version]
- Pallone, T.L.; Zhang, Z.; Rhinehart, K. Physiology of the Renal Medullary Microcirculation. Am. J. Physiol. Renal Physiol. 2003, 284, F253–F266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauerle, J.D.; Grenz, A.; Kim, J.-H.; Lee, H.T.; Eltzschig, H.K. Adenosine Generation and Signaling during Acute Kidney Injury. J. Am. Soc. Nephrol. 2011, 22, 14–20. [Google Scholar] [CrossRef]
- Murphy, S.; Williams, J.M. Impaired Renal Autoregulation in Susceptible Models of Renal Disease. Curr. Vasc. Pharmacol. 2014, 12, 859–866. [Google Scholar] [CrossRef]
- Schnermann, J.; Levine, D.Z. Paracrine Factors in Tubuloglomerular Feedback: Adenosine, ATP, and Nitric Oxide. Annu. Rev. Physiol. 2003, 65, 501–529. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; D’Ambrosio, M.A.; Garvin, J.L.; Ren, Y.; Carretero, O.A. Connecting Tubule Glomerular Feedback in Hypertension. Hypertension 2013, 62, 738–745. [Google Scholar] [CrossRef] [Green Version]
- Welch, W.J.; Wilcox, C.S. Role of Nitric Oxide in Tubuloglomerular Feedback: Effects of Dietary Salt. Clin. Exp. Pharmacol. Physiol. 1997, 24, 582–586. [Google Scholar] [CrossRef]
- Briggs, J. The Macula Densa Sensing Mechanism for Tubuloglomerular Feedback. Fed. Proc. 1981, 40, 99–103. [Google Scholar]
- Romero, C.A.; Carretero, O.A. A Novel Mechanism of Renal Microcirculation Regulation: Connecting Tubule-Glomerular Feedback. Curr. Hypertens. Rep. 2019, 21, 8. [Google Scholar] [CrossRef] [PubMed]
- Frindt, G.; Palmer, L.G. Na Channels in the Rat Connecting Tubule. Am. J. Physiol. Renal Physiol. 2004, 286, F669–F6743. [Google Scholar] [CrossRef] [Green Version]
- Ren, Y.; D’Ambrosio, M.A.; Garvin, J.L.; Wang, H.; Carretero, O.A. Possible Mediators of Connecting Tubule Glomerular Feedback. Hypertension 2009, 53, 319–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, Y.; D’Ambrosio, M.A.; Garvin, J.L.; Wang, H.; Carretero, O.A. Prostaglandin E2 Mediates Connecting Tubule Glomerular Feedback. Hypertension 2013, 62, 1123–1128. [Google Scholar] [CrossRef] [Green Version]
- Just, A. Mechanisms of Renal Blood Flow Autoregulation: Dynamics and Contributions. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R1–R17. [Google Scholar] [CrossRef]
- Mompeo, B.; Maranillo, E.; Garcia-Touchard, A.; Larkin, T.; Sanudo, J. The Gross Anatomy of the Renal Sympathetic Nerves Revisited. Clin. Anat. 2016, 29, 660–664. [Google Scholar] [CrossRef]
- Barajas, L.; Müller, J. The Innervation of the Juxtaglomerular Apparatus and Surrounding Tubules: A Quantitative Analysis by Serial Section Electron Microscopy. J. Ultrastruct. Res. 1973, 43, 107–132. [Google Scholar] [CrossRef]
- Barajas, L.; Liu, L.; Powers, K. Anatomy of the Renal Innervation: Intrarenal Aspects and Ganglia of Origin. Can. J. Physiol. Pharmacol. 1992, 70, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Genovesi, S.; Pieruzzi, F.; Wijnmaalen, P.; Centonza, L.; Golin, R.; Zanchetti, A.; Stella, A. Renal Afferents Signaling Diuretic Activity in the Cat. Circ. Res. 1993, 73, 906–913. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, A.; Langberg, H.; Holdaas, H.; Kiil, F. Glomerulotubular Balance during Renal Sympathetic Stimulation. Acta Physiol. Scand. 1986, 127, 187–195. [Google Scholar] [CrossRef]
- Kopp, U.C.; Cicha, M.Z.; Smith, L.A. Endogenous Angiotensin Modulates PGE(2)-Mediated Release of Substance P from Renal Mechanosensory Nerve Fibers. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 282, R19–R30. [Google Scholar] [CrossRef]
- Krüger-Genge, A.; Blocki, A.; Franke, R.-P.; Jung, F. Vascular Endothelial Cell Biology: An Update. Int. J. Mol. Sci. 2019, 20, 4411. [Google Scholar] [CrossRef] [Green Version]
- Mehta, D.; Malik, A.B. Signaling Mechanisms Regulating Endothelial Permeability. Physiol. Rev. 2006, 86, 279–367. [Google Scholar] [CrossRef]
- Garland, C.J.; Hiley, C.R.; Dora, K.A. EDHF: Spreading the Influence of the Endothelium. Br. J. Pharmacol. 2011, 164, 839–852. [Google Scholar] [CrossRef] [PubMed]
- Marti, C.N.; Gheorghiade, M.; Kalogeropoulos, A.P.; Georgiopoulou, V.V.; Quyyumi, A.A.; Butler, J. Endothelial Dysfunction, Arterial Stiffness, and Heart Failure. J. Am. Coll Cardiol. 2012, 60, 1455–1469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lubrano, V.; Balzan, S. Roles of LOX-1 in Microvascular Dysfunction. Microvasc. Res. 2016, 105, 132–140. [Google Scholar] [CrossRef]
- Gardiner, B.S.; Thompson, S.L.; Ngo, J.P.; Smith, D.W.; Abdelkader, A.; Broughton, B.R.S.; Bertram, J.F.; Evans, R.G. Diffusive Oxygen Shunting between Vessels in the Preglomerular Renal Vasculature: Anatomic Observations and Computational Modeling. Am. J. Physiol. Renal Physiol. 2012, 303, F605–F618. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Edwards, A. Oxygen Transport across Vasa Recta in the Renal Medulla. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H1042–H1055. [Google Scholar] [CrossRef] [Green Version]
- Sands, J.M.; Layton, H.E. The Physiology of Urinary Concentration: An Update. Semin. Nephrol. 2009, 29, 178–195. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, P.M.; Evans, R.G. Structural Antioxidant Defense Mechanisms in the Mammalian and Nonmammalian Kidney: Different Solutions to the Same Problem? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R723–R727. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, P.M.; Anderson, W.P.; Kett, M.M.; Evans, R.G. Renal Preglomerular Arterial-Venous O2 Shunting Is a Structural Anti-Oxidant Defence Mechanism of the Renal Cortex. Clin. Exp. Pharmacol. Physiol. 2006, 33, 637–641. [Google Scholar] [CrossRef]
- Lankadeva, Y.R.; Kosaka, J.; Evans, R.G.; Bailey, S.R.; Bellomo, R.; May, C.N. Intrarenal and Urinary Oxygenation during Norepinephrine Resuscitation in Ovine Septic Acute Kidney Injury. Kidney Int. 2016, 90, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Langenberg, C.; Bellomo, R.; May, C.; Wan, L.; Egi, M.; Morgera, S. Renal Blood Flow in Sepsis. Crit. Care 2005, 9, R363–R374. [Google Scholar] [CrossRef] [Green Version]
- Langenberg, C.; Wan, L.; Egi, M.; May, C.N.; Bellomo, R. Renal Blood Flow and Function during Recovery from Experimental Septic Acute Kidney Injury. Intensive Care Med. 2007, 33, 1614–1618. [Google Scholar] [CrossRef] [PubMed]
- Benes, J.; Chvojka, J.; Sykora, R.; Radej, J.; Krouzecky, A.; Novak, I.; Matejovic, M. Searching for Mechanisms That Matter in Early Septic Acute Kidney Injury: An Experimental Study. Crit. Care 2011, 15, R256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, R.G.; Gardiner, B.S.; Smith, D.W.; O’Connor, P.M. Intrarenal Oxygenation: Unique Challenges and the Biophysical Basis of Homeostasis. Am. J. Physiol. Renal Physiol. 2008, 295, F1259–F1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonventre, J.V.; Yang, L. Cellular Pathophysiology of Ischemic Acute Kidney Injury. J. Clin. Investig. 2011, 121, 4210–4221. [Google Scholar] [CrossRef] [PubMed]
- Cecconi, M.; Evans, L.; Levy, M.; Rhodes, A. Sepsis and Septic Shock. Lancet 2018, 392, 75–87. [Google Scholar] [CrossRef]
- Ergin, B.; Kapucu, A.; Demirci-Tansel, C.; Ince, C. The Renal Microcirculation in Sepsis. Nephrol. Dial. Transplant. 2015, 30, 169–177. [Google Scholar] [CrossRef] [Green Version]
- Grondman, I.; Pirvu, A.; Riza, A.; Ioana, M.; Netea, M.G. Biomarkers of Inflammation and the Etiology of Sepsis. Biochem. Soc. Trans. 2020, 48, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Cai, S.; Su, J. The Pathogenesis of Sepsis and Potential Therapeutic Targets. Int. J. Mol. Sci 2019, 20, 5376. [Google Scholar] [CrossRef] [Green Version]
- Calzavacca, P.; Evans, R.G.; Bailey, M.; Bellomo, R.; May, C.N. Cortical and Medullary Tissue Perfusion and Oxygenation in Experimental Septic Acute Kidney Injury. Crit. Care Med. 2015, 43, e431–e439. [Google Scholar] [CrossRef]
- Heemskerk, A.E.; Huisman, E.; van Lambalgen, A.A.; van den Bos, G.C.; Hennekes, M.; Thijs, L.G.; Tangelder, G.J. Renal Function and Oxygen Consumption during Bacteraemia and Endotoxaemia in Rats. Nephrol. Dial. Transplant. 1997, 12, 1586–1594. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, D.; Mendonca, M.; Schwartz, I.; Xia, Y.; Satriano, J.; Wilson, C.B.; Blantz, R.C. Inhibition of Constitutive Nitric Oxide Synthase (NOS) by Nitric Oxide Generated by Inducible NOS after Lipopolysaccharide Administration Provokes Renal Dysfunction in Rats. J. Clin. Investig. 1997, 100, 439–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ince, C. The Microcirculation Is the Motor of Sepsis. Crit. Care 2005, 9 (Suppl. 4), S13–S19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutton, T.A.; Mang, H.E.; Campos, S.B.; Sandoval, R.M.; Yoder, M.C.; Molitoris, B.A. Injury of the Renal Microvascular Endothelium Alters Barrier Function after Ischemia. Am. J. Physiol. Renal Physiol. 2003, 285, F191–F198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.; Ramnath, R.D.; Foster, R.R.; Wylie, E.C.; Fridén, V.; Dasgupta, I.; Haraldsson, B.; Welsh, G.I.; Mathieson, P.W.; Satchell, S.C. Reactive Oxygen Species Modulate the Barrier Function of the Human Glomerular Endothelial Glycocalyx. PLoS ONE 2013, 8, e55852. [Google Scholar] [CrossRef] [Green Version]
- Reggiori, G.; Occhipinti, G.; De Gasperi, A.; Vincent, J.-L.; Piagnerelli, M. Early Alterations of Red Blood Cell Rheology in Critically Ill Patients. Crit. Care Med. 2009, 37, 3041–3046. [Google Scholar] [CrossRef]
- Cosby, K.; Partovi, K.S.; Crawford, J.H.; Patel, R.P.; Reiter, C.D.; Martyr, S.; Yang, B.K.; Waclawiw, M.A.; Zalos, G.; Xu, X.; et al. Nitrite Reduction to Nitric Oxide by Deoxyhemoglobin Vasodilates the Human Circulation. Nat. Med. 2003, 9, 1498–1505. [Google Scholar] [CrossRef]
- Ince, C. Hemodynamic Coherence and the Rationale for Monitoring the Microcirculation. Crit. Care 2015, 19 (Suppl. 3), S8. [Google Scholar] [CrossRef] [Green Version]
- Ergin, B.; Guerci, P.; Zafrani, L.; Nocken, F.; Kandil, A.; Gurel-Gurevin, E.; Demirci-Tansel, C.; Ince, C. Effects of N-Acetylcysteine (NAC) Supplementation in Resuscitation Fluids on Renal Microcirculatory Oxygenation, Inflammation, and Function in a Rat Model of Endotoxemia. Intensive Care Med. Exp. 2016, 4, 29. [Google Scholar] [CrossRef] [Green Version]
- Aksu, U.; Bezemer, R.; Demirci, C.; Ince, C. Acute Effects of Balanced versus Unbalanced Colloid Resuscitation on Renal Macrocirculatory and Microcirculatory Perfusion during Endotoxemic Shock. Shock 2012, 37, 205–209. [Google Scholar] [CrossRef]
- Johannes, T.; Mik, E.G.; Nohé, B.; Raat, N.J.H.; Unertl, K.E.; Ince, C. Influence of Fluid Resuscitation on Renal Microvascular PO2 in a Normotensive Rat Model of Endotoxemia. Crit. Care 2006, 10, R88. [Google Scholar] [CrossRef] [Green Version]
- Lima, A.; van Rooij, T.; Ergin, B.; Sorelli, M.; Ince, Y.; Specht, P.A.C.; Mik, E.G.; Bocchi, L.; Kooiman, K.; de Jong, N.; et al. Dynamic Contrast-Enhanced Ultrasound Identifies Microcirculatory Alterations in Sepsis-Induced Acute Kidney Injury. Crit. Care Med. 2018, 46, 1284–1292. [Google Scholar] [CrossRef]
- Zayed, Y.; Alzghoul, B.N.; Banifadel, M.; Venigandla, H.; Hyde, R.; Sutchu, S.; Khasawneh, M.; Borok, Z.; Urbine, D.; Jantz, M.; et al. Vitamin C, Thiamine, and Hydrocortisone in the Treatment of Sepsis: A Meta-Analysis and Trial Sequential Analysis of Randomized Controlled Trials. J. Intensive Care Med. 2021, 885066620987809. [Google Scholar] [CrossRef]
- Zafrani, L.; Ergin, B.; Kapucu, A.; Ince, C. Blood Transfusion Improves Renal Oxygenation and Renal Function in Sepsis-Induced Acute Kidney Injury in Rats. Crit. Care 2016, 20, 406. [Google Scholar] [CrossRef] [Green Version]
- Guerci, P.; Ergin, B.; Kandil, A.; Ince, Y.; Heeman, P.; Hilty, M.P.; Bakker, J.; Ince, C. Resuscitation with PEGylated Carboxyhemoglobin Preserves Renal Cortical Oxygenation and Improves Skeletal Muscle Microcirculatory Flow during Endotoxemia. Am. J. Physiol. Renal Physiol. 2020, 318, F1271–F1283. [Google Scholar] [CrossRef] [PubMed]
- Peters, E.; Ergin, B.; Kandil, A.; Gurel-Gurevin, E.; van Elsas, A.; Masereeuw, R.; Pickkers, P.; Ince, C. Effects of a Human Recombinant Alkaline Phosphatase on Renal Hemodynamics, Oxygenation and Inflammation in Two Models of Acute Kidney Injury. Toxicol. Appl. Pharmacol. 2016, 313, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Lankadeva, Y.R.; Peiris, R.M.; Okazaki, N.; Birchall, I.E.; Trask-Marino, A.; Dornom, A.; Vale, T.A.M.; Evans, R.G.; Yanase, F.; Bellomo, R.; et al. Reversal of the Pathophysiological Responses to Gram-Negative Sepsis by Megadose Vitamin C. Crit. Care Med. 2021, 49, e179–e190. [Google Scholar] [CrossRef] [PubMed]
- Johannes, T.; Mik, E.G.; Klingel, K.; Dieterich, H.-J.; Unertl, K.E.; Ince, C. Low-Dose Dexamethasone-Supplemented Fluid Resuscitation Reverses Endotoxin-Induced Acute Renal Failure and Prevents Cortical Microvascular Hypoxia. Shock 2009, 31, 521–528. [Google Scholar] [CrossRef]
- Brouwer, W.P.; Duran, S.; Kuijper, M.; Ince, C. Hemoadsorption with CytoSorb Shows a Decreased Observed versus Expected 28-Day All-Cause Mortality in ICU Patients with Septic Shock: A Propensity-Score-Weighted Retrospective Study. Crit. Care 2019, 23, 317. [Google Scholar] [CrossRef] [Green Version]
- De Backer, D.; Creteur, J.; Dubois, M.-J.; Sakr, Y.; Vincent, J.-L. Microvascular Alterations in Patients with Acute Severe Heart Failure and Cardiogenic Shock. Am. Heart J. 2004, 147, 91–99. [Google Scholar] [CrossRef]
- Ashruf, J.F.; Bruining, H.A.; Ince, C. New Insights into the Pathophysiology of Cardiogenic Shock: The Role of the Microcirculation. Curr. Opin. Crit. Care 2013, 19, 381–386. [Google Scholar] [CrossRef]
- Den Uil, C.A.; Maat, A.P.; Lagrand, W.K.; van der Ent, M.; Jewbali, L.S.D.; van Thiel, R.J.; Spronk, P.E.; Simoons, M.L. Mechanical Circulatory Support Devices Improve Tissue Perfusion in Patients with End-Stage Heart Failure or Cardiogenic Shock. J. Heart Lung Transplant. 2009, 28, 906–911. [Google Scholar] [CrossRef]
- Den Uil, C.A.; Lagrand, W.K.; van der Ent, M.; Jewbali, L.S.D.; Cheng, J.M.; Spronk, P.E.; Simoons, M.L. Impaired Microcirculation Predicts Poor Outcome of Patients with Acute Myocardial Infarction Complicated by Cardiogenic Shock. Eur. Heart J. 2010, 31, 3032–3039. [Google Scholar] [CrossRef] [Green Version]
- Muslem, R.; Caliskan, K.; Akin, S.; Sharma, K.; Gilotra, N.A.; Brugts, J.J.; Houston, B.; Whitman, G.; Tedford, R.J.; Hesselink, D.A.; et al. Pre-Operative Proteinuria in Left Ventricular Assist Devices and Clinical Outcome. J. Heart Lung Transplant. 2018, 37, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Muslem, R.; Caliskan, K.; Akin, S.; Sharma, K.; Gilotra, N.A.; Constantinescu, A.A.; Houston, B.; Whitman, G.; Tedford, R.J.; Hesselink, D.A.; et al. Acute Kidney Injury and 1-Year Mortality after Left Ventricular Assist Device Implantation. J. Heart Lung Transplant. 2018, 37, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Muslem, R.; Caliskan, K.; Akin, S.; Yasar, Y.E.; Sharma, K.; Gilotra, N.A.; Kardys, I.; Houston, B.; Whitman, G.; Tedford, R.J.; et al. Effect of Age and Renal Function on Survival After Left Ventricular Assist Device Implantation. Am. J. Cardiol. 2017, 120, 2221–2225. [Google Scholar] [CrossRef] [PubMed]
- Wijntjens, G.W.; Fengler, K.; Fuernau, G.; Jung, C.; den Uil, C.; Akin, S.; van de Hoef, T.P.; Šerpytis, R.; Diletti, R.; Henriques, J.P.; et al. Prognostic Implications of Microcirculatory Perfusion versus Macrocirculatory Perfusion in Cardiogenic Shock: A CULPRIT-SHOCK Substudy. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, 108–119. [Google Scholar] [CrossRef] [Green Version]
- Kara, A.; Akin, S.; Dos Reis Miranda, D.; Struijs, A.; Caliskan, K.; van Thiel, R.J.; Dubois, E.A.; de Wilde, W.; Zijlstra, F.; Gommers, D.; et al. Microcirculatory Assessment of Patients under VA-ECMO. Crit. Care 2016, 20, 344. [Google Scholar] [CrossRef] [Green Version]
- Akil, A.; Ziegeler, S.; Reichelt, J.; Rehers, S.; Abdalla, O.; Semik, M.; Fischer, S. Combined Use of CytoSorb and ECMO in Patients with Severe Pneumogenic Sepsis. Thorac. Cardiovasc. Surg. 2021, 69, 246–251. [Google Scholar] [CrossRef]
- Bruenger, F.; Kizner, L.; Weile, J.; Morshuis, M.; Gummert, J.F. First Successful Combination of ECMO with Cytokine Removal Therapy in Cardiogenic Septic Shock: A Case Report. Int. J. Artif. Organs 2015, 38, 113–1162. [Google Scholar] [CrossRef]
- Träger, K.; Skrabal, C.; Fischer, G.; Schroeder, J.; Marenski, L.; Liebold, A.; Reinelt, H.; Datzmann, T. Hemoadsorption Treatment with CytoSorb® in Patients with Extracorporeal Life Support Therapy: A Case Series. Int. J. Artif. Organs 2020, 43, 422–429. [Google Scholar] [CrossRef]
- Arslan, M.; Comu, F.M.; Alkan, M.; Kiraz, H.A.; Kip, G.; Ozer, A.; Sivgin, V. Effect of Levosimendan on Erythrocyte Deformability during Myocardial Ischaemia-Reperfusion Injury. Bratisl Lek Listy 2015, 116, 47–50. [Google Scholar] [CrossRef] [Green Version]
- Jia, T.; Wang, S.; Luo, C.; Wang, Z.; Liu, G.; Shang, Z.; Lu, X.; Yang, Q.; Zhu, C. Levosimendan Ameliorates Post-Resuscitation Acute Intestinal Microcirculation Dysfunction Partly Independent of Its Effects on Systemic Circulation: A Pilot Study On Cardiac Arrest In A Rat Model. Shock 2021. [Google Scholar] [CrossRef]
- Onody, P.; Aranyi, P.; Turoczi, Z.; Stangl, R.; Fulop, A.; Dudas, E.; Lotz, G.; Szijarto, A. Levosimendan Administration in Limb Ischemia: Multicomponent Signaling Serving Kidney Protection. PLoS ONE 2016, 11, e01636755. [Google Scholar] [CrossRef] [Green Version]
- Shao, W.; Rosales, C.B.; Gonzalez, C.; Prieto, M.C.; Navar, L.G. Effects of Serelaxin on Renal Microcirculation in Rats under Control and High-Angiotensin Environments. Am. J. Physiol. Renal Physiol. 2018, 314, F70–F80. [Google Scholar] [CrossRef]
- Ergin, B.; Heger, M.; Kandil, A.; Demirci-Tansel, C.; Ince, C. Mycophenolate Mofetil Improves Renal Haemodynamics, Microvascular Oxygenation, and Inflammation in a Rat Model of Supra-Renal Aortic Clamping-Mediated Renal Ischaemia Reperfusion Injury. Clin. Exp. Pharmacol. Physiol. 2017, 44, 294–304. [Google Scholar] [CrossRef]
- Ergin, B.; Bezemer, R.; Kandil, A.; Demirci-Tansel, C.; Ince, C. TEMPOL Has Limited Protective Effects on Renal Oxygenation and Hemodynamics but Reduces Kidney Damage and Inflammation in a Rat Model of Renal Ischemia/Reperfusion by Aortic Clamping. J. Clin. Transl. Res. 2015, 116–128. [Google Scholar]
- Ergin, B.; Zuurbier, C.J.; Bezemer, R.; Kandil, A.; Almac, E.; Demirci, C.; Ince, C. Ascorbic Acid Improves Renal Microcirculatory Oxygenation in a Rat Model of Renal I/R Injury. J. Transl. Int. Med. 2015, 3, 116–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, P.D.; Lapointe, J.Y.; Peti-Peterdi, J. Macula Densa Cell Signaling. Annu. Rev. Physiol. 2003, 65, 481–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breyer, M.D.; Breyer, R.M. Prostaglandin E Receptors and the Kidney. Am. J. Physiol. Renal Physiol. 2000, 279, F12–F23. [Google Scholar] [CrossRef] [PubMed]
- Schramek, H.; Coroneos, E.; Dunn, M.J. Interactions of the Vasoconstrictor Peptides, Angiotensin II and Endothelin-1, with Vasodilatory Prostaglandins. Semin. Nephrol. 1995, 15, 195–204. [Google Scholar]
- Edwards, R.M. Effects of Prostaglandins on Vasoconstrictor Action in Isolated Renal Arterioles. Am. J. Physiol. 1985, 248, F779–F784. [Google Scholar] [CrossRef] [PubMed]
- Kucuk, A.; Kabadere, S.; Tosun, M.; Koken, T.; Kinaci, M.K.; Isikli, B.; Erkasap, N. Protective Effects of Doxycycline in Ischemia/Reperfusion Injury on Kidney. J. Physiol. Biochem. 2009, 65, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Erkasap, S.; Erkasap, N.; Koken, T.; Kahraman, A.; Uzuner, K.; Yazihan, N.; Ates, E. Effect of Leptin on Renal Ischemia-Reperfusion Damage in Rats. J. Physiol. Biochem. 2004, 60, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Grossini, E.; Molinari, C.; Pollesello, P.; Bellomo, G.; Valente, G.; Mary, D.; Vacca, G.; Caimmi, P. Levosimendan Protection against Kidney Ischemia/Reperfusion Injuries in Anesthetized Pigs. J. Pharmacol. Exp. Ther. 2012, 342, 376–388. [Google Scholar] [CrossRef] [Green Version]
- Döşlüoğlu, H.H.; Aktan, A.O.; Yeğen, C.; Okboy, N.; Yalçm, A.S.; Yahn, R.; Ercan, S. The Cytoprotective Effects of Verapamil and Iloprost (ZK 36374) on Ischemia/Reperfusion Injury of Kidneys. Transpl. Int. 1993, 6, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, A.; Kolankaya, D. The Protective Effects of Ascorbic Acid against Renal Ischemia-Reperfusion Injury in Male Rats. Ren. Fail. 2009, 31, 36–43. [Google Scholar] [CrossRef]
- Ergin, B.; Zuurbier, C.J.; Kapucu, A.; Ince, C. Divergent Effects of Hypertonic Fluid Resuscitation on Renal Pathophysiological and Structural Parameters in Rat Model of Lower Body Ischemia/Reperfusion-Induced Sterile Inflammation. Shock 2018, 50, 655–663. [Google Scholar] [CrossRef]
- Legrand, M.; Almac, E.; Mik, E.G.; Johannes, T.; Kandil, A.; Bezemer, R.; Payen, D.; Ince, C. L-NIL Prevents Renal Microvascular Hypoxia and Increase of Renal Oxygen Consumption after Ischemia-Reperfusion in Rats. Am. J. Physiol. Renal Physiol. 2009, 296, F1109–F1117. [Google Scholar] [CrossRef] [Green Version]
- Skrypnyk, N.I.; Voziyan, P.; Yang, H.; de Caestecker, C.R.; Theberge, M.-C.; Drouin, M.; Hudson, B.; Harris, R.C.; de Caestecker, M.P. Pyridoxamine Reduces Postinjury Fibrosis and Improves Functional Recovery after Acute Kidney Injury. Am. J. Physiol. Renal Physiol. 2016, 311, F268–F277. [Google Scholar] [CrossRef]
- De Bragança, A.C.; Volpini, R.A.; Canale, D.; Gonçalves, J.G.; Shimizu, M.H.M.; Sanches, T.R.; Seguro, A.C.; Andrade, L. Vitamin D Deficiency Aggravates Ischemic Acute Kidney Injury in Rats. Physiol. Rep. 2015, 3, e12331. [Google Scholar] [CrossRef]
- Cortes, A.L.; Gonsalez, S.R.; Rioja, L.S.; Oliveira, S.S.C.; Santos, A.L.S.; Prieto, M.C.; Melo, P.A.; Lara, L.S. Protective Outcomes of Low-Dose Doxycycline on Renal Function of Wistar Rats Subjected to Acute Ischemia/Reperfusion Injury. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 102–114. [Google Scholar] [CrossRef]
- Tögel, F.; Hu, Z.; Weiss, K.; Isaac, J.; Lange, C.; Westenfelder, C. Administered Mesenchymal Stem Cells Protect against Ischemic Acute Renal Failure through Differentiation-Independent Mechanisms. Am. J. Physiol. Renal Physiol. 2005, 289, F31–F42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hultström, M. Neurohormonal Interactions on the Renal Oxygen Delivery and Consumption in Haemorrhagic Shock-Induced Acute Kidney Injury. Acta Physiol. (Oxf.) 2013, 209, 11–25. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Yeh, Y.-C.; Chien, C.-T.; Chao, A.; Sun, W.-Z.; Cheng, Y.-J. NTUH Center of Microcirculation Medical Research (NCMMR) Laser Speckle Contrast Imaging for Assessing Microcirculatory Changes in Multiple Splanchnic Organs and the Gracilis Muscle during Hemorrhagic Shock and Fluid Resuscitation. Microvasc. Res. 2015, 101, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Aksu, U.; Bezemer, R.; Yavuz, B.; Kandil, A.; Demirci, C.; Ince, C. Balanced vs Unbalanced Crystalloid Resuscitation in a Near-Fatal Model of Hemorrhagic Shock and the Effects on Renal Oxygenation, Oxidative Stress, and Inflammation. Resuscitation 2012, 83, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Ergin, B.; Kapucu, A.; Guerci, P.; Ince, C. The Role of Bicarbonate Precursors in Balanced Fluids during Haemorrhagic Shock with and without Compromised Liver Function. Br. J. Anaesth. 2016, 117, 521–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legrand, M.; Mik, E.G.; Balestra, G.M.; Lutter, R.; Pirracchio, R.; Payen, D.; Ince, C. Fluid Resuscitation Does Not Improve Renal Oxygenation during Hemorrhagic Shock in Rats. Anesthesiology 2010, 112, 119–127. [Google Scholar] [CrossRef] [Green Version]
- Guerci, P.; Ergin, B.; Kapucu, A.; Hilty, M.P.; Jubin, R.; Bakker, J.; Ince, C. Effect of Polyethylene-Glycolated Carboxyhemoglobin on Renal Microcirculation in a Rat Model of Hemorrhagic Shock. Anesthesiology 2019, 131, 1110–1124. [Google Scholar] [CrossRef] [Green Version]
- Guerci, P.; Ergin, B.; Uz, Z.; Ince, Y.; Westphal, M.; Heger, M.; Ince, C. Glycocalyx Degradation Is Independent of Vascular Barrier Permeability Increase in Nontraumatic Hemorrhagic Shock in Rats. Anesth. Analg. 2018, 129, 607–698. [Google Scholar] [CrossRef]
- Yamamoto, M.; Horinouchi, H.; Kobayashi, K.; Seishi, Y.; Sato, N.; Itoh, M.; Sakai, H. Fluid Resuscitation of Hemorrhagic Shock with Hemoglobin Vesicles in Beagle Dogs: Pilot Study. Artif. Cells Blood Substit. Immobil. Biotechnol. 2012, 40, 179–195. [Google Scholar] [CrossRef]
- Terajima, K.; Tsueshita, T.; Sakamoto, A.; Ogawa, R. Fluid Resuscitation with Hemoglobin Vesicles in a Rabbit Model of Acute Hemorrhagic Shock. Shock 2006, 25, 184–189. [Google Scholar] [CrossRef]
- Marhefka, J.N.; Zhao, R.; Wu, Z.J.; Velankar, S.S.; Antaki, J.F.; Kameneva, M.V. Drag Reducing Polymers Improve Tissue Perfusion via Modification of the RBC Traffic in Microvessels. Biorheology 2009, 46, 281–292. [Google Scholar] [CrossRef]
- Kameneva, M.V.; Wu, Z.J.; Uraysh, A.; Repko, B.; Litwak, K.N.; Billiar, T.R.; Fink, M.P.; Simmons, R.L.; Griffith, B.P.; Borovetz, H.S. Blood Soluble Drag-Reducing Polymers Prevent Lethality from Hemorrhagic Shock in Acute Animal Experiments. Biorheology 2004, 41, 53–64. [Google Scholar] [PubMed]
- Macias, C.A.; Kameneva, M.V.; Tenhunen, J.J.; Puyana, J.-C.; Fink, M.P. Survival in a Rat Model of Lethal Hemorrhagic Shock Is Prolonged Following Resuscitation with a Small Volume of a Solution Containing a Drag-Reducing Polymer Derived from Aloe Vera. Shock 2004, 22, 151–156. [Google Scholar] [CrossRef] [PubMed]
- McCloskey, C.A.; Kameneva, M.V.; Uryash, A.; Gallo, D.J.; Billiar, T.R. Tissue Hypoxia Activates JNK in the Liver during Hemorrhagic Shock. Shock 2004, 22, 380–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Huang, T.; Dong, Z. Protective Effects of Polyethylene Oxide on the Vascular and Organ Function of Rats with Severe Hemorrhagic Shock. Can. J. Physiol. Pharmacol. 2015, 93, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Lehot, J.J.; Villard, J.; Piriz, H.; Philbin, D.M.; Carry, P.Y.; Gauquelin, G.; Claustrat, B.; Sassolas, G.; Galliot, J.; Estanove, S. Hemodynamic and Hormonal Responses to Hypothermic and Normothermic Cardiopulmonary Bypass. J. Cardiothorac. Vasc. Anesth. 1992, 6, 132–139. [Google Scholar] [CrossRef]
- Kono, K.; Philbin, D.M.; Coggins, C.H.; Slater, E.E.; Triantafillou, A.; Levine, F.H.; Buckley, M.J. Adrenocortical Hormone Levels during Cardiopulmonary Bypass with and without Pulsatile Flow. J. Thorac. Cardiovasc. Surg. 1983, 85, 129–133. [Google Scholar] [CrossRef]
- Taylor, K.M.; Bain, W.H.; Russell, M.; Brannan, J.J.; Morton, I.J. Peripheral Vascular Resistance and Angiotensin II Levels during Pulsatile and Non-Pulsatile Cardiopulmonary Bypass. Thorax 1979, 34, 594–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majid, D.S.; Navar, L.G. Nitric Oxide in the Control of Renal Hemodynamics and Excretory Function. Am. J. Hypertens. 2001, 14, 74S–82S. [Google Scholar] [CrossRef] [Green Version]
- Denton, K.M.; Shweta, A.; Finkelstein, L.; Flower, R.L.; Evans, R.G. Effect of Endothelin-1 on Regional Kidney Blood Flow and Renal Arteriole Calibre in Rabbits. Clin. Exp. Pharmacol. Physiol. 2004, 31, 494–501. [Google Scholar] [CrossRef]
- Omoro, S.A.; Majid, D.S.; El-Dahr, S.S.; Navar, L.G. Kinin Influences on Renal Regional Blood Flow Responses to Angiotensin-Converting Enzyme Inhibition in Dogs. Am. J. Physiol. 1999, 276, F271–F277. [Google Scholar] [CrossRef]
- Lannemyr, L.; Bragadottir, G.; Krumbholz, V.; Redfors, B.; Sellgren, J.; Ricksten, S.-E. Effects of Cardiopulmonary Bypass on Renal Perfusion, Filtration, and Oxygenation in Patients Undergoing Cardiac Surgery. Anesthesiology 2017, 126, 205–213. [Google Scholar] [CrossRef]
- Johannes, T.; Mik, E.G.; Nohé, B.; Unertl, K.E.; Ince, C. Acute Decrease in Renal Microvascular PO2 during Acute Normovolemic Hemodilution. Am. J. Physiol. Renal Physiol. 2007, 292, F796–F803. [Google Scholar] [CrossRef] [PubMed]
- Van Bommel, J.; Siegemund, M.; Henny, C.P.; Ince, C. Heart, Kidney, and Intestine Have Different Tolerances for Anemia. Transl. Res. 2008, 151, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Koning, N.J.; de Lange, F.; Vonk, A.B.A.; Ahmed, Y.; van den Brom, C.E.; Bogaards, S.; van Meurs, M.; Jongman, R.M.; Schalkwijk, C.G.; Begieneman, M.P.V.; et al. Impaired Microcirculatory Perfusion in a Rat Model of Cardiopulmonary Bypass: The Role of Hemodilution. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H550–H558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morariu, A.M.; Maathuis, M.-H.J.; Asgeirsdottir, S.A.; Leuvenink, H.G.D.; Boonstra, P.W.; van Oeveren, W.; Ploeg, R.J.; Molema, I.; Rakhorst, G. Acute Isovolemic Hemodilution Triggers Proinflammatory and Procoagulatory Endothelial Activation in Vital Organs: Role of Erythrocyte Aggregation. Microcirculation 2006, 13, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Konrad, F.M.; Mik, E.G.; Bodmer, S.I.A.; Ates, N.B.; Willems, H.F.E.M.; Klingel, K.; de Geus, H.R.H.; Stolker, R.J.; Johannes, T. Acute Normovolemic Hemodilution in the Pig Is Associated with Renal Tissue Edema, Impaired Renal Microvascular Oxygenation, and Functional Loss. Anesthesiology 2013, 119, 256–269. [Google Scholar] [CrossRef] [Green Version]
- Perazella, M.A. Drug-Induced Acute Kidney Injury: Diverse Mechanisms of Tubular Injury. Curr. Opin. Crit. Care 2019, 25, 550–557. [Google Scholar] [CrossRef]
- Schetz, M.; Dasta, J.; Goldstein, S.; Golper, T. Drug-Induced Acute Kidney Injury. Curr. Opin. Crit. Care 2005, 11, 555–565. [Google Scholar] [CrossRef]
- Wu, H.; Huang, J. Drug-Induced Nephrotoxicity: Pathogenic Mechanisms, Biomarkers and Prevention Strategies. Curr. Drug Metab. 2018, 19, 559–567. [Google Scholar] [CrossRef]
- Lopez-Novoa, J.M.; Quiros, Y.; Vicente, L.; Morales, A.I.; Lopez-Hernandez, F.J. New Insights into the Mechanism of Aminoglycoside Nephrotoxicity: An Integrative Point of View. Kidney Int. 2011, 79, 33–45. [Google Scholar] [CrossRef] [Green Version]
- Deray, G. Amphotericin B Nephrotoxicity. J. Antimicrob Chemother. 2002, 49 (Suppl. 1), 37–41. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, G.; Reeves, W.B. Inflammatory Cytokines in Acute Renal Failure. Kidney Int. Suppl. 2004, 66, S56–S61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perazella, M.A. Drug-Induced Nephropathy: An Update. Expert Opin. Drug Saf. 2005, 4, 689–706. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.; Justo, P.; Sanz, A.; Melero, R.; Caramelo, C.; Guerrero, M.F.; Strutz, F.; Müller, G.; Barat, A.; Egido, J. Tubular Cell Apoptosis and Cidofovir-Induced Acute Renal Failure. Antivir. Ther. 2005, 10, 185–190. [Google Scholar]
- Izzedine, H.; Hulot, J.S.; Launay-Vacher, V.; Marcellini, P.; Hadziyannis, S.J.; Currie, G.; Brosgart, C.L.; Westland, C.; Arterbrun, S.; Deray, G.; et al. Renal Safety of Adefovir Dipivoxil in Patients with Chronic Hepatitis B: Two Double-Blind, Randomized, Placebo-Controlled Studies. Kidney Int. 2004, 66, 1153–1158. [Google Scholar] [CrossRef] [Green Version]
- Heyman, S.N.; Reichman, J.; Brezis, M. Pathophysiology of Radiocontrast Nephropathy: A Role for Medullary Hypoxia. Investig. Radiol. 1999, 34, 685–691. [Google Scholar] [CrossRef]
- Chang, C.-F.; Lin, C.-C. Current Concepts of Contrast-Induced Nephropathy: A Brief Review. J. Chin. Med. Assoc. 2013, 76, 673–681. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Tang, Y.; Liu, T.; Zhang, N.; Yang, X.; Yang, D.; Hong, G. A Novel Antioxidant Protects Against Contrast Medium-Induced Acute Kidney Injury in Rats. Front. Pharmacol. 2020, 11, 599577. [Google Scholar] [CrossRef]
- Brown, J.R.; Block, C.A.; Malenka, D.J.; O’Connor, G.T.; Schoolwerth, A.C.; Thompson, C.A. Sodium Bicarbonate plus N-Acetylcysteine Prophylaxis: A Meta-Analysis. JACC Cardiovasc. Interv. 2009, 2, 1116–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Z.; Cheng, D.; Yin, J.; Wu, R.; Zhang, G.; Zhao, Q.; Wang, N.; Wang, F.; Liang, M. Antithrombin III Protects Against Contrast-Induced Nephropathy. EBioMedicine 2017, 17, 101–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morigi, M.; Benigni, A. Mesenchymal Stem Cells and Kidney Repair. Nephrol. Dial. Transplant. 2013, 28, 788–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruno, S.; Grange, C.; Deregibus, M.C.; Calogero, R.A.; Saviozzi, S.; Collino, F.; Morando, L.; Busca, A.; Falda, M.; Bussolati, B.; et al. Mesenchymal Stem Cell-Derived Microvesicles Protect against Acute Tubular Injury. J. Am. Soc. Nephrol. 2009, 20, 1053–10678. [Google Scholar] [CrossRef] [Green Version]
- Kaushal, G.P.; Shah, S.V. Challenges and Advances in the Treatment of AKI. J. Am. Soc. Nephrol. 2014, 25, 877–883. [Google Scholar] [CrossRef]


| Types of AKI | Innovative Therapies |
|---|---|
| Sepsis-induced AKI | BTx, HBOCs, vitamins (NAC, vitamin C, and thiamine), alkaline phosphatase, corticosteroids (hydrocortisone and dexamethasone), hemoadsorption |
| Cardiogenic shock-induced AKI | ECMO, LVAD, hemoadsorption Serelaxin |
| I/R-induced AKI | iNOS inhibition (L-NIL), antioxidant agents (TEMPOL, vitamin C, pyridoxamine, and vit D), anti-inflammatory agents (MFM), iloprost, levosimendan, and leptin |
| Hypovolemic/hemorrhagic shock-induced AKI | HBOCs, HbV, DRPs |
| Hemodilution-induced AKI | HBOCs, HbV, DRPs, cell salvage |
| Drug-induced AKI | Antioxidant and anti-inflammatory supports, MSCs |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ergin, B.; Akin, S.; Ince, C. Kidney Microcirculation as a Target for Innovative Therapies in AKI. J. Clin. Med. 2021, 10, 4041. https://doi.org/10.3390/jcm10184041
Ergin B, Akin S, Ince C. Kidney Microcirculation as a Target for Innovative Therapies in AKI. Journal of Clinical Medicine. 2021; 10(18):4041. https://doi.org/10.3390/jcm10184041
Chicago/Turabian StyleErgin, Bülent, Sakir Akin, and Can Ince. 2021. "Kidney Microcirculation as a Target for Innovative Therapies in AKI" Journal of Clinical Medicine 10, no. 18: 4041. https://doi.org/10.3390/jcm10184041






