Selective Arterial Embolization of Renal Angiomyolipomas with a N-Butyl Cyanoacrylate-Lipiodol Mixture: Efficacy, Safety, Short- and Mid-Term Outcomes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Diagnosis and Tumor Size
2.3. Selective Arterial Embolization (SAE)
2.4. Follow-Up
2.5. Statistical Analyses
3. Results
3.1. Patients
3.2. Selective Arterial Embolization (SAE)
3.3. Complications
3.4. Other Outcomes
3.5. Prognostic Factors of Angiomyolipoma Shrinkage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hajdu, S.I.; Foote, F.W. Angiomyolipoma of the kidney: Report of 27 cases and review of the literature. J. Urol. 1969, 102, 396–401. [Google Scholar] [CrossRef]
- Morgan, G.S.; Straumfjord, J.V.; Hall, E.J. Angiomyolipoma of the kidney. J. Urol. 1951, 65, 525–527. [Google Scholar] [CrossRef]
- Nelson, C.P.; Sanda, M.G. Contemporary diagnosis and management of renal angiomyolipoma. J. Urol. 2002, 168, 1315–1325. [Google Scholar] [CrossRef]
- Bhatt, J.R.; Richard, P.O.; Kim, N.S.; Finelli, A.; Manickavachagam, K.; Legere, L.; Evans, A.; Pei, Y.; Sykes, J.; Jhaveri, K.; et al. Natural history of renal angiomyolipoma (AML): Most patients with large AMLs >4 cm can be offered active surveillance as an initial management strategy. Eur. Urol. 2016, 70, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Seyam, R.M.; Bissada, N.K.; Kattan, S.A.; Mokhtar, A.A.; Aslam, M.; Fahmy, W.E.; Mourad, W.A.; Binmahfouz, A.A.; Alzahrani, H.M.; Hanash, K.A. Changing trends in presentation, diagnosis and management of renal angiomyolipoma: Comparison of sporadic and tuberous sclerosis complex-associated forms. Urology 2008, 72, 1077–1782. [Google Scholar] [CrossRef]
- Lendvay, T.S.; Marshall, F.F. The tuberous sclerosis complex and its highly variable manifestations. J. Urol. 2003, 169, 1635–1642. [Google Scholar] [CrossRef]
- Ramon, J.; Rimon, U.; Garniek, A.; Golan, G.; Bensaid, P.; Kitrey, N.D.; Nadu, A.; Dotan, Z.A. Renal angiomyolipoma: Long-term results following selective arterial embolization. Eur. Urol. 2009, 55, 1155–1161. [Google Scholar] [CrossRef]
- Yamakado, K.; Tanaka, N.; Nakagawa, T.; Kobayashi, S.; Yanagawa, M.; Takeda, K. Renal angiomyolipoma: Relationships between tumor size, aneurysm formation, and rupture. Radiology 2002, 225, 78–82. [Google Scholar] [CrossRef]
- Chang, Y.H.; Wang, L.L.; Chuang, C.K.; Wong, Y.C.; Wu, C.T.; Hsieh, M.L. The efficacy and outcomes of urgent superselective transcatheter arterial embolization of patients with ruptured renal angiomyolipomas. J. Trauma Acute Care Surg. 2007, 62, 1487–1490. [Google Scholar] [CrossRef]
- Murray, T.E.; Doyle, F.; Lee, M. Transarterial embolization of angiomyolipoma: A systematic review. J. Urol. 2015, 194, 635–639. [Google Scholar] [CrossRef]
- Villalta, J.D.; Sorensen, M.D.; Durack, J.C.; Kerlan, R.K.; Stoller, M.L. Selective arterial embolization of angiomyolipomas: A comparison of smaller and larger embolic agents. J. Urol. 2011, 186, 921–927. [Google Scholar] [CrossRef] [Green Version]
- Urbano, J.; Paul, L.; Cabrera, M.; Alonso-Burgos, A.; Gómez, D. Elective and emergency renal angiomyolipoma embolization with ethylene vinyl alcohol copolymer: Feasibility and initial experience. J. Vasc. Interv. Radiol. 2017, 28, 832–839. [Google Scholar] [CrossRef]
- Thulasidasan, N.; Sriskandakumar, S.; Ilyas, S.; Sabharwal, T. Renal angiomyolipoma: Mid- to long-term results following embolization with Onyx. Cardiovasc. Interv. Radiol. 2016, 39, 1759–1764. [Google Scholar] [CrossRef]
- Katsanos, K.; Sabharwal, T.; Ahmad, F.; Dourado, R.; Adam, A. Onyx embolization of sporadic angiomyolipoma. Cardiovasc. Interv. Radiol. 2009, 32, 1291–1295. [Google Scholar] [CrossRef]
- Dotter, C.T.; Goldman, M.L.; Rösch, J. Instant selective arterial occlusion with isobutyl 2-cyanoacrylate. Radiology 1975, 114, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Loffroy, R.; Guiu, B.; Cercueil, J.P.; Krausé, D. Endovascular therapeutic embolisation: An overview of occluding agents and their effects on embolised tissues. Curr. Vasc. Pharmacol. 2009, 7, 250–263. [Google Scholar] [CrossRef]
- Davenport, M.S.; Neville, A.M.; Ellis, J.H.; Cohan, R.H.; Chaudhry, H.S.; Leder, R.A. Diagnosis of renal angiomyolipoma with hounsfield unit thresholds: Effect of size of region of interest and nephrographic phase imaging. Radiology 2011, 260, 158–165. [Google Scholar] [CrossRef]
- Tomita, K.; Matsumoto, T.; Kamei, S.; Yamamoto, S.; Suda, S.; Zakoji, H.; Hasebe, T. Transcatheter arterial embolization for unruptured renal angiomyolipoma using a 1.8-Fr tip microballoon catheter with a mixture of ethanol and Lipiodol. CVIR Endovasc. 2020, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Kothary, N.; Soulen, M.C.; Clark, T.W.I.; Wein, A.J.; Shlansky-Goldberg, R.D.; Crino, P.B.; Stavropoulos, S.W. Renal angiomyolipoma: Long-term results after arterial embolization. J. Vasc. Interv. Radiol. 2005, 16, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Angle, J.F.; Siddiqi, N.H.; Wallace, M.J.; Kundu, S.; Stokes, L.; Wojak, J.C.; Cardella, J.F.; Society of Interventional Radiology Standards of Practice Committee. Quality improvement guidelines for percutaneous transcatheter embolization: Society of Interventional Radiology Standards of Practice Committee. J. Vasc. Interv. Radiol. 2010, 21, 1479–1486. [Google Scholar] [CrossRef]
- Adler, J.; Greweldinger, J.; Litzky, G. « Macro » aneurysm in renal angiomyolipoma: Two cases, with therapeutic embolization in one patient. Urol. Radiol. 1984, 6, 201–203. [Google Scholar] [CrossRef]
- Chan, C.K.; Yu, S.; Yip, S.; Lee, P. The efficacy, safety and durability of selective renal arterial embolization in treating symptomatic and asymptomatic renal angiomyolipoma. Urology 2011, 77, 642–648. [Google Scholar] [CrossRef]
- Lin, L.; Li, X.; Guan, H.; Wang, J.; Tong, X.; Yang, M.; Zou, Y. Renal function, complications, and outcomes of a reduction in tumor size after transarterial embolization for renal angiomyolipomas: A meta-analysis. J. Int. Med. Res. 2019, 47, 1417–1428. [Google Scholar] [CrossRef] [PubMed]
- Kocakgol, D.O.; Cayli, E.; Oguz, S.; Dinc, H. Selective arterial embolization of giant renal angiomyolipoma associated with tuberous sclerosis complex using particular and liquid embolic agents. Eurasian J. Med. 2018, 50, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Caloggero, S.; Catanzariti, F.; Stagno, A.; Silipigni, S.; Bottari, A. Use of a mixture of lipiodol and cyanoacrylate in percutaneous embolization treatment of symptomatic renal angiomyolipomas: Our experience. Radiol. Case Rep. 2019, 14, 343–347. [Google Scholar] [CrossRef]
- Vinters, H.V.; Galil, K.A.; Lundie, M.J.; Kaufmann, J.C. The histotoxicity of cyanoacrylates. A selective review. Neuroradiology 1985, 27, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Hocquelet, A.; Cornelis, F.; Le Bras, Y.; Meyer, M.; Tricaud, E.; Lasserre, A.S.; Ferrière, J.M.; Robert, G.; Grenier, N. Long-term results of preventive embolization of renal angiomyolipomas: Evaluation of predictive factors of volume decrease. Eur. Radiol. 2014, 24, 1785–1793. [Google Scholar] [CrossRef]
- Steiner, M.S.; Goldman, S.M.; Fishman, E.K.; Marshall, F.F. The natural history of renal angiomyolipoma. J. Urol. 1993, 150, 1782–1786. [Google Scholar] [CrossRef]
- Oesterling, J.E.; Fishman, E.K.; Goldman, S.M.; Marshall, F.F. The management of renal angiomyolipoma. J. Urol. 1986, 135, 1121–1124. [Google Scholar] [CrossRef]
- Castle, S.M.; Gorbatiy, V.; Ekwenna, O.; Young, E.; Leveillee, R.J. Radiofrequency ablation (RFA) therapy for renal angiomyolipoma (AML): An alternative to angio-embolization and nephron-sparing surgery. BJU 2012, 109, 384–387. [Google Scholar] [CrossRef] [Green Version]
- Sasiwimonphan, K.; Takahashi, N.; Leibovich, B.C.; Carter, R.E.; Atwell, T.D.; Kawashima, A. Small (<4 cm) renal mass: Differentiation of angiomyolipoma without visible fat from renal cell carcinoma utilizing MR imaging. Radiology 2012, 263, 160–168. [Google Scholar] [CrossRef]
- Bardin, F.; Chevallier, O.; Bertaut, A.; Delorme, E.; Moulin, M.; Pottecher, P.; Di Marco, L.; Gehin, S.; Mourey, E.; Cormier, L.; et al. Selective arterial embolization of symptomatic and asymptomatic renal angiomyolipomas: A retrospective study of safety, outcomes and tumor size reduction. Quant. Imaging Med. Surg. 2017, 7, 8–23. [Google Scholar] [CrossRef] [Green Version]
- Sheth, R.A.; Feldman, A.S.; Paul, E.; Thiele, E.A.; Walker, T.G. Sporadic versus Tuberous Sclerosis Complex-associated angiomyolipomas: Predictors for long-term outcomes following transcatheter embolization. J. Vasc. Interv. Radiol. 2016, 27, 1542–1549. [Google Scholar] [CrossRef]
- Flum, A.S.; Hamoui, N.; Said, M.A.; Yang, X.J.; Casalino, D.D.; McGuire, B.B.; Perry, K.T.; Nadler, R.B. Update on the diagnosis and management of renal angiomyolipoma. J. Urol. 2016, 195, 834–846. [Google Scholar] [CrossRef] [PubMed]
- Planché, O.; Correas, J.M.; Mader, B.; Joly, D.; Méjean, A.; Hélénon, O. Prophylactic embolization of renal angiomyolipomas: Evaluation of therapeutic response using CT 3D volume calculation and density histograms. J. Vasc. Interv. Radiol. 2011, 22, 1388–1395. [Google Scholar] [CrossRef] [PubMed]
- Chatziioannou, A.; Gargas, D.; Malagari, K.; Kornezos, I.; Ioannidis, I.; Primetis, E.; Moschouris, H.; Gouliamos, A.; Mourikis, D. Transcatheter arterial embolization as therapy of renal angiomyolipomas: The evolution in 15 years of experience. Eur. J. Radiol. 2012, 81, 2308–2312. [Google Scholar] [CrossRef] [PubMed]
- Rimon, U.; Duvdevani, M.; Garniek, A.; Golan, G.; Bensaid, P.; Ramon, J.; Morag, B. Large renal angiomyolipomas: Digital subtraction angiographic grading and presentation with bleeding. Clin. Radiol. 2006, 61, 520–526. [Google Scholar] [CrossRef]
- Patatas, K.; Robinson, G.J.; Ettles, D.F.; Lakshminarayan, R. Patterns of renal angiomyolipoma regression post embolisation on medium- to long-term follow-up. Br. J. Radiol. 2013, 86, 20120633. [Google Scholar] [CrossRef] [Green Version]
- Williams, J.M.; Racadio, J.M.; Johnson, N.D.; Donnelly, L.F.; Bissler, J.J. Embolization of renal angiomyolipomata in patients with tuberous sclerosis complex. Am. J. Kidney Dis. 2006, 47, 95–102. [Google Scholar] [CrossRef]
- Edelman, M.A.; Mitty, H.A.; Dan, S.J.; Birns, D.R. Angiomyolipoma: Postembolization liquefaction and percutaneous drainage. Urol. Radiol. 1990, 12, 145–147. [Google Scholar] [CrossRef]
- Boorjian, S.A.; Frank, I.; Inman, B.; Lohse, C.M.; Cheville, J.C.; Leibovich, B.C.; Blute, M.L. The role of partial nephrectomy for the management of sporadic renal angiomyolipoma. Urology 2007, 70, 1064–1068. [Google Scholar] [CrossRef] [PubMed]
- Baba, Y.; Hayashi, S.; Ikeda, S.; Jinguji, M.; Nakajo, M.; Nakajo, M. Evaluation of split renal function before and after renal arterial embolization for angiomyolipoma using absolute ethanol. Cardiovasc. Interv. Radiol. 2014, 37, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Hsu, H.H.; Chen, Y.C.; Huang, C.C.; Wong, Y.C.; Wang, L.J.; Chuang, C.K.; Yang, C.W. Evaluation of renal function of angiomyolipoma patients after selective transcatheter arterial embolization. Am. J. Med. Sci. 2009, 337, 103–108. [Google Scholar] [CrossRef]
- Bissler, J.J.; Kingswood, J.C.; Radzikowska, E.; Zonnenberg, B.A.; Frost, M.; Belousova, E.; Sauter, M.; Nonomura, N.; Brakemeier, S.; de Vries, P.J.; et al. Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): A multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2013, 381, 817–824. [Google Scholar] [CrossRef]
- Bissler, J.J.; Budde, K.; Sauter, M.; Franz, D.N.; Zonnenberg, B.A.; Frost, M.D.; Belousova, E.; Berkowitz, N.; Ridolfi, A.; Kingswood, J.C. Effect of everolimus on renal function in patients with tuberous sclerosis complex: Evidence from EXIST-1 and EXIST-2. Nephrol. Dial. Transplant. 2019, 34, 1000–1008. [Google Scholar] [CrossRef]
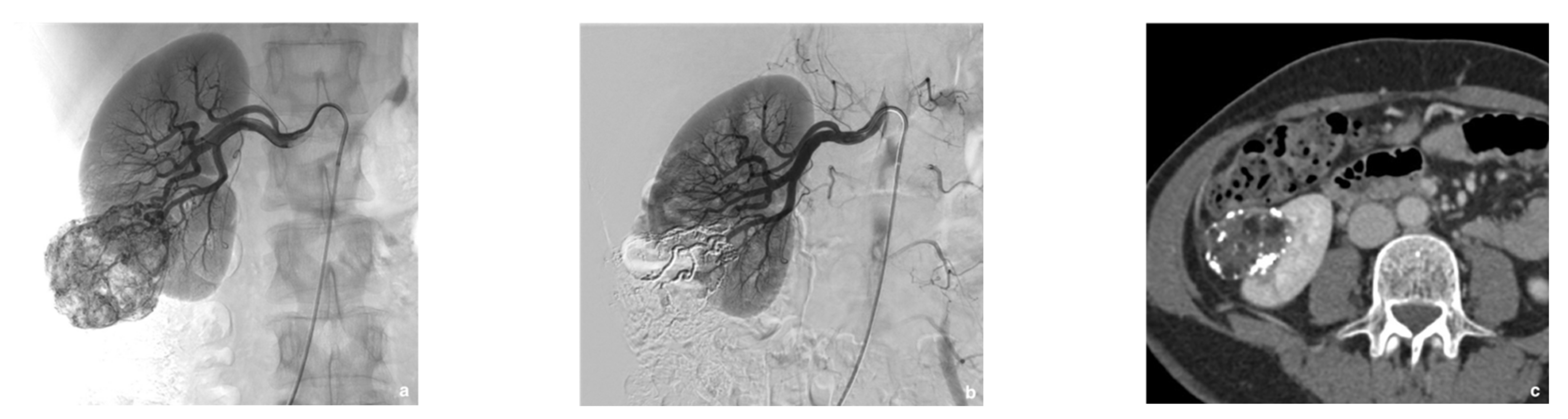
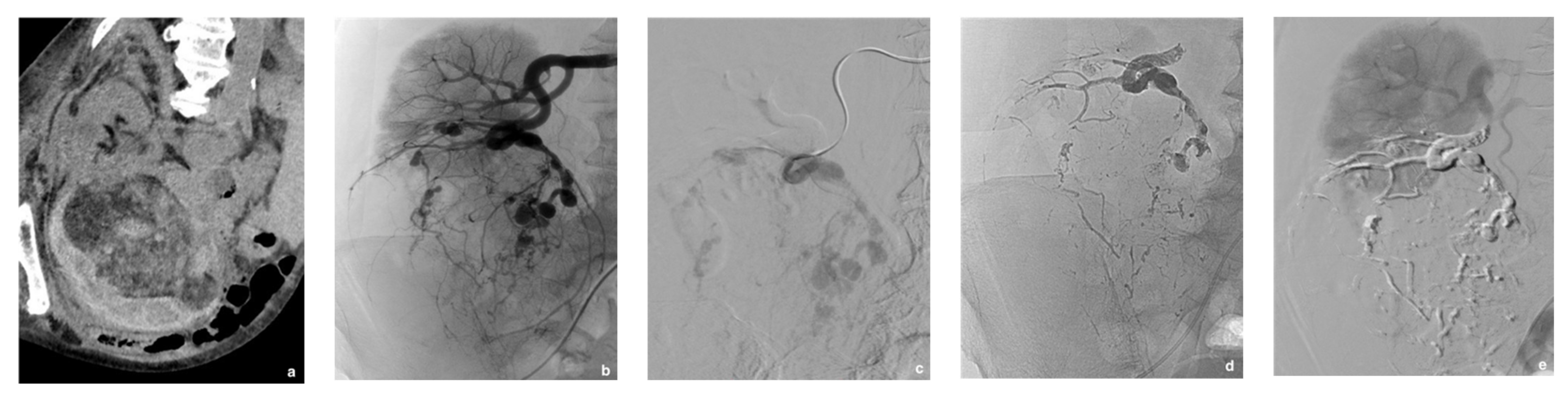
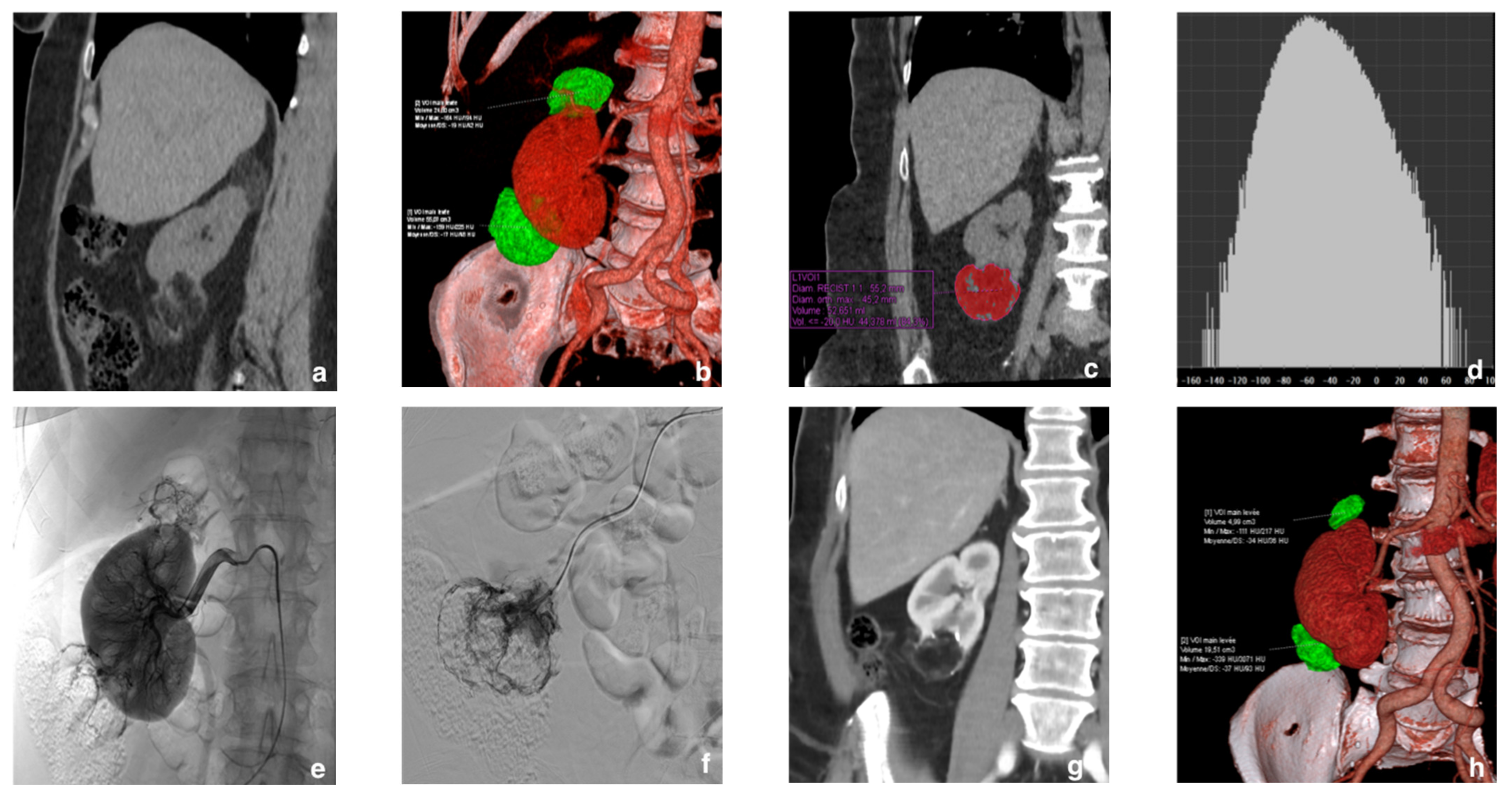
| Variable | Data |
|---|---|
| No. of patients | 24 |
| No. of embolized rAMLs | 27 |
| Females/Males, n (%) | 22 (91.6)/2 (8.3) |
| Age, y, mean ± SD | 51 ± 20.8 |
| Tuberous sclerosis, n (%) | 5 (20.8) |
| Everolimus treatment, n (%) | 5 (20.8) |
| Indication of SAE, n (%) | |
| Prophylactic SAE: prevention of bleeding a | 20 (83.3) |
| Bleeding requiring emergency treatment | 4 (16.7) |
| Clinical presentation of rAML, n (%) | |
| Shock | 3 (12.5) |
| Retroperitoneal bleeding | 4 (16.7) |
| Hematuria | 2 (8.3) |
| Pain | 13 (54.2) |
| No symptoms | 11 (45.8) |
| Multiple rAMLs, n (%) | 7 (29.2) |
| Bilateral rAMLs, n (%) | 7 (29.2) |
| Presence of aneurysms, n (%) | 9 (33.3) |
| Side of embolized rAMLs, n (%) | |
| Right | 14 (51.9) |
| Left | 13 (48.1) |
| Location of embolized rAMLs | |
| Central | 2 (7.4) |
| Exophytic | 25 (92.6) |
| Variables | Data |
|---|---|
| rAMLs before SAE | |
| Size, cm, mean ± SD | 8.0 ± 2.9 |
| Size, cm, median (range) | 7.6 (4.2–15.4) |
| Volume, cm3, mean ± SD | 143.3 ± 162.2 |
| Volume, cm3, median (range) | 82.3 (21.0–646.0) |
| Radiologic follow-up, months, mean (range) | 15 (1–72) |
| rAMLs after SAE (at mean follow-up) | |
| Size, cm, mean ± SD | 6.1 ± 3.0 |
| Size, cm, median (range) | 5.0 (2.5–13.1) |
| Volume, cm3, mean ± SD | 78.8 ± 110.0 |
| Volume, cm3, median (range) | 28.1 (2.7–348.0) |
| Tumor size reduction at mean follow-up | |
| cm, mean ± SD | 1.9 ± 1.4 |
| %, mean ± SD | 25.2 ± 16.0 |
| Tumor volume reduction at mean follow-up | |
| cm3, mean ± SD | 64.5 ± 69.0 |
| %, mean ± SD | 55.1 ± 24.9 |
| Serum creatinine level | |
| Before SAE, µmol/L, mean ± SD | 69.3 ± 16.8 |
| After SAE, µmol/L, mean ± SD | 73.4 ± 22.6 |
| Technical success a rate, n (%) | 25 (92.6) |
| Need for re-embolization, n (%) | 1 (3.7) |
| Renal surgery after SAE, n (%) | 1 (3.7) |
| Minor complications within 1 month, n (%) | 16 (61.5) |
| PES | 15 (57.7) |
| Allergic reaction | 1 (3.7) |
| Major complications within 1 month, n (%) | 3 (11.1) |
| Pseudoaneurysm | 1 (3.7) |
| Abscess | 2 (7.4) |
| Death | 1 (3.7) |
| Variables | p Value |
|---|---|
| Aging | 0.054 |
| Lower % of fat | 0.001 |
| Greater initial volume | 0.014 |
| Longer of follow-up time | 0.0001 |
| Tuberous sclerosis complex | 0.059 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prigent, F.-V.; Guillen, K.; Comby, P.-O.; Pellegrinelli, J.; Falvo, N.; Midulla, M.; Majbri, N.; Chevallier, O.; Loffroy, R. Selective Arterial Embolization of Renal Angiomyolipomas with a N-Butyl Cyanoacrylate-Lipiodol Mixture: Efficacy, Safety, Short- and Mid-Term Outcomes. J. Clin. Med. 2021, 10, 4062. https://doi.org/10.3390/jcm10184062
Prigent F-V, Guillen K, Comby P-O, Pellegrinelli J, Falvo N, Midulla M, Majbri N, Chevallier O, Loffroy R. Selective Arterial Embolization of Renal Angiomyolipomas with a N-Butyl Cyanoacrylate-Lipiodol Mixture: Efficacy, Safety, Short- and Mid-Term Outcomes. Journal of Clinical Medicine. 2021; 10(18):4062. https://doi.org/10.3390/jcm10184062
Chicago/Turabian StylePrigent, François-Victor, Kévin Guillen, Pierre-Olivier Comby, Julie Pellegrinelli, Nicolas Falvo, Marco Midulla, Nabil Majbri, Olivier Chevallier, and Romaric Loffroy. 2021. "Selective Arterial Embolization of Renal Angiomyolipomas with a N-Butyl Cyanoacrylate-Lipiodol Mixture: Efficacy, Safety, Short- and Mid-Term Outcomes" Journal of Clinical Medicine 10, no. 18: 4062. https://doi.org/10.3390/jcm10184062
APA StylePrigent, F.-V., Guillen, K., Comby, P.-O., Pellegrinelli, J., Falvo, N., Midulla, M., Majbri, N., Chevallier, O., & Loffroy, R. (2021). Selective Arterial Embolization of Renal Angiomyolipomas with a N-Butyl Cyanoacrylate-Lipiodol Mixture: Efficacy, Safety, Short- and Mid-Term Outcomes. Journal of Clinical Medicine, 10(18), 4062. https://doi.org/10.3390/jcm10184062






