Aerobic Threshold Identification in a Cardiac Disease Population Based on Correlation Properties of Heart Rate Variability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Exercise Testing Protocol
2.3. Exercise Training Intervention
2.4. Gas Exchange Testing
2.5. RR Measurements and Calculation of DFA-a1-Derived Threshold
2.6. Statistics
3. Results
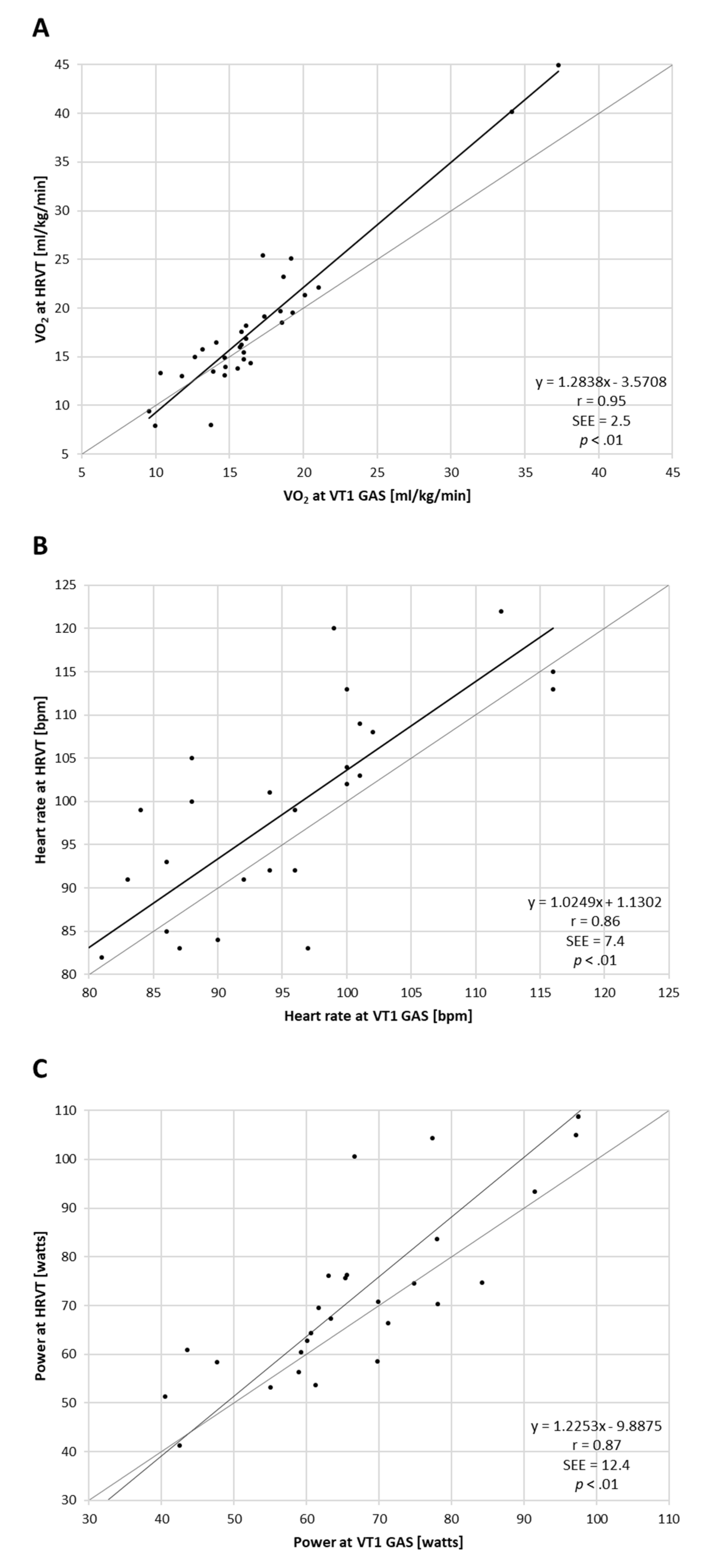
3.1. Comparison of VT1 and HRVT
3.2. PRE vs. POST Training Comparisons
4. Discussion
5. Limitations and Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bourgois, J.G.; Bourgois, G.; Boone, J. Perspectives and Determinants for Training-Intensity Distribution in Elite Endurance Athletes. Int. J. Sports Physiol. Perform. 2019, 14, 1151–1156. [Google Scholar] [CrossRef]
- Mezzani, A.; Hamm, L.F.; Jones, A.M.; McBride, P.E.; Moholdt, T.; Stone, J.A.; Williams, M.A. Aerobic exercise intensity assessment and prescription in cardiac rehabilitation: A joint position statement of the European Association for Cardiovascular Prevention and Rehabilitation, the American Association of Cardiovascular and Pulmonary Rehabilitation and the Canadian Association of Cardiac Rehabilitation. Eur. J. Prev. Cardiol. 2013, 20, 442–467. [Google Scholar]
- Hannan, A.L.; Hing, W.; Simas, V.; Climstein, M.; Coombes, J.S.; Jayasinghe, R.; Furness, J. High-intensity interval training versus moderate-intensity continuous training within cardiac rehabilitation: A systematic review and meta-analysis. Open Access. J. Sports Med. 2018, 26, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcin, T.; Eser, P.; Prescott, E.; Prins, L.F.; Kolkman, E.; Bruins, W.; van der Velde, A.E.; Gil, C.P.; Iliou, M.-C.; Ardissino, D.; et al. Training intensity and improvements in exercise capacity in elderly patients undergoing European cardiac rehabilitation—The EU-CaRE multicenter cohort study. PLoS ONE 2020, 15, e0242503. [Google Scholar] [CrossRef] [PubMed]
- Seiler, K.S.; Kjerland, G.Ø. Quantifying training intensity distribution in elite endurance athletes: Is there evidence for an “optimal” distribution? Scand. J. Med. Sci. Sports 2006, 16, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Esteve-Lanao, J.; Foster, C.; Seiler, S.; Lucia, A. Impact of training intensity distribution on performance in endurance athletes. J. Strength Cond. Res. 2007, 21, 943–949. [Google Scholar]
- Meyer, K.; Hajric, R.; Westbrook, S.; Samek, L.; Lehmann, M.; Schwaibold, M.; Roskamm, H. Ventilatory and lactate threshold determinations in healthy normals and cardiac patients: Methodological problems. Eur. J. Appl. Physiol. Occupat. Physiol. 1996, 72, 387–393. [Google Scholar] [CrossRef]
- Faude, O.; Kindermann, W.; Meyer, T. Lactate threshold concepts. Sports Med. 2009, 39, 469–490. [Google Scholar] [CrossRef]
- Jamnick, N.A.; Botella, J.; Pyne, D.B.; Bishop, D.J. Manipulating graded exercise test variables affects the validity of the lactate threshold and VO2peak. PLoS ONE 2018, 13, e0199794. [Google Scholar] [CrossRef] [Green Version]
- Jamnick, N.A.; Pettitt, R.W.; Granata, C.; Pyne, D.B.; Bishop, D.J. An Examination and Critique of Current Methods to Determine Exercise Intensity. Sports Med. 2020, 50, 1729–1756. [Google Scholar] [CrossRef]
- Karapetian, G.K.; Engels, H.J.; Gretebeck, R.J. Use of heart rate variability to estimate LT and VT. Int. J. Sports Med. 2008, 29, 652–657. [Google Scholar] [CrossRef]
- Michael, S.; Graham, K.S.; Davis Oam, G.M. Cardiac Autonomic Responses during Exercise and Post-exercise Recovery Using Heart Rate Variability and Systolic Time Intervals-A Review. Front. Physiol. 2017, 8, 301. [Google Scholar] [CrossRef] [Green Version]
- Blasco-Lafarga, C.; Camarena, B.; Mateo-March, M. Cardiovascular and Autonomic Responses to a Maximal Exercise Test in Elite Youngsters. Int. J. Sports Med. 2017, 38, 666–674. [Google Scholar] [CrossRef]
- Rogers, B.; Giles, D.; Draper, N.; Hoos, O.; Gronwald, T. A New Detection Method Defining the Aerobic Threshold for Endurance Exercise and Training Prescription Based on Fractal Correlation Properties of Heart Rate Variability. Front. Physiol. 2021, 11, 596567. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, V.O.; Mezzani, A. Aerobic exercise training intensity in patients with chronic heart failure: Principles of assessment and prescription. Eur. J. Cardiovasc. Prev. Rehabil. 2011, 18, 5–14. [Google Scholar] [CrossRef]
- Franklin, B.A.; Lavie, C.J.; Squires, R.W.; Milani, R.V. Exercise-based cardiac rehabilitation and improvements in cardiorespiratory fitness: Implications regarding patient benefit. Mayo Clin. Proc. 2013, 88, 431–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gronwald, T.; Hoos, O. Correlation properties of heart rate variability during endurance exercise: A systematic review. Ann. Noninvasive Electrocardiol. 2020, 25, e12697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gronwald, T.; Rogers, B.; Hoos, O. Fractal correlation properties of heart rate variability: A new biomarker for intensity distribution in endurance exercise and training prescription? Front. Physiol. 2020, 10, 3389. [Google Scholar] [CrossRef]
- Balagué, N.; Hristovski, R.; Almarcha, M.; Garcia-Retortillo, S.; Ivanov, P.C. Network Physiology of Exercise: Vision and Perspectives. Front. Physiol. 2020, 11, 611550. [Google Scholar] [CrossRef]
- Tulppo, M.P.; Kiviniemi, A.M.; Hautala, A.J.; Kallio, M.; Seppänen, T.; Mäkikallio, T.H.; Huikuri, H.V. Physiological background of the loss of fractal heart rate dynamics. Circulation 2005, 112, 314–319. [Google Scholar] [CrossRef] [Green Version]
- Voss, A.; Schulz, S.; Schroeder, R.; Baumert, M.; Caminal, P. Methods derived from nonlinear dynamics for analysing heart rate variability. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2009, 367, 277–296. [Google Scholar] [CrossRef]
- White, D.W.; Raven, P.B. Autonomic neural control of heart rate during dynamic exercise: Revisited. J. Physiol. 2014, 592, 2491–2500. [Google Scholar] [CrossRef]
- American College of Sports Medicine. Acsm’s Guidelines for Exercise Testing and Prescription, 11th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2021. [Google Scholar]
- Teffaha, D.; Mourot, L.; Vernochet, P.; Ounissi, F.; Regnard, J.; Monpère, C.; Dugué, B. Relevance of water gymnastics in rehabilitation programs in patients with chronic heart failure or coronary artery disease with normal left ventricular function. J. Card. Fail. 2011, 17, 676–683. [Google Scholar] [CrossRef]
- Gaskill, S.E.; Ruby, B.C.; Walker, A.J.; Sanchez, O.A.; Serfass, R.C.; Leon, A.S. Validity and reliability of combining three methods to determine ventilatory threshold. Med. Sci. Sports. Exerc. 2001, 33, 1841–1848. [Google Scholar] [CrossRef] [PubMed]
- Bentley, D.J.; McNaughton, L.R. Comparison of W(peak), VO2(peak) and the ventilation threshold from two different incremental exercise tests: Relationship to endurance performance. J. Sci. Med. Sport 2003, 6, 422–435. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.H. Biostatistics 104: Correlational analysis. Singap. Med. J. 2003, 44, 614–619. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Mourot, L.; Tordi, N.; Bouhaddi, M.; Teffaha, D.; Monpere, C.; Regnard, J. Heart rate variability to assess ventilatory thresholds: Reliable in cardiac disease? Eur. J. Prev. Cardiol. 2012, 19, 1272–1280. [Google Scholar] [CrossRef] [PubMed]
- Leprêtre, P.M.; Bulvestre, M.; Ghannem, M.; Ahmaidi, S.; Weissland, T.; Lopes, P. Determination of Ventilatory Threshold using Heart Rate Variability in Patients with Heart Failure. Surgery 2013, S12, 003. [Google Scholar] [CrossRef] [Green Version]
- Gronwald, T.; Berk, S.; Altini, M.; Mourot, L.; Hoos, O.; Rogers, B. Real-Time Estimation of Aerobic Threshold and Exercise Intensity Distribution Using Fractal Correlation Properties of Heart Rate Variability: A Single-Case Field Application in a Former Olympic Triathlete. Front. Sports Act. Living 2021, 3, 668812. [Google Scholar] [CrossRef]
- Nichols, S.; Taylor, C.; Goodman, T.; Page, R.; Kallvikbacka-Bennett, A.; Nation, F.; Ingle, L. Routine exercise-based cardiac rehabilitation does not increase aerobic fitness: A CARE CR study. Int. J. Cardiol. 2020, 15, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Bouchard, C.; An, P.; Rice, T.; Skinner, J.S.; Wilmore, J.H.; Gagnon, J.; Rao, D.C. Familial aggregation of VO(2max) response to exercise training: Results from the HERITAGE Family Study. J. Appl. Physiol. 1999, 87, 1003–1008. [Google Scholar] [CrossRef] [Green Version]
- Castiglioni, P.; Parati, G.; Di Rienzo, M.; Carabalona, R.; Cividjian, A.; Quintin, L. Scale exponents of blood pressure and heart rate during autonomic blockade as assessed by detrended fluctuation analysis. J. Physiol. 2011, 589, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Huikuri, H.V.; Stein, P.K. Heart rate variability in risk stratification of cardiac patients. Prog. Cardiovasc. Dis. 2013, 56, 153–159. [Google Scholar] [CrossRef]
- Ridha, M.; Mäkikallio, T.H.; Lopera, G.; Pastor, J.; De Marchena, E.; Chakko, S.; Myerburg, R.J. Effects of carvedilol on heart rate dynamics in patients with congestive heart failure. Ann. Noninvasive Electrocardiol. 2002, 7, 133–138. [Google Scholar] [CrossRef]
- Merri, M.; Farden, D.C.; Mottley, J.G.; Titlebaum, E.L. Sampling frequency of the electrocardiogram for spectral analysis of the heart rate variability. IEEE Trans. Biomed. Eng. 1990, 37, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Tapanainen, J.M.; Seppänen, T.; Laukkanen, R.; Loimaala, A.; Huikuri, H.V. Significance of the Accuracy of RR Interval Detection for the Analysis of New Dynamic Measures of Heart Rate Variability. Ann. Noninvasive Electrocardiol. 1999, 4, 10–17. [Google Scholar] [CrossRef]
- Rincon Soler, A.I.; Silva, L.E.V.; Fazan, R., Jr.; Murta, L.O., Jr. The impact of artefact correction methods of RR series on heart rate variability parameters. J. Appl. Physiol. 2018, 124, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Rogers, B.; Giles, D.; Draper, N.; Mourot, L.; Gronwald, T. Influence of Artefact Correction and Recording Device Type on the Practical Application of a Non-Linear Heart Rate Variability Biomarker for Aerobic Threshold Determination. Sensors 2021, 21, 821. [Google Scholar] [CrossRef]
- Pallarés, J.G.; Morán-Navarro, R.; Ortega, J.F.; Fernández-Elías, E.; Mora-Rodriguez, R. Validity and reliability of ventilatory and blood lactate thresholds in well-trained cyclists. PLoS ONE 2016, 11, e0163389. [Google Scholar] [CrossRef] [PubMed]


| Age (Years) | Ht (cm) | BW (kg) | HRPEAK (bpm) | VO2PEAK (mL/kg/min) | Etiology |
|---|---|---|---|---|---|
| 52 | 173 | 71 | 117 | 17.36 | CHF |
| 59 | 168 | 73 | 109 | 23.05 | CAD |
| 55 | 176 | 89 | 141 | 31.42 | CAD |
| 62 | 172 | 85 | 129 | 28.96 | CAD |
| 56 | 167 | 72 | 131 | 42.50 | CAD |
| 55 | 176 | 94 | 112 | 24.84 | CAD |
| 54 | 178 | 94 | 95 | 25.89 | CAD |
| 59 | 163 | 58 | 110 | 24.93 | CAD |
| 41 | 176 | 58 | 128 | 51.23 | CAD |
| 53 | 171 | 96 | 131 | 27.21 | CHF |
| 44 | 173 | 67 | 133 | 33.05 | CAD |
| 70 | 174 | 81 | 108 | 21.52 | CHF |
| 64 | 170 | 74 | 114 | 35.67 | CHF |
| 40 | 182 | 89 | 162 | 27.88 | CHF * |
| 62 | 175 | 97 | 132 | 28.53 | CAD |
| 58 | 175 | 67 | 131 | 22.28 | CHF |
| 55 (±8) | 173 (±5) | 79.0 (±13) | 124 (±16) | 29.15 (±8.42) | - |
| PRE | POST | Paired t Testing and Effect Size | |
|---|---|---|---|
| BW (kg) | 79.0 (±13.0) | 78.1 (±12.5) | p = 0.03, d = 0.61 |
| HRPEAK (bpm) | 124 (±16) | 135 (±18) | p < 0.01, d = 1.66 |
| VO2PEAK (mL/kg/min) | 29.15 (±8.42) | 30.73 (±10.01) | p = 0.07, d = 0.49 |
| VT1 VO2 (mL/kg/min) | 16.61 (±5.54) | 17.02 (±6.06) | p = 0.58, d = 0.14 |
| HRVT VO2 (mL/kg/min) | 17.15 (±7.61) | 18.88 (±8.02) | p = 0.05, d = 0.54 |
| VT1 HR (bpm) | 90.5 (±11.7) | 92.0 (±12.3) | p = 0.30, d = 0.27 |
| HRVT HR (bpm) | 92.1 (±13.6) | 97.3 (±14.7) | p = 0.02, d = 0.64 |
| PPEAK (watts) | 119.5 (±29.0) | 135.8 (±34.0) | p < 0.01, d = 1.92 |
| VT1 P (watts) | 64.7 (±18.2) | 70.9 (±17.5) | p = 0.05, d = 0.54 |
| HRVT P (watts) | 67.1 (±25.1) | 79.4 (±24.2) | p < 0.01, d = 0.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogers, B.; Mourot, L.; Gronwald, T. Aerobic Threshold Identification in a Cardiac Disease Population Based on Correlation Properties of Heart Rate Variability. J. Clin. Med. 2021, 10, 4075. https://doi.org/10.3390/jcm10184075
Rogers B, Mourot L, Gronwald T. Aerobic Threshold Identification in a Cardiac Disease Population Based on Correlation Properties of Heart Rate Variability. Journal of Clinical Medicine. 2021; 10(18):4075. https://doi.org/10.3390/jcm10184075
Chicago/Turabian StyleRogers, Bruce, Laurent Mourot, and Thomas Gronwald. 2021. "Aerobic Threshold Identification in a Cardiac Disease Population Based on Correlation Properties of Heart Rate Variability" Journal of Clinical Medicine 10, no. 18: 4075. https://doi.org/10.3390/jcm10184075
APA StyleRogers, B., Mourot, L., & Gronwald, T. (2021). Aerobic Threshold Identification in a Cardiac Disease Population Based on Correlation Properties of Heart Rate Variability. Journal of Clinical Medicine, 10(18), 4075. https://doi.org/10.3390/jcm10184075








