Itch as Major Mediator of Effect of Tofacitinib on Health-Related Quality of Life in Psoriatic Arthritis: A Mediation Analysis
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Patients
3.2. Initial Mediation Model
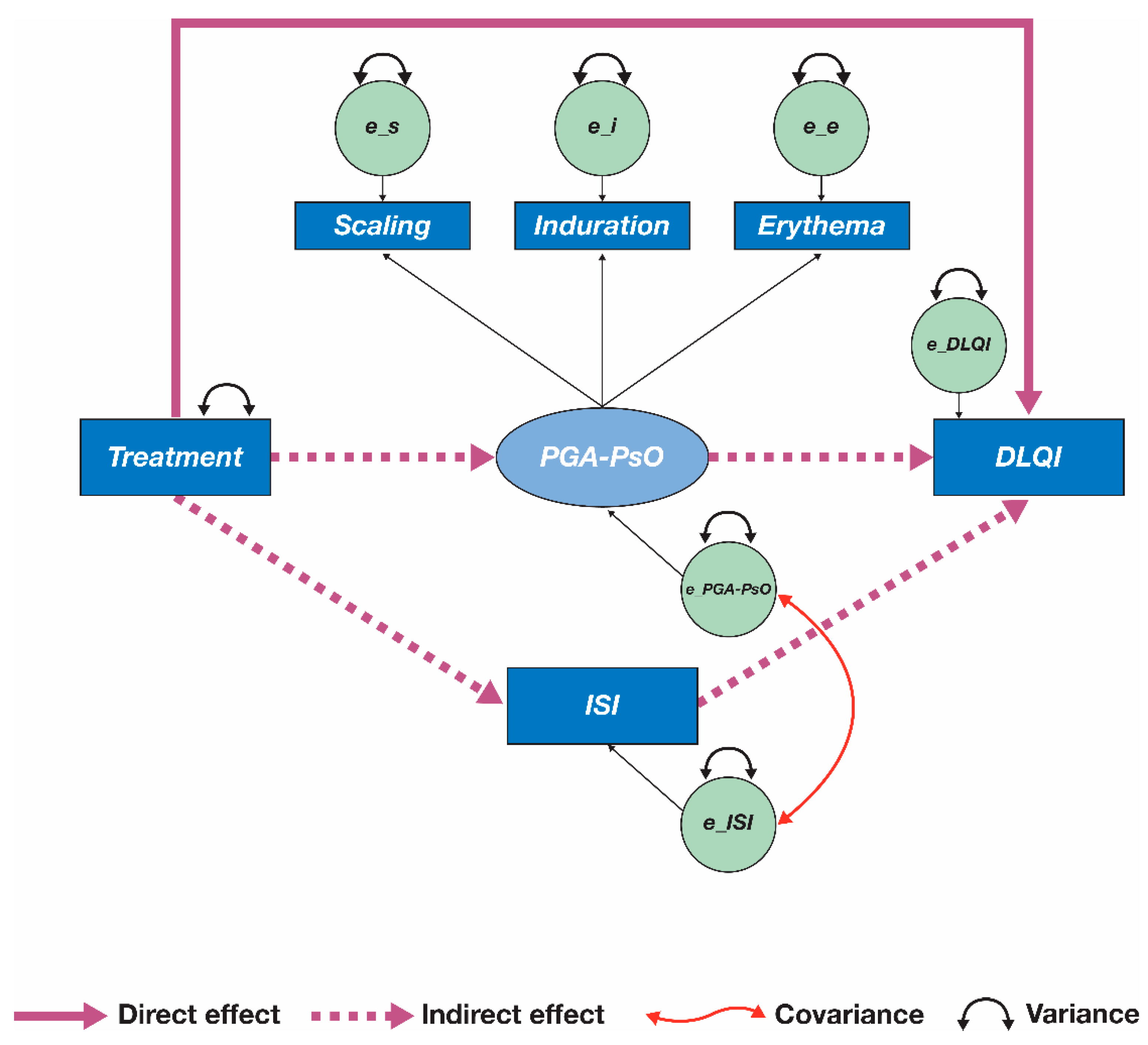
3.3. Re-Specified Mediation Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Veale, D.J.; Fearon, U. The pathogenesis of psoriatic arthritis. Lancet 2018, 391, 2273–2284. [Google Scholar] [CrossRef]
- Ritchlin, C.T.; Colbert, R.A.; Gladman, D.D. Psoriatic arthritis. N. Engl. J. Med. 2017, 376, 957–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gladman, D.D.; Antoni, C.; Mease, P.; Clegg, D.O.; Nash, P. Psoriatic arthritis: Epidemiology, clinical features, course, and outcome. Ann. Rheum. Dis. 2005, 64, ii14–ii17. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, M.G.; Bachelez, H.; Barker, J.; Girolomoni, G.; Kavanaugh, A.; Langley, R.G.; Paul, C.F.; Puig, L.; Reich, K.; van de Kerkhof, P.C.M. Patient perspectives in the management of psoriasis: Results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J. Am. Acad. Dermatol. 2014, 70, 871–881.e30. [Google Scholar] [CrossRef]
- Stolwijk, C.; van Onna, M.; Boonen, A.; van Tubergen, A. Global prevalence of spondyloarthritis: A systematic review and meta-regression analysis. Arthritis Care Res. (Hoboken) 2016, 68, 1320–1331. [Google Scholar] [CrossRef]
- Villani, A.P.; Rouzaud, M.; Sevrain, M.; Barnetche, T.; Paul, C.; Richard, M.-A.; Beylot-Barry, M.; Misery, L.; Joly, P.; Le Maitre, M.; et al. Prevalence of undiagnosed psoriatic arthritis among psoriasis patients: Systematic review and meta-analysis. J. Am. Acad. Dermatol. 2015, 73, 242–248. [Google Scholar] [CrossRef]
- Mease, P.J.; Menter, M.A. Quality-of-life issues in psoriasis and psoriatic arthritis: Outcome measures and therapies from a dermatological perspective. J. Am. Acad. Dermatol. 2006, 54, 685–704. [Google Scholar] [CrossRef]
- Husted, J.A.; Gladman, D.D.; Farewell, V.T.; Long, J.A.; Cook, R.J. Validating the SF-36 health survey questionnaire in patients with psoriatic arthritis. J. Rheumatol. 1997, 24, 511–517. [Google Scholar] [PubMed]
- Merola, J.F.; Shrom, D.; Eaton, J.; Dworkin, C.; Krebsbach, C.; Shah-Manek, B.; Birt, J. Patient perspective on the burden of skin and joint symptoms of psoriatic arthritis: Results of a multi-national patient survey. Rheumatol. Ther. 2019, 6, 33–45. [Google Scholar] [CrossRef] [Green Version]
- de Vlam, K.; Merola, J.F.; Birt, J.A.; Sandoval, D.M.; Lobosco, S.; Moon, R.; Milligan, G.; Boehncke, W.H. Skin involvement in psoriatic arthritis worsens overall disease activity, patient-reported outcomes, and increases healthcare resource utilization: An observational, cross-sectional study. Rheumatol. Ther. 2018, 5, 423–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kavanaugh, A.; McInnes, I.B.; Krueger, G.G.; Gladman, D.D.; Beutler, A.; Gathany, T.; Mack, M.; Tandon, N.; Han, C.; Mease, P. Patient-reported outcomes and the association with clinical response in patients with active psoriatic arthritis treated with golimumab: Findings through 2 years of a phase III, multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Care Res. (Hoboken) 2013, 65, 1666–1673. [Google Scholar] [CrossRef]
- Gossec, L.; de Wit, M.; Kiltz, U.; Braun, J.; Kalyoncu, U.; Scrivo, R.; Maccarone, M.; Carton, L.; Otsa, K.; Sooäär, I.; et al. A patient-derived and patient-reported outcome measure for assessing psoriatic arthritis: Elaboration and preliminary validation of the Psoriatic Arthritis Impact of Disease (PsAID) questionnaire, a 13-country EULAR initiative. Ann. Rheum. Dis. 2014, 73, 1012–1019. [Google Scholar] [CrossRef] [Green Version]
- Oetjen, L.K.; Mack, M.R.; Feng, J.; Whelan, T.M.; Niu, H.; Guo, C.J.; Chen, S.; Trier, A.M.; Xu, A.Z.; Tripathi, S.V.; et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell 2017, 171, 217–228.e13. [Google Scholar] [CrossRef] [Green Version]
- Busch-Dienstfertig, M.; González-Rodríguez, S. IL-4, JAK-STAT signaling, and pain. JAKSTAT 2013, 2, e27638. [Google Scholar] [CrossRef] [Green Version]
- Han, L.; Ma, C.; Liu, Q.; Weng, H.-J.; Cui, Y.; Tang, Z.; Kim, Y.; Nie, H.; Qu, L.; Patel, K.N.; et al. A subpopulation of nociceptors specifically linked to itch. Nat. Neurosci. 2013, 16, 174–182. [Google Scholar] [CrossRef]
- Mease, P.; Hall, S.; FitzGerald, O.; van der Heijde, D.; Merola, J.F.; Avila-Zapata, F.; Ciéslak, D.; Graham, D.; Wang, C.; Menon, S.; et al. Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N. Engl. J. Med. 2017, 377, 1537–1550. [Google Scholar] [CrossRef]
- Gladman, D.; Rigby, W.; Azevedo, V.F.; Behrens, F.; Blanco, R.; Kaszuba, A.; Kudlacz, E.; Wang, C.; Menon, S.; Hendrikx, T.; et al. Tofacitinib for psoriatic arthritis in patients with an inadequate response to TNF inhibitors. N. Engl. J. Med. 2017, 377, 1525–1536. [Google Scholar] [CrossRef]
- Strand, V.; de Vlam, K.; Covarrubias-Cobos, J.A.; Mease, P.J.; Gladman, D.D.; Chen, L.; Kudlacz, E.; Wu, J.; Cappelleri, J.C.; Hendrikx, T.; et al. Effect of tofacitinib on patient-reported outcomes in patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors in the phase III, randomised controlled trial: OPAL Beyond. RMD Open 2019, 5, e000808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strand, V.; de Vlam, K.; Covarrubias-Cobos, J.A.; Mease, P.J.; Gladman, D.D.; Graham, D.; Wang, C.; Cappelleri, J.C.; Hendrikx, T.; Hsu, M.-A. Tofacitinib or adalimumab versus placebo: Patient-reported outcomes from OPAL Broaden—A phase III study of active psoriatic arthritis in patients with an inadequate response to conventional synthetic disease-modifying antirheumatic drugs. RMD Open 2019, 5, e000806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merola, J.F.; Papp, K.A.; Nash, P.; Gratacós, J.; Boehncke, W.H.; Thaçi, D.; Graham, D.; Hsu, M.-A.; Wang, C.; Wu, J.; et al. Tofacitinib in psoriatic arthritis patients: Skin signs and symptoms and health-related quality of life from two randomized phase 3 studies. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2809–2820. [Google Scholar] [CrossRef] [PubMed]
- Finlay, A.Y.; Khan, G.K. Dermatology Life Quality Index (DLQI)—A simple practical measure for routine clinical use. Clin. Exp. Dermatol 1994, 19, 210–216. [Google Scholar] [CrossRef]
- Cappelleri, J.C.; Zou, K.H.; Bushmakin, A.G.; Alvir, J.M.J.; Alemayehu, D.; Symonds, T. Patient-Reported Outcomes: Measurement, Implementation and Interpretation; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Bushmakin, A.G.; Mamolo, C.; Cappelleri, J.C.; Stewart, M. The relationship between pruritus and the clinical signs of psoriasis in patients receiving tofacitinib. J. Dermatol. Treat. 2015, 26, 19–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panés, J.; Su, C.; Bushmakin, A.G.; Cappelleri, J.C.; Healey, P. Direct and indirect effects of tofacitinib on treatment satisfaction in patients with ulcerative colitis. J. Crohn’s Colitis 2016, 10, 1310–1315. [Google Scholar] [CrossRef] [Green Version]
- Hongbo, Y.; Thomas, C.L.; Harrison, M.A.; Salek, M.S.; Finlay, A.Y. Translating the science of quality of life into practice: What do dermatology life quality index scores mean? J. Invest. Dermatol. 2005, 125, 659–664. [Google Scholar] [CrossRef] [Green Version]
- Ständer, S.; Luger, T.; Cappelleri, J.C.; Bushmakin, A.G.; Mamolo, C.; Zielinski, M.A.; Tallman, A.M.; Yosipovitch, G. Validation of the Itch Severity Item as a measurement tool for pruritus in patients with psoriasis: Results from a phase 3 tofacitinib program. Acta Derm. Venereol. 2018, 98, 340–345. [Google Scholar] [CrossRef] [Green Version]
- Langley, R.G.B.; Feldman, S.R.; Nyirady, J.; van de Kerkhof, P.; Papavassilis, C. The 5-point Investigator’s Global Assessment (IGA) scale: A modified tool for evaluating plaque psoriasis severity in clinical trials. J. Dermatol. Treat. 2015, 26, 23–31. [Google Scholar] [CrossRef]
- Gudu, T.; Gossec, L. Quality of life in psoriatic arthritis. Expert Rev. Clin. Immunol. 2018, 14, 405–417. [Google Scholar] [CrossRef]
- Kini, S.P.; DeLong, L.K.; Veledar, E.; McKenzie-Brown, A.M.; Schaufele, M.; Chen, S.C. The impact of pruritus on quality of life: The skin equivalent of pain. Arch. Dermatol. 2011, 147, 1153–1156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yosipovitch, G.; Goon, A.; Wee, J.; Chan, Y.H.; Goh, C.L. The prevalence and clinical characteristics of pruritus among patients with extensive psoriasis. Br. J. Dermatol. 2000, 143, 969–973. [Google Scholar] [CrossRef] [PubMed]
- Zachariae, R.; Zachariae, C.O.C.; Lei, U.; Pedersen, A.F. Affective and sensory dimensions of pruritus severity: Associations with psychological symptoms and quality of life in psoriasis patients. Acta Derm. Venereol. 2008, 88, 121–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, M.A.; Gupta, A.K.; Kirkby, S.; Weiner, H.K.; Mace, T.M.; Schork, N.J.; Johnson, E.H.; Ellis, C.N.; Voorhees, J.J. Pruritus in psoriasis. A prospective study of some psychiatric and dermatologic correlates. Arch. Dermatol. 1988, 124, 1052–1057. [Google Scholar] [CrossRef] [PubMed]
- Hawro, T.; Hawro, M.; Zalewska-Janowska, A.; Weller, K.; Metz, M.; Maurer, M. Pruritus and sleep disturbances in patients with psoriasis. Arch. Dermatol. Res. 2020, 312, 103–111. [Google Scholar] [CrossRef]
- Taylor, P.C.; De Vlam, K.; Bushmakin, A.G.; Fallon, L.; Merola, J.F.; Cappelleri, J.C.; Hsu, M.A.; Mease, P.J. Identifying mediators of pain reduction in patients with psoriatic arthritis treated with tofacitinib: Role of inflammation associated with peripheral arthritis, enthesitis and skin disease. Ann. Rheum. Dis. 2020, 79, 1724–1725, Abstract AB0838. [Google Scholar] [CrossRef]
- Taylor, P.C.; Lee, Y.C.; Fleischmann, R.; Takeuchi, T.; Perkins, E.L.; Fautrel, B.; Zhu, B.; Quebe, A.K.; Gaich, C.L.; Zhang, X.; et al. Achieving pain control in rheumatoid arthritis with baricitinib or adalimumab plus methotrexate: Results from the RA-BEAM trial. J. Clin. Med. 2019, 8, 831. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taylor, P.C.; Bushmakin, A.G.; Cappelleri, J.C.; Young, P.; Germino, R.; Merola, J.F.; Yosipovitch, G. Itch as Major Mediator of Effect of Tofacitinib on Health-Related Quality of Life in Psoriatic Arthritis: A Mediation Analysis. J. Clin. Med. 2021, 10, 4081. https://doi.org/10.3390/jcm10184081
Taylor PC, Bushmakin AG, Cappelleri JC, Young P, Germino R, Merola JF, Yosipovitch G. Itch as Major Mediator of Effect of Tofacitinib on Health-Related Quality of Life in Psoriatic Arthritis: A Mediation Analysis. Journal of Clinical Medicine. 2021; 10(18):4081. https://doi.org/10.3390/jcm10184081
Chicago/Turabian StyleTaylor, Peter C., Andrew G. Bushmakin, Joseph C. Cappelleri, Pamela Young, Rebecca Germino, Joseph F. Merola, and Gil Yosipovitch. 2021. "Itch as Major Mediator of Effect of Tofacitinib on Health-Related Quality of Life in Psoriatic Arthritis: A Mediation Analysis" Journal of Clinical Medicine 10, no. 18: 4081. https://doi.org/10.3390/jcm10184081
APA StyleTaylor, P. C., Bushmakin, A. G., Cappelleri, J. C., Young, P., Germino, R., Merola, J. F., & Yosipovitch, G. (2021). Itch as Major Mediator of Effect of Tofacitinib on Health-Related Quality of Life in Psoriatic Arthritis: A Mediation Analysis. Journal of Clinical Medicine, 10(18), 4081. https://doi.org/10.3390/jcm10184081







