A Novel Diagnostic Score Integrating Atrial Dimensions to Differentiate between the Athlete’s Heart and Arrhythmogenic Right Ventricular Cardiomyopathy
Abstract
:1. Introduction
2. Methods
2.1. Population
2.2. Echocardiography
2.3. Ergometry
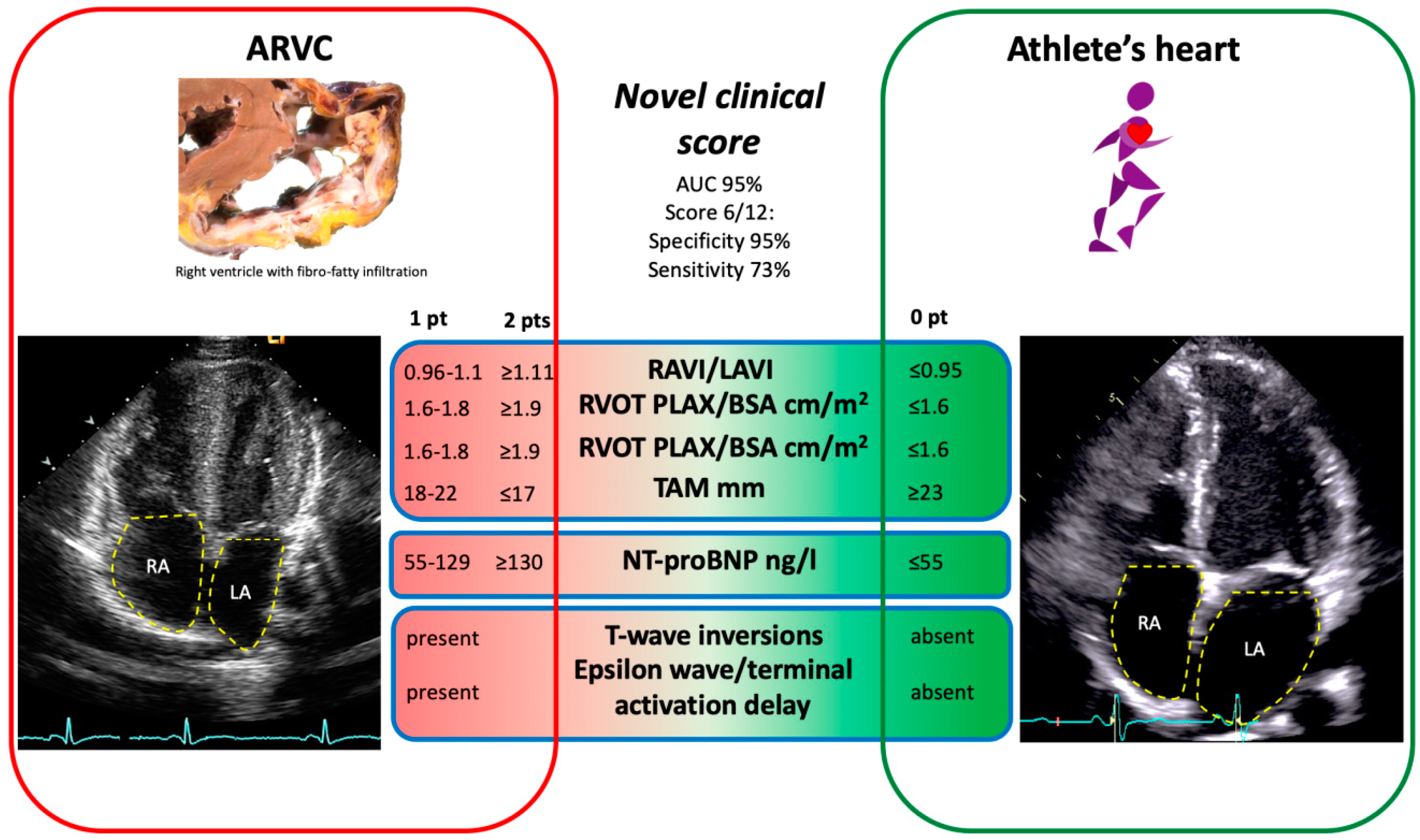
2.4. Clinical Score to Differentiate the Athlete’s Heart from ARVC
2.5. Statistical Analyses
3. Results
3.1. Demographics and Classification of Sports Activity
3.2. Echocardiographic Measurements
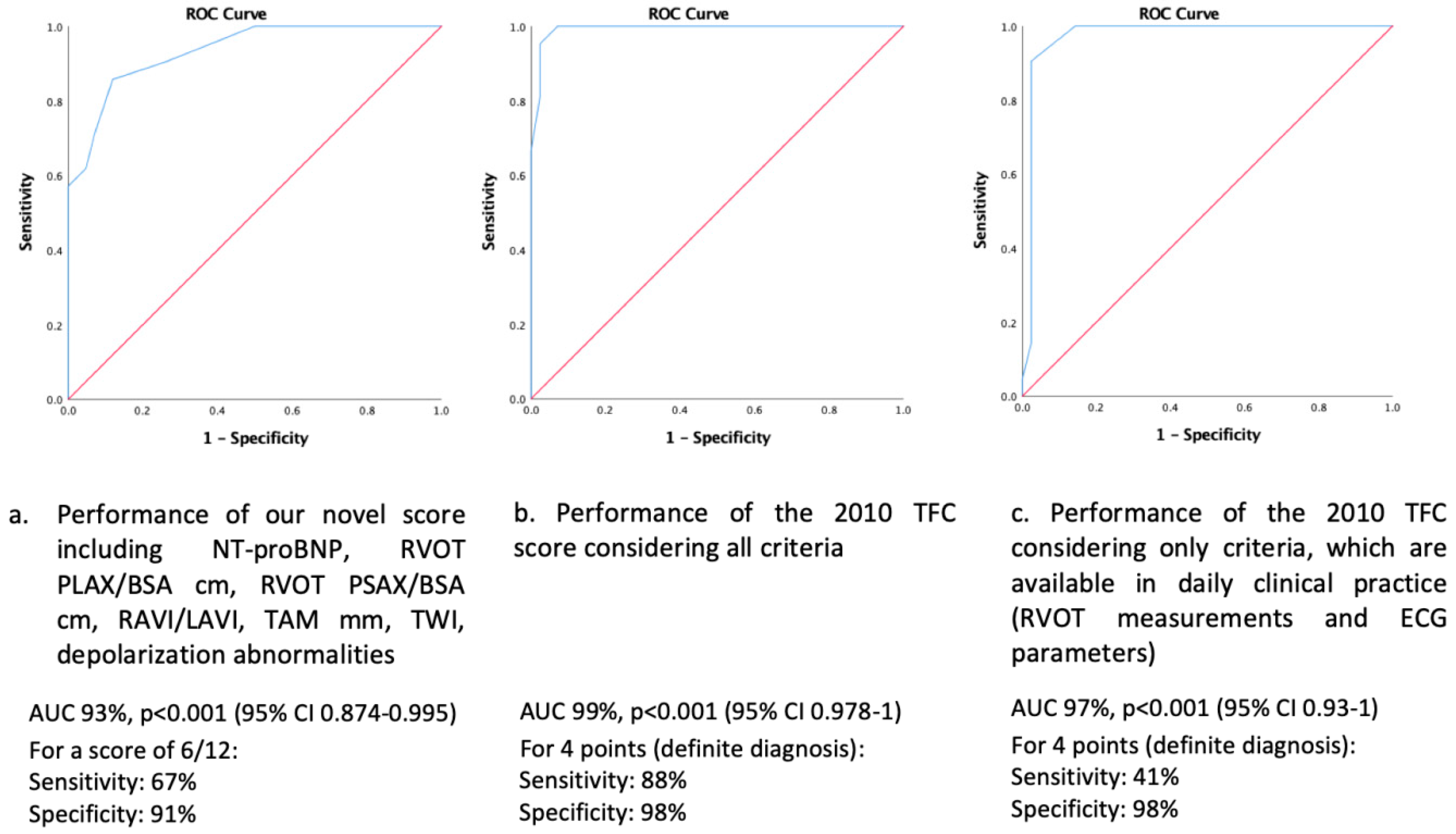
3.3. 2010 TFC and Novel Clinical Score
3.4. Laboratory Testing
3.5. Arrhythmia and ECG Alterations
4. Discussion
- ARVC patients presented with significantly larger RA, but smaller LA dimensions as compared to athletes, resulting in a greater RAVI/LAVI ratio.
- The best novel diagnostic model to discriminate between ARVC (diagnosed by the full 2010 TFC serving as the diagnostic gold standard) and the athlete’s heart was obtained when including the following parameters: RAVI/LAVI ratio, RVOT in PLAX and PSAX, TAM, TWI and depolarization abnormalities on 12-lead ECG, and serum NT-proBNP with a diagnostic accuracy of 93%. A score value of ≥6 out of a maximum of 12 points was specific for a diagnosis of ARVC.
- Our novel diagnostic model showed a higher sensitivity to diagnose ARVC as compared to the 2010 TFC restricting to echocardiography and 12-lead ECG only, which are easy to obtain and readily available in daily clinical practice.
4.1. Rationale to Develop a Novel Diagnostic Score to Differentiate between ARVC and the Athlete’s Heart
4.2. Cardiac Remodeling in the Athlete’s Heart and ARVC
4.3. NT-proBNP
4.4. ECG Depolarization and Repolarization Alterations
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| ARVC | arrhythmogenic right ventricular cardiomyopathy |
| AUC | Area Under the Curve |
| BSA | body surface area |
| CMR | cardiac magnetic resonance imaging |
| ECG | electrocardiogram |
| Fac | fractional area change |
| GFR | glomerular filtration rate |
| LA | left atrium |
| LV | left ventricle |
| NT-proBNP | N-terminal fragment of brain natriuretic peptide |
| PLAX | parasternal long axis |
| PSAX | parasternal short axis |
| RA | right atrium |
| RAVI/LAVI | right - left atrial volume indexed |
| RBBB | right bundle branch block |
| ROC | receiver operator curve |
| RV | right ventricle |
| RVOT | right ventricular outflow tract |
| TAM | tricuspid annular motion |
| TFC | Task Force Criteria |
| TTE | transthoracic echocardiography |
| TSH | thyroid-stimulating hormon |
| TWI | T-wave inversions |
References
- Bohm, P.; Schneider, G.; Linneweber, L.; Rentzsch, A.; Krämer, N.; Abdul-Khaliq, H.; Kindermann, W.; Meyer, T.; Scharhag, J. Right and Left Ventricular Function and Mass in Male Elite Master Athletes: A Controlled Contrast-Enhanced Cardiovascular Magnetic Resonance Study. Circulation 2016, 133, 1927–1935. [Google Scholar] [CrossRef]
- Gasperetti, A.; James, C.A.; Cerrone, M.; Delmar, M.; Calkins, H.; Duru, F. Arrhythmias right ventricular cardiomyopathy and sports activity: From molecular pathways in diseased hearts to new insights into the athletic heart mimicry. Eur. Heart J. 2020, 42, 1231–1243. [Google Scholar] [CrossRef]
- Corrado, D.; Van Tintelen, P.J.; McKenna, W.J.; Hauer, R.N.W.; Anastastakis, A.; Asimaki, A.; Basso, C.; Bauce, B.; Brunckhorst, C.; Bucciarelli-Ducci, C.; et al. Arrhythmogenic right ventricular cardiomyopathy: Evaluation of the current diagnostic criteria and differential diagnosis. Eur. Heart J. 2020, 41, 1414–1429. [Google Scholar] [CrossRef] [Green Version]
- Gotschy, A.; Saguner, A.M.; Niemann, M.; Hamada, S.; Akdis, D.; Yoon, J.-N.; Parmon, E.; Delgado, V.; Bax, J.J.; Kozerke, S.; et al. Right ventricular outflow tract dimensions in arrhythmogenic right ventricular cardiomyopathy/dysplasia—A multicentre study comparing echocardiography and cardiovascular magnetic resonance. Eur. Heart J. Cardiovasc. Imaging 2017, 19, 516–523. [Google Scholar] [CrossRef]
- Boczar, K.E.; Alqarawi, W.; Green, M.S.; Redpath, C.; Burwash, I.G.; Dwivedi, G. The echocardiographic assessment of the right ventricle in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia compared with athletes and matched controls. Echocardiography 2019, 36, 666–670. [Google Scholar] [CrossRef]
- Qasem, M.; George, K.; Somauroo, J.; Forsythe, L.; Brown, B.; Oxborough, D. Right ventricular function in elite male athletes meeting the structural echocardiographic task force criteria for arrhythmogenic right ventricular cardiomyopathy. J. Sports Sci. 2018, 37, 306–312. [Google Scholar] [CrossRef] [Green Version]
- Bauce, B.; Frigo, G.; Benini, G.; Michieli, P.; Basso, C.; Folino, A.F.; Rigato, I.; Mazzotti, E.; Daliento, L.; Thiene, G.; et al. Differences and similarities between arrhythmogenic right ventricular cardiomyopathy and athlete’s heart adaptations. Br. J. Sports Med. 2010, 44, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Saguner, A.M.; Vecchiati, A.; Baldinger, S.H.; Rüeger, S.; Medeiros-Domingo, A.; Mueller-Burri, A.S.; Haegeli, L.M.; Biaggi, P.; Manka, R.; Lüscher, T.F.; et al. Different prognostic value of functional right ventricular parameters in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circ. Cardiovasc. Imaging 2014, 7, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, A.; Caselli, S.; Sharma, S.; Basso, C.; Bax, J.J.; Corrado, D.; D’Andrea, A.; D’Ascenzi, F.; Di Paolo, F.M.; Edvardsen, T.; et al. European Association of Preventive Cardiology (EAPC) and European Association of Cardiovascular Imaging (EACVI) joint position statement: Recommendations for the indication and interpretation of cardiovascular imaging in the evaluation of the athlete’s heart. Eur. Heart J. 2018, 39, 1949–1969. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.H.; Haskell, W.; Snell, P.; Van Camp, S.P. Task Force 8: Classification of sports. J. Am. Coll. Cardiol. 2005, 45, 1364–1367. [Google Scholar] [CrossRef] [Green Version]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American society of echocardiography and the European association of cardiovascular imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef]
- Brosnan, M.J.; Riele, A.S.T.; Bosman, L.P.; Hoorntje, E.T.; Berg, M.V.D.; Hauer, R.N.; Flannery, M.D.; Kalman, J.M.; Prior, D.L.; Tichnell, C.; et al. Electrocardiographic Features Differentiating Arrhythmogenic Right Ventricular Cardiomyopathy From an Athlete’s Heart. JACC Clin. Electrophysiol. 2018, 4, 1613–1625. [Google Scholar] [CrossRef]
- Mertens, L.L.; Friedberg, M. Imaging the right ventricle—current state of the art. Nat. Rev. Cardiol. 2010, 7, 551–563. [Google Scholar] [CrossRef]
- Oezcan, S.; Jost, C.A.; Pfyffer, M.; Kellenberger, C.; Jenni, R.; Binggeli, C.; Faeh-Gunz, A.; Seifert, B.; Scharf, C.; Kretschmar, O.; et al. Pectus excavatum: Echocardiography and cardiac MRI reveal frequent pericardial effusion and right-sided heart anomalies. Eur. Heart J. Cardiovasc. Imaging 2012, 13, 673–679. [Google Scholar] [CrossRef] [Green Version]
- Venlet, J.; Piers, S.R.; Jongbloed, J.D.; Androulakis, A.F.; Naruse, Y.; den Uijl, D.W.; Kapel, G.F.; de Riva, M.; van Tintelen, J.P.; Barge-Schaapveld, D.Q.; et al. Isolated Subepicardial Right Ventricular Outflow Tract Scar in Athletes with Ventricular Tachycardia. J. Am. Coll. Cardiol. 2017, 69, 497–507. [Google Scholar] [CrossRef]
- Corrado, D.; Pelliccia, A.; Heidbuchel, H.; Sharma, S.; Link, M.; Basso, C.; Biffi, A.; Buja, G.; Delise, P.; Gussac, I.; et al. Recommendations for interpretation of 12-lead electrocardiogram in the athlete. Eur. Heart J. 2010, 31, 243–259. [Google Scholar] [CrossRef] [Green Version]
- Ohno, S. The genetic background of arrhythmogenic right ventricular cardiomyopathy. J. Arrhythmia 2016, 32, 398–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, S.; Medeiros-Domingo, A.; Gasperetti, A.; Akdis, D.; Berger, W.; James, C.A.; Ruschitzka, F.; Brunckhorst, C.B.; Duru, F.; Saguner, A.M. Impact of Genetic Variant Reassessment on the Diagnosis of Arrhythmogenic Right Ventricular Cardiomyopathy Based on the 2010 Task Force Criteria. Circ. Genom. Precis. Med. 2021, 14. [Google Scholar] [CrossRef] [PubMed]
- D’Ascenzi, F.; Cameli, M.; Padeletti, M.; Lisi, M.; Zacà, V.; Natali, B.; Malandrino, A.; Alvino, F.; Morelli, M.; Vassallo, G.M.; et al. Characterization of right atrial function and dimension in top-level athletes: A speckle tracking study. Int. J. Cardiovasc. Imaging 2012, 29, 87–94. [Google Scholar] [CrossRef]
- Li, G.; Fontaine, G.H.; Fan, S.; Yan, Y.; Bode, P.K.; Duru, F.; Frank, R.; Saguner, A.M. Right atrial pathology in arrhythmogenic right ventricular dysplasia. Cardiol. J. 2020, 26, 736–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abhayaratna, W.; Seward, J.B.; Appleton, C.P.; Douglas, P.S.; Oh, J.K.; Tajik, A.J.; Tsang, T.S. Left Atrial Size: Physiologic Determinants and Clinical Applications. J. Am. Coll. Cardiol. 2006, 47, 2357–2363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelliccia, A.; Culasso, F.; Di Paolo, F.M.; Maron, B.J. Physiologic Left Ventricular Cavity Dilatation in Elite Athletes. Ann. Intern. Med. 1999, 130, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Iskandar, A.; Mujtaba, M.T.; Thompson, P.D. Left Atrium Size in Elite Athletes. JACC Cardiovasc. Imaging 2015, 8, 753–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Ascenzi, F.; Anselmi, F.; Focardi, M.; Mondillo, S. Atrial Enlargement in the Athlete’s Heart: Assessment of Atrial Function May Help Distinguish Adaptive from Pathologic Remodeling. J. Am. Soc. Echocardiogr. 2018, 31, 148–157. [Google Scholar] [CrossRef] [PubMed]
- D’Ascenzi, F.; Pisicchio, C.; Caselli, S.; Di Paolo, F.M.; Spataro, A.; Pelliccia, A. RV Remodeling in Olympic Athletes. JACC Cardiovasc. Imaging 2017, 10, 385–393. [Google Scholar] [CrossRef] [PubMed]
- D’Ascenzi, F.; Pelliccia, A.; Solari, M.; Piu, P.; Loiacono, F.; Anselmi, F.; Caselli, S.; Focardi, M.; Bonifazi, M.; Mondillo, S. Normative Reference Values of Right Heart in Competitive Athletes: A Systematic Review and Meta-Analysis. J. Am. Soc. Echocardiogr. 2017, 30, 845–858.e2. [Google Scholar] [CrossRef] [PubMed]
- Scharhag, J.; Herrmann, M.; Urhausen, A.; Haschke, M.; Herrmann, W.; Kindermann, W. Independent elevations of N-terminal pro–brain natriuretic peptide and cardiac troponins in endurance athletes after prolonged strenuous exercise. Am. Heart J. 2005, 150, 1128–1134. [Google Scholar] [CrossRef]
- Baggish, A.L.; Wood, M.J. Athlete’s heart and cardiovascular care of the athlete: Scientific and clinical update. Circulation 2011, 123, 2723–2735. [Google Scholar] [CrossRef] [Green Version]
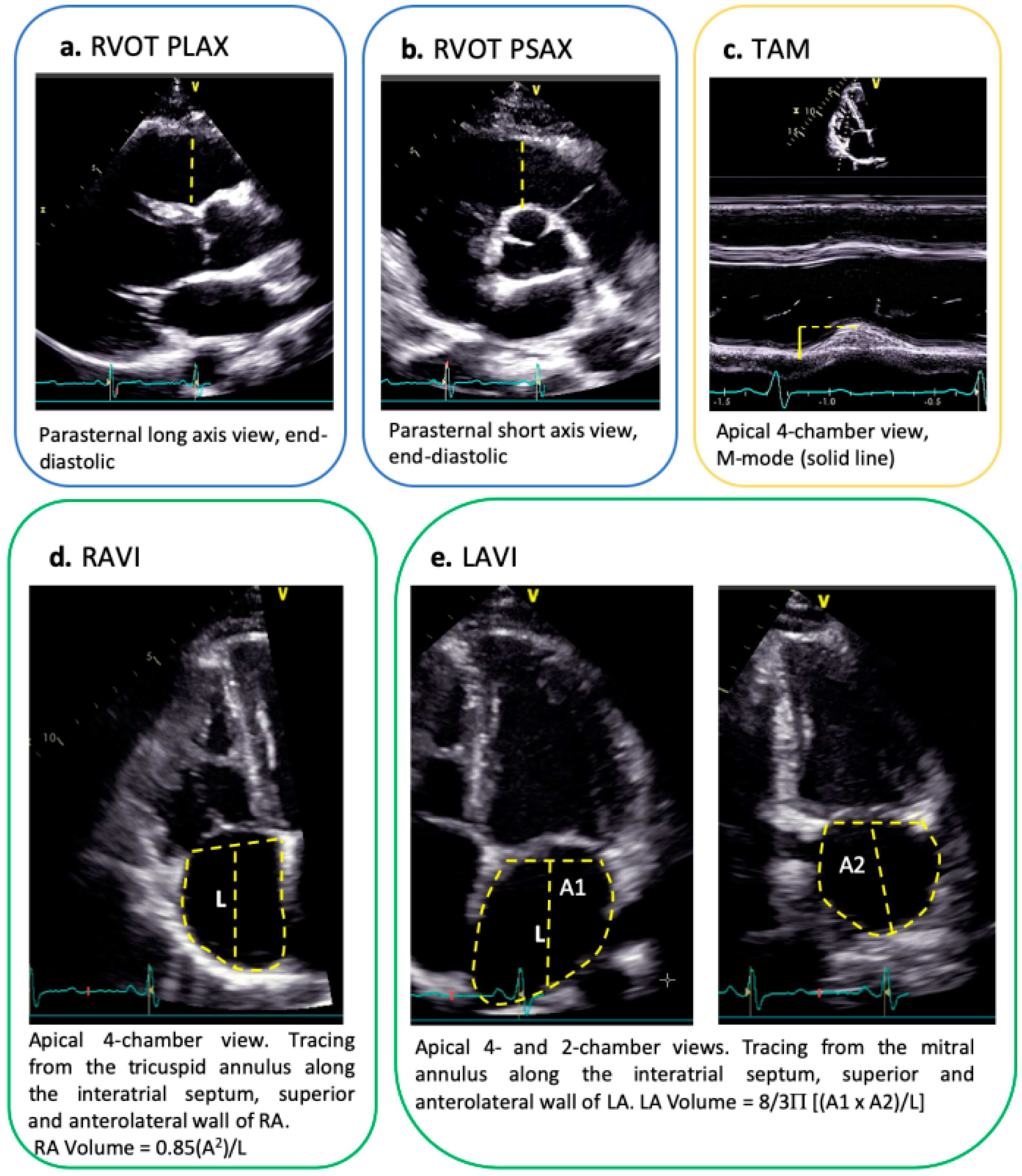
| ARVC n = 21 | Athletes n = 42 | ||
|---|---|---|---|
| Mean (SD) or % | Mean (SD) or % | p-Value | |
| Gender (male/female) | 16/5 | 33/9 | 0.830 |
| Age (years) | 37.3 (17.6) | 32.4 (12.5) | 0.204 |
| BMI (kg/m2) | 22.7 (5.9) | 22.7 (2.5) | 0.992 |
| Sports % | 57 | 100 | |
| endurance/mixed/strength (n) | 8/4/0 | 23/19/0 | 0.462 |
| Among these: competitive athletes or professional (n) | 1 | 8 | 0.417 |
| Co-morbidities/medication | |||
| Atrial fibrillation/flutter % | 4.8 | 2.4 | 0.624 |
| Arterial hypertension % | 4.8 | 11.9 | 0.349 |
| Beta-blocker | 61.9 | 4.8 | <0.001 |
| Amiodarone | 14.3 | 0 | 0.013 |
| Diuretics | 9.5 | 0 | 0.045 |
| Aldosterone-Antagonists | 14.3 | 0 | 0.013 |
| ACE-I/Sartans | 23.8 | 9.5 | 0.137 |
| ECG at baseline | |||
| HR at rest (bpm) | 56.9 (8.9) | 55.8 (11.5) | 0.691 |
| AV-block 1st degree % | 14.3 | 9.5 | 0.571 |
| T-wave inversions beyond V2 % | 90.5 | 19 | <0.001 |
| Right bundle branch block % | 23.8 | 9.5 | 0.127 |
| 24 h Holter ECG | n = 21 | n = 24 | |
| SVPB count/24 h | 768 (1013) | 768 (1550) | 0.999 |
| PVC count/24 h | 2331 (2812) | 111 (219) | <0.001 |
| Blood test | |||
| Leucocyte total G/L | 6.7 (2.3) | 5.9 (1.7) | 0.194 |
| Hb g/dl | 144.6 (7.3) | 143.8 (13.6) | 0.806 |
| NT-proBNP ng/L | 345 (612) | 48 (57) | <0.001 |
| CRP mg/L | 2 (2.6) | 0.8 (0.6) | 0.021 |
| Cholesterin total mmol/L | 4.3 (0.7) | 5.4 (5.7) | 0.518 |
| GFR (CKD-EPI, mL/min) | 94.1 (21.9) | 100.3 (18.6) | 0.296 |
| TSH mU/L | 1.6 (1) | 2.1 (1) | 0.152 |
| Ergometry | n = 13 | n = 33 | |
| Maximum Watt | 179.8 (75.3) | 301 (86.8) | <0.001 |
| % of expected maximum Watt | 109 (35.6) | 161.5 (36.5) | <0.001 |
| HR max (bpm) | 149.1 (30.5) | 171.2 (23.1) | 0.007 |
| % of expected HR max | 85.1 (14.7) | 95.6 (12.8) | 0.017 |
| Double product factor | 3.3 (0.9) | 3.6 (0.8) | 0.262 |
| BP syst max mmHg | 181.4 (30.8) | 205.2 (25) | 0.007 |
| BP diast max mmHg | 82.5 (11.3) | 88.8 (12.5) | 0.099 |
| ECG alteration/arrhythmia % | 61.5 | 9.1 | <0.001 |
| ARVC Patients n = 21 Mean (SD) or % | Athletes n = 42 Mean (SD) or % | p-Value | |
|---|---|---|---|
| Echocardiography | |||
| LAVI mL/m2 | 27.2 (17.7) | 33.4 (7.8) | 0.059 |
| RAVI mL/m2 | 40.7 (29.8) | 28.5 (8.7) | 0.017 |
| RAVI/LAVI ratio | 1.76 (1.5) | 0.87 (0.2) | <0.001 |
| RA/LA axis ratio | 1.2 (0.4) | 0.97 (0.1) | 0.004 |
| PLAX RVOT cm | 3.6 (0.7) | 3.1 (0.5) | 0.002 |
| PLAX RVOT/BSA cm/m2 | 2.2 (1.2) | 1.7 (0.3) | 0.013 |
| PSAX RVOT cm | 3.7 (0.8) | 3 (0.5) | <0.001 |
| PSAX RVOT/BSA cm/m2 | 2.2 (1.2) | 1.6 (0.3) | 0.003 |
| RVIT cm | 4.4 (0.9) | 3.8 (0.4) | 0.001 |
| RVIT/BSA cm/m2 | 2.5 (1) | 2 (0.3) | 0.003 |
| RV ED short axis cm | 4.2 (0.9) | 4.1 (0.5) | 0.478 |
| RV Area D cm2 | 31.2 (8.7) | 24 (4.1) | <0.001 |
| fac% | 29 (10.1) | 42.2 (5) | <0.001 |
| TAM mm | 19.8 (5.4) | 23.8 (3.8) | 0.001 |
| LV ED short axis cm | 4.9 (0.6) | 5.2 (0.6) | 0.078 |
| RV/LV ratio | 0.9 (0.2) | 0.8 (0.1) | 0.029 |
| LV shortening % | 32.7 (10.5) | 35.7 (6.2) | 0.155 |
| LV posterior wall mm | 0.8 (0.1) | 0.8 (0.1) | 0.171 |
| LV septal wall mm | 0.9 (0.2) | 0.9 (0.1) | 0.575 |
| LVEF % | 55.8 (11.4) | 56.9 (9.2) | 0.683 |
| LVEDVI mL/m2 | 60.8 (12.5) | 73.7 (14.2) | 0.001 |
| LVMNI g/m2 | 79.5 (32.2) | 83.6 (24.2) | 0.580 |
| rTh | 0.3 (0.1) | 0.3 (0.1) | 0.893 |
| LV wall motion anomalies % | 33.3 | 0 | <0.001 |
| Diastolic dysfunction % | 23.8 | 0 | <0.001 |
| E/e’ | 8.1 (2.6) | 6.8 (1.6) | 0.022 |
| Criterion | ARVC Patients | Athletes |
|---|---|---|
| I. Global or regional dysfunction and structural alterations on TTE | ||
| Major (n) | 20 | 1 |
| Minor (n) | 1 | |
| II. Tissue characterization of RV wall* | ||
| Major (n) | 3 | - |
| Minor (n) | 1 | - |
| Biopsy available in n = 5 ARVC patients and none among athletes | ||
| III. Repolarization abnormalities | ||
| Major (n) | 11 | 6 |
| Minor (n) | 6 | 1 |
| IV. Depolarization abnormalities | ||
| Major (n) | 3 | 0 |
| Minor (n) | 4 | 1 |
| V. Ventricular Arrhythmias | ||
| Major (n) | 3 | 1 |
| Minor (n) | 13 | 1 |
| VI. Family history | ||
| Major (n) | 10 | 0 |
| Minor (n) | 1 | |
| Pathogenic/likely pathogenic genetic variants | n = 16 | |
| PKP-2 n = 8 DSG-2 n = 4 DSP n = 1 DSC-2 n = 1 SCN5A n = 1 TTN n = 1 | ||
| Value | Sensitivity, % | Specificity, % | p-Value | 0 Points | 1 Point | 2 Points | |
|---|---|---|---|---|---|---|---|
| NT-proBNP ng/L | 116 | 52 | 91 | <0.001 | ≤35 | 36–115 | ≥116 |
| RVOT PLAX/BSA cm/m2 | 1.9 | 43 | 80 | 0.004 | ≤1.6 | 1.6–1.8 | ≥1.9 |
| RVOT PSAX/BSA cm/m2 | 2.1 | 33 | 93 | 0.001 | ≤1.8 | 1.8–2.0 | ≥2.1 |
| RAVI/LAVI | 1.11 | 63 | 91 | <0.001 | ≤0.95 | 0.96–1.1 | ≥1.11 |
| TAM mm | 17 | 77 | 99 | 0.003 | ≥23 | 18–22 | ≤17 |
| TWI * | <0.001 | absent | present | ||||
| Depolarization abnormalities ** | <0.001 | absent | present |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossi, V.A.; Niederseer, D.; Sokolska, J.M.; Kovacs, B.; Costa, S.; Gasperetti, A.; Brunckhorst, C.; Akdis, D.; Tanner, F.C.; Duru, F.; et al. A Novel Diagnostic Score Integrating Atrial Dimensions to Differentiate between the Athlete’s Heart and Arrhythmogenic Right Ventricular Cardiomyopathy. J. Clin. Med. 2021, 10, 4094. https://doi.org/10.3390/jcm10184094
Rossi VA, Niederseer D, Sokolska JM, Kovacs B, Costa S, Gasperetti A, Brunckhorst C, Akdis D, Tanner FC, Duru F, et al. A Novel Diagnostic Score Integrating Atrial Dimensions to Differentiate between the Athlete’s Heart and Arrhythmogenic Right Ventricular Cardiomyopathy. Journal of Clinical Medicine. 2021; 10(18):4094. https://doi.org/10.3390/jcm10184094
Chicago/Turabian StyleRossi, Valentina A., David Niederseer, Justyna M. Sokolska, Boldizsar Kovacs, Sarah Costa, Alessio Gasperetti, Corinna Brunckhorst, Deniz Akdis, Felix C. Tanner, Firat Duru, and et al. 2021. "A Novel Diagnostic Score Integrating Atrial Dimensions to Differentiate between the Athlete’s Heart and Arrhythmogenic Right Ventricular Cardiomyopathy" Journal of Clinical Medicine 10, no. 18: 4094. https://doi.org/10.3390/jcm10184094
APA StyleRossi, V. A., Niederseer, D., Sokolska, J. M., Kovacs, B., Costa, S., Gasperetti, A., Brunckhorst, C., Akdis, D., Tanner, F. C., Duru, F., Schmied, C. M., & Saguner, A. M. (2021). A Novel Diagnostic Score Integrating Atrial Dimensions to Differentiate between the Athlete’s Heart and Arrhythmogenic Right Ventricular Cardiomyopathy. Journal of Clinical Medicine, 10(18), 4094. https://doi.org/10.3390/jcm10184094








