Active Ingredients of Voice Therapy for Muscle Tension Voice Disorders: A Retrospective Data Audit
Abstract
1. Introduction
1.1. Behavioural Voice Therapy Is the First-Line Treatment for MTVD
1.2. What Is an Active Ingredient in Voice Therapy?
1.3. VoiceCraft® Sob Voice Therapy
1.4. Retrospective Cohort Analysis vs. Randomised Control Trial
- (1)
- evaluate the overall treatment effects of the Sob Voice Therapy program on MTVD with and without mucosal lesions of the vocal folds;
- (2)
- investigate the effects of ingredients within the Sob Voice Therapy program on treatment outcomes for patients with MTVD; and
- (3)
- identify any diagnostic or service delivery factors that influence the efficacy of a specific technique or process.
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.2.1. Selection Criteria
2.2.2. Sample Size Calculation
2.3. Voice Therapy Programs under Review: Sob Voice Therapy
2.4. Data Extraction
2.4.1. Demographic Characteristics
2.4.2. Extraction of Voice Recordings
2.5. Auditory–Perceptual Outcome Measures
2.5.1. Listeners
2.5.2. Stimuli
2.5.3. Procedure
2.5.4. Reliability of Auditory–Perceptual Analyses
2.6. Acoustic Outcome Measures
2.6.1. Harmonics-to-Noise Ratio (HNR)
2.6.2. Fundamental Frequency (F0)
2.6.3. Cepstral Peak Prominence: Non-Smoothed (CPP) and Smoothed (CPPS)
2.6.4. Cepstral/Spectral Index of Dysphonia
2.6.5. Vocal Intensity
2.6.6. Reliability Analysis of Acoustic Measurements
2.7. Statistical Analyses
3. Results
3.1. Characteristics of the Study Population
3.2. Treatment Effects of Sob Voice Therapy on MTVD
3.2.1. Auditory-Perceptual Outcomes
3.2.2. Acoustic Outcomes
3.3. Estimates of Active Ingredients within the Sob Voice Therapy Program
3.3.1. Effect Size for Auditory–Perceptual Outcomes
3.3.2. Effect Size for Acoustic Outcomes
3.4. Impact of Service Delivery Factors on the Treatment Outcome
3.4.1. Number of Sessions and Duration of Sob Voice Therapy
3.4.2. Clinician Effects
3.5. Drop-Out Rate
4. Discussion
4.1. Treatment Effects of Sob Voice Therapy on Patients with MTVD
4.2. Active Ingredients of the Sob Voice Therapy Program
4.2.1. Effects of OPT
4.2.2. Effects of SVQ
4.2.3. Effects of SVQ Task Variation
4.2.4. Effects of Negative Practice
4.3. Effect of Diagnosis and Service Delivery
4.4. Comparison with Other Voice Therapy Outcomes Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
Appendix A. Settings for Acoustic Measurements
Appendix A.1. Settings for the Fundamental Frequency Measurement in Praat
Appendix A.2. Settings for the CPP Measurement in the Analysis of Dysphonia in Speech and Voice (ADSV)
Appendix A.3. Settings for the CPPS Measurement in Praat
Appendix B
| Vocal Load | Frequency | Percent |
|---|---|---|
| Below 2 h | 4 | 6.5 |
| 2–4 h | 9 | 14.5 |
| 4–6 h | 16 | 25.8 |
| Above 6 h | 33 | 53.2 |
| Total | 62 | 100.0 |
| Comorbidities | Frequency | Percent | |
|---|---|---|---|
| Reflux | No | 37 | 54.4 |
| Yes | 31 | 45.6 | |
| Sinusitis | No | 52 | 76.5 |
| Yes | 16 | 23.5 | |
| Asthma | No | 57 | 83.8 |
| Yes | 11 | 16.2 | |
| Cough | No | 33 | 48.5 |
| Yes | 35 | 51.5 | |
| Stress (missing data n = 4) | No | 22 | 34.4 |
| Yes | 42 | 65.6 | |
| Factors | Frequency | Percent | |
|---|---|---|---|
| Caffein (missing data n = 2) | No | 7 | 10.6 |
| Yes | 59 | 89.4 | |
| Alcohol (missing data n = 1) | No | 14 | 20.9 |
| Yes | 53 | 79.1 | |
| Smoking (missing data n = 1) | No | 65 | 97.0 |
| Yes | 2 | 3.0 | |
Appendix C
| Measure | Normative Cut-Off | Time Point | Mean (SD) | 95% CI | MD | F | p | Partial η2 |
|---|---|---|---|---|---|---|---|---|
| CPP vowel | 11.74 dB [87] | Baseline | 11.4 (2.6) | 10.0–12.7 | ||||
| OPT | 9.7 (2.6) | 8.4–11.1 | 1.7 | 0.722 | 0.399 | 0.011 | ||
| SVQ | 10.3 (2.3) | 9.0–11.5 | 1.1 | 0.096 | 0.759 | 0.002 | ||
| SVQ variant | 10.4 (2.1) | 9.2–11.4 | 1.0 | 0.514 | 0.479 | 0.017 | ||
| Post NP | 10.9 (2.1) | 9.7–12.0 | 0.5 | 0.599 | 0.448 | 0.029 | ||
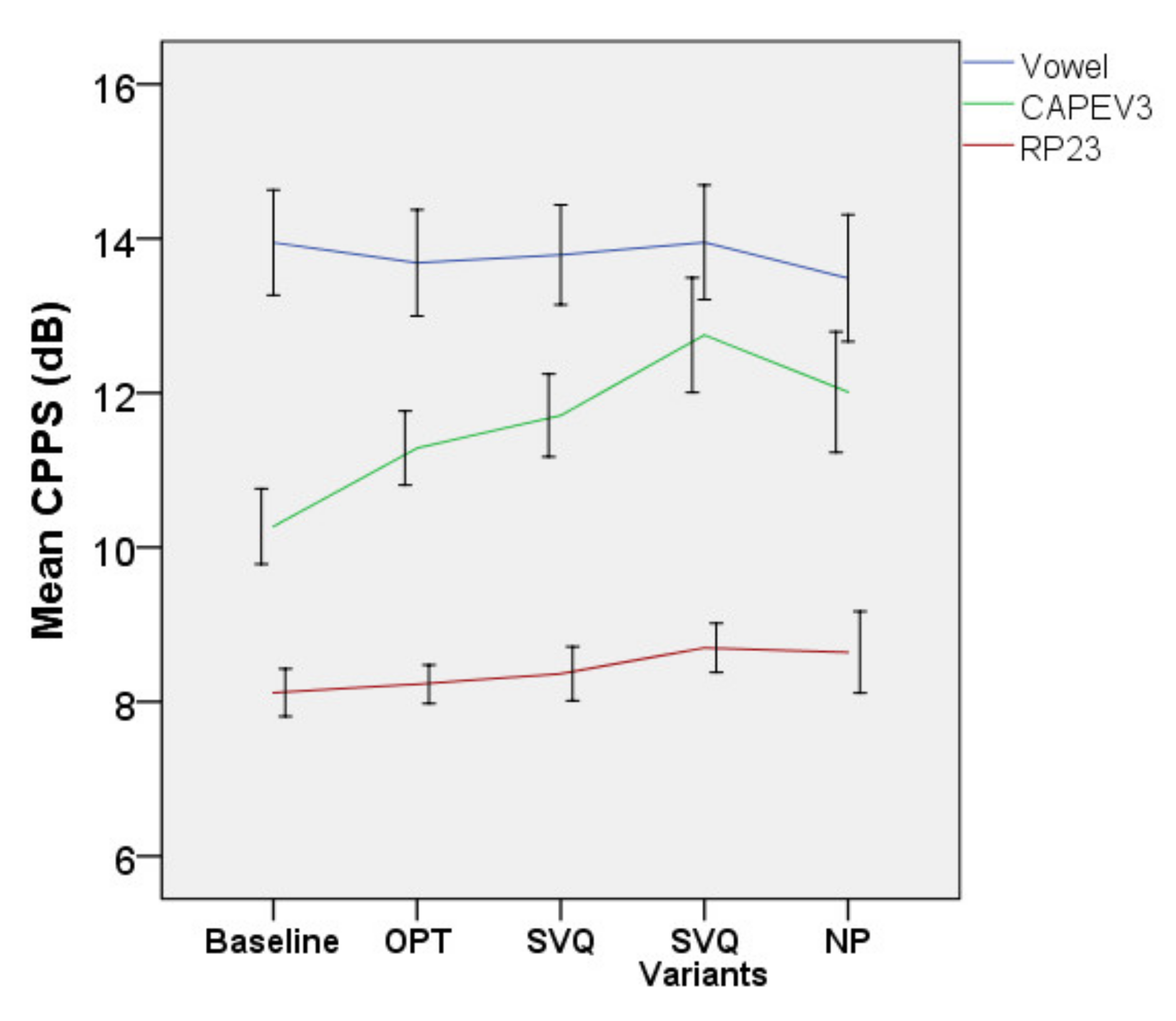
| CPPS vowel | 14.45 dB [111] | Baseline | 14.5 (3.2) | 12.8–16.1 | ||||
| OPT | 13.3 (3.9) | 11.4–15.3 | 1.2 | 0.658 | 0.420 | 0.010 | ||
| SVQ | 13.4 (2.6) | 12.1–14.7 | 1.1 | 1.215 | 0.277 | 0.029 | ||
| SVQ variant | 13.6 (1.8) | 12.6–14.5 | 0.9 | 0.197 | 0.661 | 0.007 | ||
| NP | 13.7 (1.8) | 12.8–14.6 | 0.8 | 1.054 | 0.317 | 0.050 | ||
| CPPS RP23 | 9.33 dB [111] | Baseline | 8.4 (1.0) | 7.9–8.9 | ||||
| OPT | 8.4 (1.0) | 8.0–8.9 | 0.0 | 0.497 | 0.483 | 0.007 | ||
| SVQ | 8.7 (1.3) | 8.1–9.3 | 0.3 | 0.647 | 0.426 | 0.015 | ||
| SVQ variant | 8.6 (1.1) | 7.7–8.8 | 0.2 | 2.622 | 0.115 | 0.076 | ||
| NP | 8.8 (1.2) | 8.2–9.4 | 0.4 | 3.108 | 0.091 | 0.119 | ||
| CSID vowel | NA | Baseline | 3.8 (16.6) | −5.1–12.6 | ||||
| OPT | 12.7 (17.3) | 3.5–22.0 | 8.9 | 0.287 | 0.594 | 0.004 | ||
| SVQ | 6.1 (11.5) | 0.0–12.2 | 2.3 | 0.094 | 0.760 | 0.002 | ||
| SVQ variant | 6.2 (12.8) | −0.6–13.0 | 2.4 | 0.935 | 0.341 | 0.030 | ||
| NP | 3.4 (11.1) | −2.6–9.3 | 0.4 | 0.001 | 0.973 | 0.000 | ||
| Intensity vowel | 73.42 dB [76] | Baseline | 57.0 (7.0) | 53.4–60.6 | ||||
| OPT | 56.4 (7.5) | 52.6–60.3 | 0.6 | 1.147 | 0.288 | 0.018 | ||
| SVQ | 57.9 (6.4) | 54.6–61.2 | 0.9 | 0.253 | 0.618 | 0.006 | ||
| SVQ variant | 58.0 (6.4) | 54.7–61.3 | 1.0 | 0.118 | 0.734 | 0.004 | ||
| NP | 56.8 (6.9) | 53.3–60.4 | 0.2 | 0.055 | 0.817 | 0.003 | ||
| Intensity CAPEV3 | NA | Baseline | 56.6 (5.3) | 53.9–59.2 | ||||
| OPT | 57.0 (6.8) | 53.6–60.4 | 0.4 | 0.474 | 0.494 | 0.007 | ||
| SVQ | 56.8 (6.0) | 53.8–59.8 | 0.2 | 0.837 | 0.366 | 0.020 | ||
| SVQ variant | 56.8 (5.6) | 54.1–59.6 | 0.2 | 0.034 | 0.854 | 0.001 | ||
| NP | 54.9 (6.3) | 51.8–58.0 | 1.7 | 1.155 | 0.294 | 0.050 | ||
| Intensity of RP | 68.37 dB [112] | Baseline | 47.9 (6.7) | 44.7–51.1 | ||||
| OPT | 49.3 (6.0) | 46.4–52.2 | 1.4 | 0.369 | 0.545 | 0.006 | ||
| SVQ | 49.5 (5.4) | 46.9–52.1 | 1.6 | 0.035 | 0.852 | 0.001 | ||
| SVQ variant | 49.0 (6.5) | 45.9–52.1 | 1.1 | 0.036 | 0.851 | 0.001 | ||
| NP | 47.8 (7.8) | 44.0–51.6 | 0.1 | 0.006 | 0.938 | 0.000 |
References
- Baker, J.; Ben-Tovim, D.I.; Butcher, A.; Esterman, A.; McLaughlin, K. Development of a modified diagnostic classification system for voice disorders with inter-rater reliability study. Logop. Phoniatr. Vocol. 2007, 32, 99–112. [Google Scholar] [CrossRef]
- Oates, J.; Winkworth, A. Current knowledge, controversies and future directions in hyperfunctional voice disorders. Int. J. Speech-Lang. Pathol. 2008, 10, 267–277. [Google Scholar] [CrossRef]
- Dabirmoghaddam, P.; Rahmaty, B.; Erfanian, R.; Taherkhani, S.; Msc, S.H.; Satariyan, A. Voice Component Relationships with High Reflux Symptom Index Scores in Muscle Tension Dysphonia. Laryngoscope 2021, 131. [Google Scholar] [CrossRef]
- Martins, R.H.G.; Amaral, H.A.D.; Tavares, E.L.M.; Martins, M.G.; Gonçalves, T.M.; Dias, N.H. Voice Disorders: Etiology and Diagnosis. J. Voice 2016, 30, 761.e1–761.e9. [Google Scholar] [CrossRef] [PubMed]
- Van Houtte, E.; Van Lierde, K.; Claeys, S. Pathophysiology and Treatment of Muscle Tension Dysphonia: A Review of the Current Knowledge. J. Voice 2011, 25, 202–207. [Google Scholar] [CrossRef]
- Nanjundeswaran, C.; Li-Jessen, N.; Chan, K.M.; Wong, K.S.R.; Yiu, E.M.-L.; Verdolini-Abbott, K. Preliminary Data on Prevention and Treatment of Voice Problems in Student Teachers. J. Voice 2012, 26, 816.e1–816.e12. [Google Scholar] [CrossRef] [PubMed]
- Mathieson, L. The Voice and Its Disorders, 6th ed.; Whurr Publishers: London, UK, 2001. [Google Scholar]
- Carding, P. Evaluating Voice Therapy. Measuring the Effectiveness of Treatment; Whurr Publishers: London, UK, 2000. [Google Scholar]
- Chan, A.K.; McCabe, P.; Madill, C. The implementation of evidence-based practice in the management of adults with functional voice disorders: A national survey of speech-language pathologists. Int. J. Speech-Lang. Pathol. 2013, 15, 334–344. [Google Scholar] [CrossRef]
- Signorelli, M.E.; Madill, C.; McCabe, P. The management of vocal fold nodules in children: A national survey of speech-language pathologists. Int. J. Speech-Lang. Pathol. 2011, 13, 227–238. [Google Scholar] [CrossRef]
- Eastwood, C.; Madill, C.; Mccabe, P. The behavioural treatment of muscle tension voice disorders: A systematic review. Int. J. Speech-Lang. Pathol. 2015, 17, 287–303. [Google Scholar] [CrossRef]
- Gartner-Schmidt, J.L.; Roth, D.F.; Zullo, T.G.; Rosen, C.A. Quantifying Component Parts of Indirect and Direct Voice Therapy Related to Different Voice Disorders. J. Voice 2013, 27, 210–216. [Google Scholar] [CrossRef] [PubMed]
- De Bodt, M.; Patteeuw, T.; Versele, A. Temporal Variables in Voice Therapy. J. Voice 2015, 29, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Zanca, J.M.; Turkstra, L.S.; Chen, C.; Packel, A.; Ferraro, M.; Hart, T.; Van Stan, J.H.; Whyte, J.; Dijkers, M.P. Advancing Rehabilitation Practice Through Improved Specification of Interventions. Arch. Phys. Med. Rehabil. 2019, 100, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Roy, N. Optimal dose–response relationships in voice therapy. Int. J. Speech-Lang. Pathol. 2012, 14, 419–423. [Google Scholar] [CrossRef]
- Baker, E. Optimal intervention intensity in speech-language pathology: Discoveries, challenges, and unchartered territories. Int. J. Speech-Lang. Pathol. 2012, 14, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Turkstra, L.S.; Norman, R.; Whyte, J.; Dijkers, M.P.; Hart, T. Knowing What We‘re Doing: Why Specification of Treatment Methods Is Critical for Evidence-Based Practice in Speech-Language Pathology. Am. J. Speech-Lang. Pathol. 2016, 25, 164–171. [Google Scholar] [CrossRef]
- Van Stan, J.H.; Whyte, J.; Duffy, J.R.; Barkmeier-Kraemer, J.M.; Doyle, P.B.; Gherson, S.; Kelchner, L.; Muise, J.; Petty, B.; Roy, N.; et al. Rehabilitation Treatment Specification System: Methodology to Identify and Describe Unique Targets and Ingredients. Arch. Phys. Med. Rehabil. 2021, 102, 521–531. [Google Scholar] [CrossRef]
- Van Stan, J.H.; Roy, N.; Awan, S.; Stemple, J.; Hillman, R.E. A taxonomy of voice therapy. Am. J. Speech-Lang. Pathol. 2015, 24, 101–125. [Google Scholar] [CrossRef]
- Verdolini-Abbott, K. Lessac-Madsen Resonant Voice Therapy Clinician Manual; Plural Publishing: San Diego, CA, USA, 2008. [Google Scholar]
- Nguyen, D.D.; Kenny, D.T. Randomized controlled trial of vocal function exercises on muscle tension dysphonia in Vietnamese female teachers. J. Otolaryngol. Head Neck Surg. 2009, 38, 261–278. [Google Scholar] [PubMed]
- Stone, R.E.; Casteel, R.I. Restoration of voice in nonorganically based dysphonia. In Phonatory Voice Disorders in Children; Filter, M., Ed.; Charles C Thomas: Springfield, IL, USA, 1982. [Google Scholar]
- Bassiouny, S. Efficacy of the accent method of voice therapy. Folia Phoniatr. Logop. 1998, 50, 146–164. [Google Scholar] [CrossRef] [PubMed]
- Fex, B.; Fex, S.; Shiromoto, O.; Hirano, M. Acoustic analysis of functional dysphonia: Before and after voice therapy (accent method). J. Voice 1994, 8, 163–167. [Google Scholar] [CrossRef]
- Iwarsson, J. Facilitating behavioral learning and habit change in voice therapy—Theoretic premises and practical strategies. Logop. Phoniatr. Vocol. 2015, 40, 179–186. [Google Scholar] [CrossRef]
- S.L. Hunter Speechworks. Resonant Voice Therapy. 2008. Available online: https://www.slhunterspeechworks.com/Therapy-Services/Voice-Therapy/Resonant-Voice-Therapy (accessed on 12 August 2021).
- Watts, C.R.; Diviney, S.S.; Hamilton, A.; Toles, L.; Childs, L.; Mau, T. The Effect of Stretch-and-Flow Voice Therapy on Measures of Vocal Function and Handicap. J. Voice 2015, 29, 191–199. [Google Scholar] [CrossRef]
- Bane, M.; Angadi, V.; Dressler, E.; Andreatta, R.; Stemple, J. Vocal function exercises for normal voice: The effects of varying dosage. Int. J. Speech-Lang. Pathol. 2019, 21, 37–45. [Google Scholar] [CrossRef]
- Bane, M.; Brown, M.; Angadi, V.; Croake, D.J.; Andreatta, R.D.; Stemple, J.C. Vocal function exercises for normal voice: With and without semi-occlusion. Int. J. Speech-Lang. Pathol. 2019, 21, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Madill, C.; Bagnall, A.D.; Beatty, J. Voicecraft Essentials Workshop Manual; Voicecraft International: Adelaide, SA, Australia, 2015. [Google Scholar]
- Bagnall, A.D. Voicecraft Workshop Manual; Voicecraft International: Adelaide, SA, Australia, 1997. [Google Scholar]
- Madill, C.; Sheard, C.; Heard, R. Differentiated Vocal Tract Control and the Reliability of Interpretations of Nasendoscopic Assessment. J. Voice 2010, 24, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Bagnall, A.D.; McCulloch, K. The Impact of Specific Exertion on the Efficiency and Ease of the Voice: A Pilot Study. J. Voice 2005, 19, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Kelso, J.A.S.; Schöner, G. Self-organization of coordinative movement patterns. Hum. Mov. Sci. 1988, 7, 27–46. [Google Scholar] [CrossRef]
- Winkworth, A.L.; Davis, P.J.; Adams, R.D.; Ellis, E. Breathing Patterns During Spontaneous Speech. J. Speech Lang. Hear. Res. 1995, 38, 124–144. [Google Scholar] [CrossRef]
- Winkworth, A.L.; Davis, P.J.; Ellis, E.; Adams, R.D. Variability and Consistency in Speech Breathing During Reading. J. Speech Lang. Hear. Res. 1994, 37, 535–556. [Google Scholar] [CrossRef]
- Sprecher, A.; Olszewski, A.; Jiang, J.J.; Zhang, Y. Updating signal typing in voice: Addition of type 4 signals. J. Acoust. Soc. Am. 2010, 127, 3710–3716. [Google Scholar] [CrossRef]
- GLIMMPSE. General Linear Mixed Model Power and Sample Size. Available online: https://glimmpse.samplesizeshop.org/ (accessed on 2 January 2021).
- Guo, Y.; Logan, H.L.; Glueck, D.H.; Muller, K.E. Selecting a sample size for studies with repeated measures. BMC Med. Res. Methodol. 2013, 13, 493–498. [Google Scholar] [CrossRef]
- Wenke, R.; Coman, L.; Walton, C.; Madill, C.; Theodoros, D.; Bishop, C.; Stabler, P.; Lawrie, M.; O‘Neill, J.; Gray, H.; et al. Effectiveness of Intensive Voice Therapy Versus Weekly Therapy for Muscle Tension Dysphonia: A Noninferiority Randomised Controlled Trial with Nested Focus Group. J. Voice 2021. [Google Scholar] [CrossRef] [PubMed]
- Maas, E.; Robin, D.A.; Hula, S.N.A.; Freedman, S.E.; Wulf, G.; Ballard, K.; Schmidt, R.A. Principles of Motor Learning in Treatment of Motor Speech Disorders. Am. J. Speech-Lang. Pathol. 2008, 17, 277–298. [Google Scholar] [CrossRef]
- Sataloff, R.T. Professional Voice: The Science and Art of Clinical Care; Plural Publishing: San Diego, CA, USA, 2017. [Google Scholar]
- Rosen, C.A.; Lee, A.S.; Osborne, J.; Zullo, T.; Murry, T. Development and Validation of the Voice Handicap Index-10. Laryngoscope 2004, 114, 1549–1556. [Google Scholar] [CrossRef]
- Belafsky, P.C.; Postma, G.N.; Koufman, J.A. Validity and Reliability of the Reflux Symptom Index (RSI). J. Voice 2002, 16, 274–277. [Google Scholar] [CrossRef]
- Fairbanks, G. Voice and Articulation Drillbook, 2nd ed.; Harper & Row: New York, NY, USA, 1960. [Google Scholar]
- Kempster, G.B.; Gerratt, B.R.; Verdolini-Abbott, K.; Barkmeier-Kraemer, J.; Hillman, R.E. Consensus Auditory-Perceptual Evaluation of Voice: Development of a Standardized Clinical Protocol. Am. J. Speech-Lang. Pathol. 2009, 18, 124–132. [Google Scholar] [CrossRef]
- AKG Acoustics. C520. Available online: https://www.akg.com/Microphones/Headset%20Microphones/C520.html (accessed on 1 June 2018).
- Roland Corp. Quad-Capture—USB 2.0. Audio Interface. Available online: https://www.roland.com/au/products/quad-capture/ (accessed on 7 March 2019).
- Audacity Team. Audacity(R): Free Audio Editor and Recorder [Computer Application]. Available online: https://www.audacityteam.org/ (accessed on 16 March 2021).
- Kreiman, J.; Gerratt, B.R.; Kempster, G.B.; Erman, A.; Berke, G.S. Perceptual Evaluation of Voice Quality. J. Speech Lang. Hear. Res. 1993, 36, 21–40. [Google Scholar] [CrossRef]
- Boersma, P.; Weenink, D. Praat: Doing Phonetics by Computer. Available online: http://www.fon.hum.uva.nl/praat/ (accessed on 1 January 2018).
- Madill, C.; So, T.; Corcoran, S. Bridge2practice: Translating Theory into Practice. Available online: https://bridge2practice.com/ (accessed on 7 March 2019).
- IBM Corp. IBM SPSS Software. Available online: https://www.ibm.com/analytics/data-science/predictive-analytics/spss-statistical-software (accessed on 1 February 2018).
- Shrout, P.E.; Fleiss, J.L. Intraclass correlations: Uses in assessing rater reliability. Psychol. Bull. 1979, 86, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Pentax Medical. Analysis of Dysphonia in Speech and Voice—ADSV [Computer Application]. Available online: https://www.pentaxmedical.com/pentax/en/99/1/Analysis-of-Dysphonia-in-Speech-and-Voice-ADSV (accessed on 1 March 2018).
- Awan, S.N.; Roy, N.; Zhang, D.; Cohen, S. Validation of the Cepstral Spectral Index of Dysphonia (CSID) as a Screening Tool for Voice Disorders: Development of Clinical Cutoff Scores. J. Voice 2016, 30, 130–144. [Google Scholar] [CrossRef]
- Maryn, Y. Recording Quality: Speech-To-Noise Ratio and Voice-To-Noise Ratio. Available online: https://www.phonanium.com/product/recording-quality/ (accessed on 24 November 2020).
- Deliyski, D.D.; Shaw, H.S.; Evans, M.K. Adverse Effects of Environmental Noise on Acoustic Voice Quality Measurements. J. Voice 2005, 19, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Yumoto, E.; Gould, W.J.; Baer, T. Harmonics-to-noise ratio as an index of the degree of hoarseness. J. Acoust. Soc. Am. 1982, 71, 1544–1550. [Google Scholar] [CrossRef]
- Warhurst, S.; Madill, C.; McCabe, P.; Heard, R.; Yiu, E. The Vocal Clarity of Female Speech-Language Pathology Students: An Exploratory Study. J. Voice 2012, 26, 63–68. [Google Scholar] [CrossRef]
- Desuter, G.; Dedry, M.; Schaar, B.; Van Lith-Bijl, J.; Van Benthem, P.P.; Sjögren, E.V. Voice outcome indicators for unilateral vocal fold paralysis surgery: A review of the literature. Eur. Arch. Oto-Rhino-Laryngol. 2018, 275, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.M.; Yumoto, E.; Kumai, Y.; Sanuki, T.; Kodama, N. Vocal outcome after arytenoid adduction and ansa cervicalis transfer. Arch. Otolaryngol. Head Neck Surg. 2012, 138, 60–65. [Google Scholar] [CrossRef][Green Version]
- Hollien, H. Vocal Fold Dynamics for Frequency Change. J. Voice 2014, 28, 395–405. [Google Scholar] [CrossRef]
- McKenna, V.S.; Murray, E.S.H.; Lien, Y.-A.S.; Stepp, C.E. The Relationship Between Relative Fundamental Frequency and a Kinematic Estimate of Laryngeal Stiffness in Healthy Adults. J. Speech Lang. Hear. Res. 2016, 59, 1283–1294. [Google Scholar] [CrossRef] [PubMed]
- Linville, S.E. Intraspeaker variability in fundamental frequency stability: An age-related phenomenon? J. Acoust. Soc. Am. 1988, 83, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Noll, A.M. Cepstrum pitch determination. J. Acoust. Soc. Am. 1967, 41, 293–309. [Google Scholar] [CrossRef]
- Hillenbrand, J.; Cleveland, R.A.; Erickson, R.L. Acoustic Correlates of Breathy Vocal Quality. J. Speech Lang. Hear. Res. 1994, 37, 769–778. [Google Scholar] [CrossRef]
- Maryn, Y.; Roy, N.; Bodt, M.; Cauwenberge, P.; Corthals, P. Acoustic measurement of overall voice quality: A meta-analysis. J. Acoust. Soc. Am. 2009, 126, 2619–2634. [Google Scholar] [CrossRef] [PubMed]
- Awan, S.N.; Roy, N. Toward the development of an objective index of dysphonia severity: A four factor acoustic model. Clin. Linguist. Phon. 2006, 20, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Watts, C.R.; Awan, S.N.; Maryn, Y. A Comparison of Cepstral Peak Prominence Measures from Two Acoustic Analysis Programs. J. Voice 2017, 31, 387.e1–387.e10. [Google Scholar] [CrossRef]
- Phadke, K.V.; Laukkanen, A.-M.; Ilomäki, I.; Kankare, E.; Geneid, A.; Švec, J.G. Cepstral and Perceptual Investigations in Female Teachers with Functionally Healthy Voice. J. Voice 2018, 34, 485.e33–485.e43. [Google Scholar] [CrossRef]
- Hillenbrand, J.M.; Houde, R.A. Acoustic Correlates of Breathy Vocal Quality: Dysphonic Voices and Continuous Speech. J. Speech Lang. Hear. Res. 1996, 39, 311–321. [Google Scholar] [CrossRef]
- Awan, S.N.; Roy, N.; Jette, M.E.; Meltzner, G.S.; Hillman, R.E. Quantifying dysphonia severity using a spectral/cepstral-based acoustic index: Comparisons with auditory-perceptual judgements from the CAPE-V. Clin. Linguist. Phon. 2010, 24, 742–758. [Google Scholar] [CrossRef] [PubMed]
- Watts, C.R.; Awan, S.N. Use of Spectral/Cepstral Analyses for Differentiating Normal from Hypofunctional Voices in Sustained Vowel and Continuous Speech Contexts. J. Speech Lang. Hear. Res. 2011, 54, 1525–1537. [Google Scholar] [CrossRef]
- Awan, S.N.; Giovinco, A.; Owens, J. Effects of Vocal Intensity and Vowel Type on Cepstral Analysis of Voice. J. Voice 2012, 26, 670.e15–670.e20. [Google Scholar] [CrossRef] [PubMed]
- Microsoft. Microsoft Excel. Available online: https://www.microsoft.com/en-us/microsoft-365/excel (accessed on 2 June 2021).
- Massey, F.J. The Kolmogorov-Smirnov Test for Goodness of Fit. J. Am. Stat. Assoc. 1951, 46, 68–78. [Google Scholar] [CrossRef]
- Murphy, K.R. Statistical Power Analysis: A Simple and General Model. for Traditional and Modern Hypothesis Tests, 4th ed.; Routledge: New York, NY, USA, 2014. [Google Scholar]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Roy, N.; Merrill, R.M.; Gray, S.D.; Smith, E.M. Voice Disorders in the General Population: Prevalence, Risk Factors, and Occupational Impact. Laryngoscope 2005, 115, 1988–1995. [Google Scholar] [CrossRef]
- Roy, N.; Merrill, R.M.; Thibeault, S.; Parsa, R.A.; Gray, S.D.; Smith, E.M. Prevalence of Voice Disorders in Teachers and the General Population. J. Speech Lang. Hear. Res. 2004, 47, 281–293. [Google Scholar] [CrossRef]
- Procter, T.; Joshi, A. Cultural Competency in Voice Evaluation: Considerations of Normative Standards for Sociolinguistically Diverse Voices. J. Voice 2020. [Google Scholar] [CrossRef] [PubMed]
- Snidecor, J.C. A comparative study of the pitch and duration characteristics of impromptu speaking and oral reading. Speech Monogr. 1943, 10, 50–56. [Google Scholar] [CrossRef]
- Stoicheff, M.L. Speaking Fundamental Frequency Characteristics of Nonsmoking Female Adults. J. Speech Lang. Hear. Res. 1981, 24, 437–441. [Google Scholar] [CrossRef]
- Saxman, J.H.; Burk, K.W. Speaking Fundamental Frequency Characteristics of Middle-Aged Females. Folia Phoniatr. Logop. 1967, 19, 167–172. [Google Scholar] [CrossRef]
- Garrett, R. Cepstral- and Spectral-Based Acoustic Measures of Normal Voices. Ph.D. Thesis, University of Wisconsin-Milwaukee, Milwaukee, WI, USA, 2013. [Google Scholar]
- Diaz, F.J. Measuring the individual benefit of a medical or behavioral treatment using generalized linear mixed-effects models. Stat. Med. 2016, 35, 4077–4092. [Google Scholar] [CrossRef]
- Maryn, Y.; Weenink, D. Objective Dysphonia Measures in the Program Praat: Smoothed Cepstral Peak Prominence and Acoustic Voice Quality Index. J. Voice 2015, 29, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Koufman, J.A.; Isaacson, G. The Spectrum of Vocal Dysfunction. Otolaryngol. Clin. N. Am. 1991, 24, 985–988. [Google Scholar] [CrossRef]
- Madill, C.; Lee, R.; Heard, R.; Roarke, R.; McCabe, T. Impact of breathing instructions on voice onset time. In Proceedings of the Voice Foundation 45th Annual Symposium, Philadelphia, PA, USA, 1–5 June 2016. [Google Scholar]
- Vennard, W. Singing: The Mechanism and the Technic, 5th ed.; Fischer: New York, NY, USA, 1968. [Google Scholar]
- Colton, R.H.; Estill, J. Elements of Voice Quality: Perceptual, Acoustic, and Physiologic Aspects. In Speech and Language: Advances in Basic Research and Practice; Lass, N.J., Ed.; Academic Press: Cambridge, MA, USA, 1981; Volume 5, pp. 311–401. [Google Scholar]
- Roy, N.; Whitchurch, M.; Merrill, R.M.; Houtz, D.; Smith, M.E. Differential Diagnosis of Adductor Spasmodic Dysphonia and Muscle Tension Dysphonia Using Phonatory Break Analysis. Laryngoscope 2008, 118, 2245–2253. [Google Scholar] [CrossRef]
- Harris, T.; Harris, S.; Rubin, J.S.; Howard, D.M. The Voice Clinic Handbook; Wiley: Chichester, UK, 1997. [Google Scholar]
- Simonyan, K.; Ackermann, H.; Chang, E.F.; Greenlee, J.D. New Developments in Understanding the Complexity of Human Speech Production. J. Neurosci. 2016, 36, 11440–11448. [Google Scholar] [CrossRef]
- Nikjeh, D.A.; Lister, J.J.; Frisch, S.A. The relationship between pitch discrimination and vocal production: Comparison of vocal and instrumental musicians. J. Acoust. Soc. Am. 2009, 125, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Estis, J.M.; Dean-Claytor, A.; Moore, R.E.; Rowell, T.L. Pitch-Matching Accuracy in Trained Singers and Untrained Individuals: The Impact of Musical Interference and Noise. J. Voice 2011, 25, 173–180. [Google Scholar] [CrossRef]
- Carey, J.R.; Bhatt, E.; Nagpal, A. Neuroplasticity promoted by task complexity. Exerc. Sport Sci. Rev. 2005, 33, 24–31. [Google Scholar] [PubMed]
- Kantak, S.S.; Zahedi, N.; McGrath, R. Complex Skill Training Transfers to Improved Performance and Control of Simpler Tasks After Stroke. Phys. Ther. 2017, 97, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.A.; Aertsen, A.; Wolpert, D.M.; Mehring, C. Motor Task Variation Induces Structural Learning. Curr. Biol. 2009, 19, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Dhawale, A.K.; Smith, M.A.; Ölveczky, B.P. The Role of Variability in Motor Learning. Annu. Rev. Neurosci. 2017, 40, 479–498. [Google Scholar] [CrossRef]
- Vecchione, J.; Madill, C.; Hodges, N. Modifying Technique in Self-Paced Motor Tasks. In Psychology of Closed Self-Paced Motor Tasks; Ziv, G.L.R., Ed.; Routledge: Philadelphia, PA, USA, 2021. [Google Scholar]
- Rutherford, B.R. The Use of Negative Practice in Speech Therapy with Children Handicapped by Cerebral Palsy, Athetoid Type. J. Speech Disord. 1940, 5, 259–264. [Google Scholar] [CrossRef]
- Lyndon, E.H.; Malcolm, B. The effects of proactive and retroactive inhibition: The Old Way/New Way methodology and its application to speech pathology. In Proceedings of the Annual Conference of the Australian Association of Speech and Hearing: Beyond 1984; University of Adelaide: Adelaide, SA, Australia, 1984. [Google Scholar]
- Gillespie, A.I.; Yabes, J.; Rosen, C.A.; Gartner-Schmidt, J.L. Efficacy of Conversation Training Therapy for Patients with Benign Vocal Fold Lesions and Muscle Tension Dysphonia Compared to Historical Matched Control Patients. J. Speech Lang. Hear. Res. 2019, 62, 4062–4079. [Google Scholar] [CrossRef] [PubMed]
- Colloca, L. How do placebo effects and patient-clinician relationships influence behaviors and clinical outcomes? PAIN Rep. 2019, 4, e758. [Google Scholar] [CrossRef]
- Berg, E.E.; Hapner, E.; Klein, A.; Johns, M.M., 3rd. Voice Therapy Improves Quality of Life in Age-Related Dysphonia: A Case-Control Study. J. Voice 2008, 22, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, M.; Hirano, S.; Tateya, I.; Kishimoto, Y.; Hiwatashi, N.; Fujiu-Kurachi, M.; Ito, J. Multidimensional Analysis on the Effect of Vocal Function Exercises on Aged Vocal Fold Atrophy. J. Voice 2015, 29, 638–644. [Google Scholar] [CrossRef]
- Angadi, V.; Croake, D.; Stemple, J. Effects of Vocal Function Exercises: A Systematic Review. J. Voice 2019, 33, 124.e13–124.e34. [Google Scholar] [CrossRef]
- Murton, O.; Hillman, R.; Mehta, D. Cepstral Peak Prominence Values for Clinical Voice Evaluation. Am. J. Speech-Lang. Pathol. 2020, 29, 1596–1607. [Google Scholar] [CrossRef] [PubMed]
- Belsky, M.A.; Rothenberger, S.D.; Gillespie, A.I.; Gartner-Schmidt, J.L. Do Phonatory Aerodynamic and Acoustic Measures in Connected Speech Differ Between Vocally Healthy Adults and Patients Diagnosed with Muscle Tension Dysphonia? J. Voice 2020, 35, 663.e1–663.e7. [Google Scholar] [CrossRef] [PubMed]








| Component | Description |
|---|---|
| Optimal phonation task (OPT) | The patient is instructed to breathe in and out, then produce a clear, effortless, and quiet/m/using the sound we make when we mean ‘yes’. Instructions are given to prime the vocal system for low effort and low impact phonation including a gradual start (simultaneous onset). Focus is on ensuring the sound has communicative intent and is not produced as in singing. The patient is cued to notice how the sound feels and sounds. Explicit instruction is provided if whole-task modelling and imitation is insufficient for the patient to acquire the task. Home practise is recommended, ten repetitions/hour for 10 h during the day. |
| Sob voice quality (SVQ) | The patient is instructed to produce a clear, quiet, and effortless/ŋ/using a gradual start to the sound and a sad, mournful expression (similar to a puppy whimper). Explicit instruction is provided to cue increased accessory muscle activation if whole-task modelling and imitation is insufficient for the patient to acquire the task. The patient is cued to notice how their voice feels and sounds. Home practise is recommended from six to eight repetitions/hour for 10 h during the day. |
| SVQ task variation (SVQ variants) | The patient is instructed to produce all voice carrier phrases beginning with a momentary/ŋ/using SVQ. Phrases begin with all voiced sounds and then phrases with voiceless sounds are introduced. The patient is taught to produce a siren using a clear, quiet, and effortless/ŋ/using SVQ, slowly, smoothly, evenly, and effortlessly sliding the pitch up and down in the middle of their comfortable vocal range. Siren extensions that gradually increase and decrease pitch in the siren are also introduced. The patient is cued to notice how their voice feels and sounds. Home practise is recommended with six phrases/hour and two to three sirens/hour for 10 h during the day. |
| Negative practice (NP) | The patient is instructed to imitate the voice quality they presented with at assessment by listening to their initial voice recording. They are instructed to use this ‘old voice’ quality in carrier phrases used in SVQ task variation and then compare this with SVQ carrier phrases (still initiated with a momentary/ŋ/), which is the ‘new voice’. They are then asked to describe the differences between the two voice qualities with a focus on the sound and feeling of the voice. Home practise is recommended using three to four negative practice pairs (old way/new way) of SVQ phrases/hour for 10 h during the day. |
| OPT-SVQ n = 64 | SVQ-SVQ Variants n = 43 | SVQ Variant-NP n = 33 | NP Post-NP n = 24 | Total OPT Post-NP | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sessions | Days | Sessions | Days | Sessions | Days | Sessions | Days | Sessions | Days | |
| Mean (SD) | 1.3 (0.6) | 28.5 (27.6) | 1.5 (0.9) | 38.3 (48.2) | 2.5 (2.3) | 37.3 (27.0) | 1.2 (0.5) | 24.0 (17.0) | 4.0 (3.0) | 83.1 (59.2) |
| 95% CI | 1.2–1.5 | 21.5–35.5 | 1.2–1.8 | 24.0–52.6 | 1.6–3.3 | 27.0–47.6 | 1.0–1.4 | 16.7–31.4 | 3.2–4.8 | 68.1–98.1 |
| Min–max | 1.0–4.0 | 4.0–173.0 | 1.0–5.0 | 6.0–248.0 | 1.0–11.0 | 7.0–105.0 | 1.0–3.0 | 7.0–84.0 | 1.0–15.0 | 7.0–283.0 |
| Median | 1.0 | 21.0 | 1.0 | 23.5 | 2.0 | 26.0 | 1.0 | 23.0 | 3.0 | 72.5 |
| Rater | Types of Measures | ICC | |||
|---|---|---|---|---|---|
| Severity | Roughness | Breathiness | Strain | ||
| Rater 1 | Single measures | 0.854 | 0.869 | 0.738 | 0.862 |
| Average measures | 0.921 | 0.930 | 0.849 | 0.926 | |
| Rater 2 | Single measures | 0.977 | 0.889 | 0.948 | 0.896 |
| Average measures | 0.988 | 0.941 | 0.974 | 0.945 | |
| Rater 3 | Single measures | 0.822 | 0.812 | 0.810 | 0.829 |
| Average measures | 0.903 | 0.896 | 0.895 | 0.906 | |
| Voice Measure | ICC Measures | ICC | 95% CI | p |
|---|---|---|---|---|
| Overall severity | Single measures | 0.703 | 0.547–0.824 | 0.000 |
| Average measures | 0.876 | 0.783–0.933 | 0.000 | |
| Roughness | Single measures | 0.696 | 0.537–0.819 | 0.000 |
| Average measures | 0.873 | 0.777–0.932 | 0.000 | |
| Breathiness | Single measures | 0.659 | 0.490–0.795 | 0.000 |
| Average measures | 0.853 | 0.743–0.921 | 0.000 | |
| Strain | Single measures | 0.691 | 0.531–0.816 | 0.000 |
| Average measures | 0.870 | 0.772–0.930 | 0.000 |
| Norms | Mean (SD) | 95% CI | Min–Max | ||
|---|---|---|---|---|---|
| F0 of CAPEV3 | Male | 108.94 [83] | 137.5 (31.2) | 111.5–163.6 | 107.3–199.7 |
| Female | 235.07 [83] | 189.2 (17.7) | 184.5–193.8 | 148.1–232.9 | |
| F0 of RP | Male | 84–178 [40] | 140.6 (43.3) | 104.4–176.7 | 97.6–236.9 |
| Female | 127–275 [40] | 185.8 (14.7) | 181.9–189.7 | 148.9–219.6 | |
| F0SD | Male | 3.3 [84] | 1.8 (1.0) | 1.0–2.6 | 0.8–4.0 |
| Female | 20–29y: 3.8 [85] 30–40y: 2.5 [86] 40–50y: 2.8 [86] 60–69y: 4.3 [85] | 2.3 (1.4) | 2.0–2.7 | 0.7–8.2 |
| Measures | Time Point | Mean (SD) | 95% CI for Mean | MD | F | p | Partial η2 |
|---|---|---|---|---|---|---|---|
| Overall severity | Baseline | 26.8 (13.7) | 20.2–33.4 | ||||
| OPT | 25.8 (11.3) | 20.4–31.3 | 1.0 | 0.376 | 0.546 | 0.018 | |
| SVQ | 20.0 (7.9) | 16.2–23.8 | 6.8 | 12.001 | 0.003 * | 0.387 | |
| SVQ variant | 20.9 (7.2) | 17.4–24.4 | 5.9 | 12.381 | 0.002 * | 0.360 | |
| NP | 21.6 (8.9) | 17.3–25.9 | 5.2 | 11.312 | 0.003 * | 0.340 | |
| Roughness | Baseline | 24.1 (12.8) | 17.9–30.3 | ||||
| OPT | 22.8 (12.4) | 16.8–28.8 | 1.3 | 0.865 | 0.363 | 0.041 | |
| SVQ | 19.5 (9.2) | 15.1–23.9 | 4.6 | 7.069 | 0.016 * | 0.271 | |
| SVQ variant | 18.8 (8.8) | 14.5–23.0 | 5.3 | 12.289 | 0.002 * | 0.358 | |
| NP | 19.4 (9.7) | 14.7–24.1 | 4.7 | 10.471 | 0.004 * | 0.322 | |
| Breathiness | Baseline | 23.4 (13.8) | 16.8–30.1 | ||||
| OPT | 23.6 (11.1) | 18.5–28.6 | 0.2 | 0.007 | 0.936 | 0.001 | |
| SVQ | 18.6 (8.2) | 14.6–22.5 | 4.9 | 6.859 | 0.017 * | 0.265 | |
| SVQ variant | 18.9 (5.9) | 16.0–21.7 | 4.6 | 6.375 | 0.019 * | 0.225 | |
| NP | 19.8 (8.1) | 15.9–23.7 | 3.6 | 5.444 | 0.029 * | 0.198 | |
| Strain | Baseline | 18.4 (11.6) | 12.8–23.9 | ||||
| OPT | 16.8 (9.5) | 12.2–21.3 | 1.6 | 1.526 | 0.231 | 0.071 | |
| SVQ | 17.0 (6.6) | 13.8–20.2 | 1.3 | 1.085 | 0.311 | 0.054 | |
| SVQ variant | 12.3 (7.0) | 8.9–15.7 | 6.1 | 17.713 | 0.001 * | 0.446 | |
| NP | 13.6 (9.2) | 9.2–18.0 | 4.8 | 9.409 | 0.006 * | 0.300 |
| Measure | Normative Cut-Off | Time Point | Mean (SD) | 95% CI | MD | F | p | Partial η2 |
|---|---|---|---|---|---|---|---|---|
| HNR | 20 dB [51] | Baseline | 23.6 (5.4) | 21.1–26.1 | ||||
| OPT | 23.5 (6.1) | 20.7–26.4 | 0.1 | 0.001 | 0.976 | 0.000 | ||
| SVQ | 24.7 (4.9) | 22.4–270.0 | 1.1 | 1.203 | 0.286 | 0.060 | ||
| SVQ variant | 25.3 (4.7) | 23.1–27.5 | 1.7 | 2.153 | 0.157 | 0.093 | ||
| NP | 25.6 (4.2) | 23.6–27.6 | 20.0 | 2.574 | 0.124 | 0.114 | ||
| CPP of CAPEV3 | >7.8 dB [87] | Baseline | 7.1 (1.3) | 6.5–7.7 | ||||
| OPT | 7.2 (1.5) | 6.5–80.0 | 0.2 | 1.147 | 0.296 | 0.052 | ||
| SVQ | 7.4 (1.7) | 6.6–8.1 | 0.3 | 0.995 | 0.331 | 0.050 | ||
| SVQ variant | 7.4 (1.8) | 6.6–8.3 | 0.4 | 1.563 | 0.224 | 0.064 | ||
| NP | 7.7 (1.7) | 6.9–8.5 | 0.6 | 50.098 | 0.034 * | 0.188 | ||
| CPPS of CAPEV3 | NA | Baseline | 10.6 (1.6) | 9.9–11.4 | ||||
| OPT | 11.2 (2.4) | 10.2–12.3 | 0.6 | 1.752 | 0.200 | 0.077 | ||
| SVQ | 11.6 (2.1) | 10.6–12.6 | 10.0 | 6.208 | 0.022 * | 0.237 | ||
| SVQ variant | 120.0 (20.0) | 11.1–12.9 | 1.4 | 10.587 | 0.003 * | 0.315 | ||
| NP | 12.1 (1.8) | 11.3–12.9 | 1.5 | 17.629 | 0.001 * | 0.445 | ||
| CPP RP23 | 6.6 dB [87] | Baseline | 5.6 (0.9) | 5.1–60.0 | ||||
| OPT | 5.5 (0.8) | 5.1–5.9 | 0.1 | 1.126 | 0.292 | 0.017 | ||
| SVQ | 5.8 (1.1) | 5.3–6.4 | 0.2 | 0.806 | 0.375 | 0.019 | ||
| SVQ variant | 5.9 (10.0) | 5.3–6.4 | 0.3 | 50.009 | 0.032 * | 0.135 | ||
| NP | 60.0 (10.0) | 5.5–6.5 | 0.4 | 3.577 | 0.071 | 0.135 | ||
| CSID of CAPEV3 | NA | Baseline | −130.0 (15.5) | (−20.2)–(−5.7) | ||||
| OPT | −7.3 (17.3) | −15.4–0.9 | 5.7 | 50.096 | 0.035 * | 0.195 | ||
| SVQ | −9.1 (14.6) | (−15.9)–(−2.2) | 3.9 | 1.490 | 0.237 | 0.073 | ||
| SVQ variant | −8.7 (16.2) | (−16.2)–(−1.1) | 4.3 | 2.416 | 0.134 | 0.095 | ||
| NP | −11.5 (16.4) | (−19.2)–(−3.8) | 1.5 | 1.157 | 0.294 | 0.050 | ||
| CSID of RP23 | <24.27 [57] | Baseline | 12.5 (12.3) | 6.9–18.1 | ||||
| OPT | 15.3 (13.9) | 90.0–21.7 | 2.8 | 1.339 | 0.260 | 0.057 | ||
| SVQ | 11.2 (14.7) | 4.6–17.9 | 1.3 | 0.247 | 0.624 | 0.012 | ||
| SVQ variant | 9.8 (14.4) | 3.3–16.4 | 2.7 | 1.382 | 0.252 | 0.057 | ||
| NP | 8.9 (13.5) | 2.7–150.0 | 3.7 | 4.396 | 0.047 * | 0.160 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madill, C.; Chacon, A.; Kirby, E.; Novakovic, D.; Nguyen, D.D. Active Ingredients of Voice Therapy for Muscle Tension Voice Disorders: A Retrospective Data Audit. J. Clin. Med. 2021, 10, 4135. https://doi.org/10.3390/jcm10184135
Madill C, Chacon A, Kirby E, Novakovic D, Nguyen DD. Active Ingredients of Voice Therapy for Muscle Tension Voice Disorders: A Retrospective Data Audit. Journal of Clinical Medicine. 2021; 10(18):4135. https://doi.org/10.3390/jcm10184135
Chicago/Turabian StyleMadill, Catherine, Antonia Chacon, Evan Kirby, Daniel Novakovic, and Duy Duong Nguyen. 2021. "Active Ingredients of Voice Therapy for Muscle Tension Voice Disorders: A Retrospective Data Audit" Journal of Clinical Medicine 10, no. 18: 4135. https://doi.org/10.3390/jcm10184135
APA StyleMadill, C., Chacon, A., Kirby, E., Novakovic, D., & Nguyen, D. D. (2021). Active Ingredients of Voice Therapy for Muscle Tension Voice Disorders: A Retrospective Data Audit. Journal of Clinical Medicine, 10(18), 4135. https://doi.org/10.3390/jcm10184135






