Associations between Clinical Findings and Severity of Diffuse Idiopathic Skeletal Hyperostosis in Patients with Ossification of the Posterior Longitudinal Ligament
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Methods
2.2. Clinical Evaluation
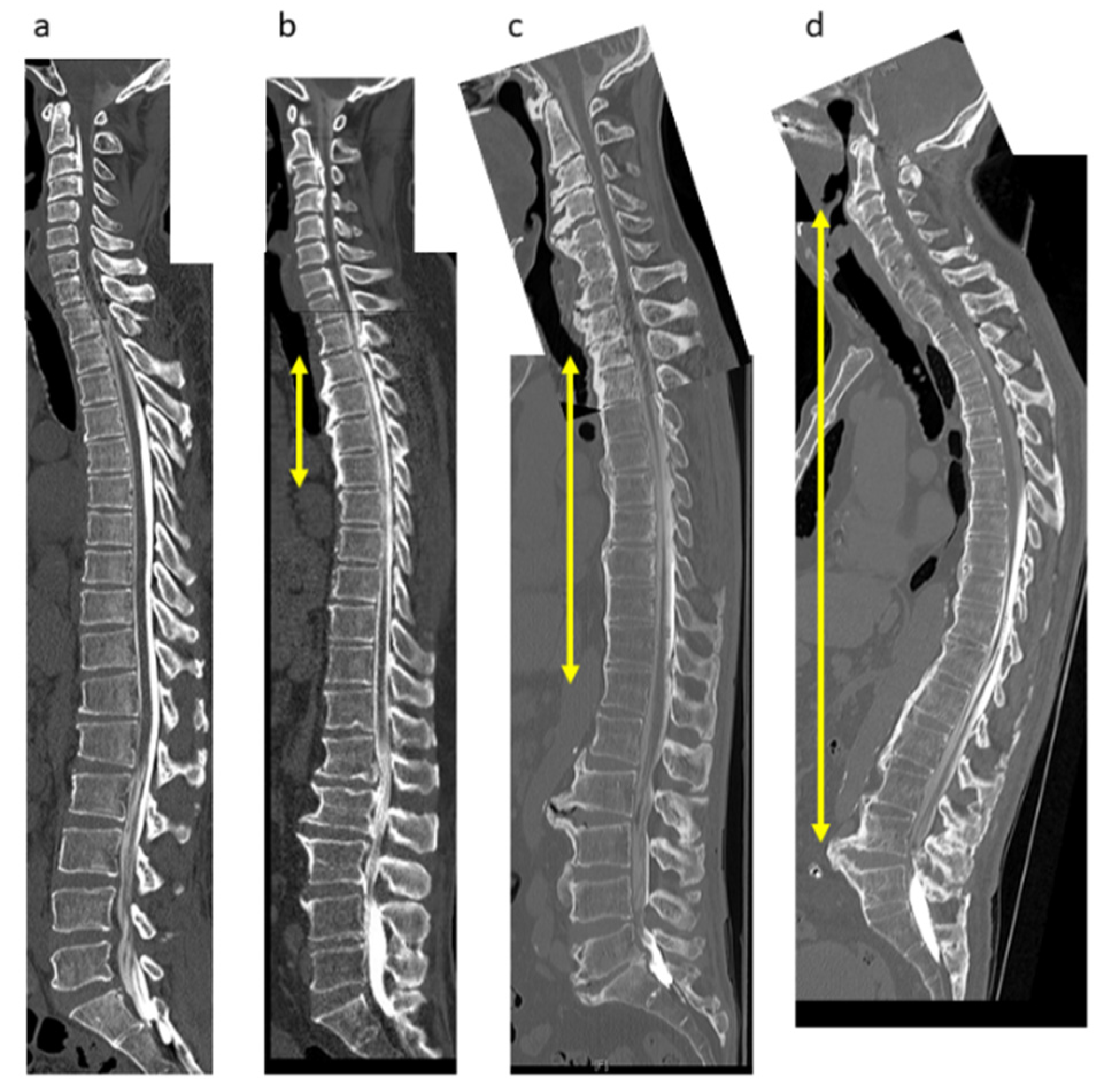
2.3. Radiologic Evaluations
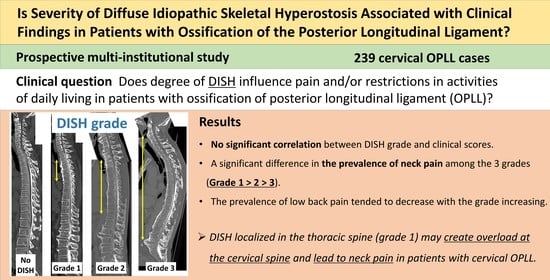
3. Results
3.1. Demographic and Clinical Data
3.2. Demographic and Clinical Characteristics by DISH Grade
3.3. Severity of DISH Was Not Associated with Myelopathic Symptoms or Lumbar Spine Function in Patients with Cervical OPLL
3.4. Degree of DISH Correlated Negatively with Prevalence of Neck Pain but Not Back Pain or LBP in Patients with Cervical OPLL
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Onishi, E.; Sakamoto, A.; Murata, S.; Matsushita, M. Risk factors for acute cervical spinal cord injury associated with ossification of the posterior longitudinal ligament. Spine 2012, 37, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, M.; Aiba, A.; Hashimoto, M.; Fujiyoshi, T.; Yamazaki, M. Cervical myelopathy in patients with ossification of the posterior longitudinal ligament. J. Neurosurg. Spine 2009, 10, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, S.; Sakou, T. Ossification of the posterior longitudinal ligament of the cervical spine: Etiology and natural history. Spine 2012, 37, E309–E314. [Google Scholar] [CrossRef] [PubMed]
- Resnick, D.; Guerra, J.; Robinson, C.A.; Vint, V.C. Association of diffuse idiopathic skeletal hyperostosis (DISH) and calcification and ossification of the posterior longitudinal ligament. AJR Am. J. Roentgenol. 1978, 131, 1049–1053. [Google Scholar] [CrossRef] [PubMed]
- Resnick, D.; Niwayama, G. Radiographic and pathologic features of spinal involvement in diffuse idiopathic skeletal hyperostosis (DISH). Radiology 1976, 119, 559–568. [Google Scholar] [CrossRef]
- Hirai, T.; Yoshii, T.; Iwanami, A.; Takeuchi, K.; Mori, K.; Yamada, T.; Wada, K.; Koda, M.; Matsuyama, Y.; Takeshita, K.; et al. Prevalence and distribution of ossified lesions in the whole spine of patients with cervical ossification of the posterior longitudinal ligament a multicenter study (JOSL CT study). PLoS ONE 2016, 11, e0160117. [Google Scholar] [CrossRef]
- Hirai, T.; Yoshii, T.; Nagoshi, N.; Takeuchi, K.; Mori, K.; Ushio, S.; Iwanami, A.; Yamada, T.; Seki, S.; Tsuji, T.; et al. Distribution of ossified spinal lesions in patients with severe ossification of the posterior longitudinal ligament and prediction of ossification at each segment based on the cervical OP index classification: A multicenter study (JOSL CT study). BMC Musculoskelet. Disord. 2018, 19, 107. [Google Scholar] [CrossRef]
- Mori, K.; Yoshii, T.; Hirai, T.; Iwanami, A.; Takeuchi, K.; Yamada, T.; Seki, S.; Tsuji, T.; Fujiyoshi, K.; Furukawa, M.; et al. Prevalence and distribution of ossification of the supra/interspinous ligaments in symptomatic patients with cervical ossification of the posterior longitudinal ligament of the spine: A CT-based multicenter cross-sectional study. BMC Musculoskelet. Disord. 2016, 17, 492. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Nakano, M.; Yasuda, T.; Seki, S.; Hori, T.; Kimura, T. Ossification of the posterior longitudinal ligament in not only the cervical spine, but also other spinal regions: Analysis using multidetector computed tomography of the whole spine. Spine 2013, 38, E1477–E1482. [Google Scholar] [CrossRef]
- Nishimura, S.; Nagoshi, N.; Iwanami, A.; Takeuchi, A.; Hirai, T.; Yoshii, T.; Takeuchi, K.; Mori, K.; Yamada, T.; Seki, S.; et al. Prevalence and distribution of diffuse idiopathic skeletal hyperostosis on whole-spine computed tomography in patients with cervical ossification of the posterior longitudinal ligament: A multicenter study. Clin. Spine Surg. 2018, 31, E460–E465. [Google Scholar] [CrossRef]
- Hirai, T.; Okawa, A.; Arai, Y.; Takahashi, M.; Kawabata, S.; Kato, T.; Enomoto, M.; Tomizawa, S.; Sakai, K.; Torigoe, I.; et al. Middle-term results of a prospective comparative study of anterior decompression with fusion and posterior decompression with laminoplasty for the treatment of cervical spondylotic myelopathy. Spine 2011, 36, 1940–1947. [Google Scholar] [CrossRef]
- Fukui, M.; Chiba, K.; Kawakami, M.; Kikuchi, S.; Konno, S.; Miyamoto, M.; Seichi, A.; Shimamura, T.; Shirado, O.; Taguchi, T.; et al. JOA Back Pain Evaluation Questionnaire (JOABPEQ)/JOA Cervical Myelopathy Evaluation Questionnaire (JOACMEQ). The report on the development of revised versions. 16 April 2007. The Subcommittee of the Clinical Outcome Committee of the Japanese Orthopaedic Association on Low Back Pain and Cervical Myelopathy Evaluation. J. Orthop. Sci. 2009, 14, 348–365. [Google Scholar] [CrossRef]
- Hirai, T.; Yoshii, T.; Ushio, S.; Hashimoto, J.; Mori, K.; Maki, S.; Katsumi, K.; Nagoshi, N.; Takeuchi, K.; Furuya, T.; et al. Associations between clinical symptoms and degree of ossification in patients with cervical ossification of the posterior longitudinal ligament: A prospective multi-institutional cross-sectional study. J. Clin. Med. 2020, 9, 4055. [Google Scholar] [CrossRef]
- Beyeler, C.H.; Schlapbach, P.; Gerber, N.J.; Fahrer, H.; Hasler, F.; van der Linden, S.M.; Bürgi, U.; Fuchs, W.A.; Ehrengruber, H. Diffuse idiopathic skeletal hyperostosis (DISH) of the elbow: A cause of elbow pain? A controlled study. Br. J. Rheumatol. 1992, 31, 319–323. [Google Scholar] [CrossRef][Green Version]
- Utsinger, P.D. Diffuse idiopathic skeletal hyperostosis. Clin. Rheum. Dis. 1985, 11, 325–351. [Google Scholar] [CrossRef]
- Verlaan, J.J.; Boswijk, P.F.E.; de Ru, J.A.; Dhert, W.J.A.; Oner, F.C. Diffuse idiopathic skeletal hyperostosis of the cervical spine: An underestimated cause of dysphagia and airway obstruction. Spine J. 2011, 11, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- Okada, E.; Yoshii, T.; Yamada, T.; Watanabe, K.; Katsumi, K.; Hiyama, A.; Watanabe, M.; Nakagawa, Y.; Okada, M.; Endo, T.; et al. Spinal fractures in patients with Diffuse idiopathic skeletal hyperostosis: A nationwide multi-institution survey. J. Orthop. Sci. 2019, 24, 601–606. [Google Scholar] [CrossRef]
- Katoh, H.; Okada, E.; Yoshii, T.; Yamada, T.; Watanabe, K.; Katsumi, K.; Hiyama, A.; Nakagawa, Y.; Okada, M.; Endo, T.; et al. A comparison of cervical and thoracolumbar fractures associated with diffuse idiopathic skeletal hyperostosis—A nationwide multicenter study. J. Clin. Med. 2020, 9, 208. [Google Scholar] [CrossRef] [PubMed]
- Ehara, S.; Shimamura, T.; Nakamura, R.; Yamazaki, K. Paravertebral ligamentous ossification: DISH, OPLL and OLF. Eur. J. Radiol. 1998, 27, 196–205. [Google Scholar] [CrossRef]
- Yoshimura, N.; Nagata, K.; Muraki, S.; Oka, H.; Yoshida, M.; Enyo, Y.; Kagotani, R.; Hashizume, H.; Yamada, H.; Ishimoto, Y.; et al. Prevalence and progression of radiographic ossification of the posterior longitudinal ligament and associated factors in the Japanese population: A 3-year follow-up of the ROAD study. Osteoporos. Int. 2014, 25, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Epstein, N.E. Simultaneous cervical diffuse idiopathic skeletal hyperostosis and ossification of the posterior longitudinal ligament resulting in dysphagia or myelopathy in two geriatric North Americans. Surg. Neurol. 2000, 53, 427–431, discussion 431. [Google Scholar] [CrossRef]
- Fujimori, T.; Watabe, T.; Iwamoto, Y.; Hamada, S.; Iwasaki, M.; Oda, T. Prevalence, concomitance, and distribution of ossification of the spinal ligaments: Results of whole spine CT scans in 1500 Japanese patients. Spine 2016, 41, 1668–1676. [Google Scholar] [CrossRef]
- Toyoda, H.; Terai, H.; Yamada, K.; Suzuki, A.; Dohzono, S.; Matsumoto, T.; Nakamura, H. Prevalence of diffuse idiopathic skeletal hyperostosis in patients with spinal disorders. Asian Spine J. 2017, 11, 63–70. [Google Scholar] [CrossRef]
- Okada, E.; Ishihara, S.; Azuma, K.; Michikawa, T.; Suzuki, S.; Tsuji, O.; Nori, S.; Nagoshi, N.; Yagi, M.; Takayama, M.; et al. Metabolic syndrome is a predisposing factor for diffuse idiopathic skeletal hyperostosis. Neurospine 2021, 18, 109–116. [Google Scholar] [CrossRef]
- Dan Lantsman, C.; Herman, A.; Verlaan, J.J.; Stern, M.; Mader, R.; Eshed, I. Abdominal fat distribution in diffuse idiopathic skeletal hyperostosis and ankylosing spondylitis patients compared to controls. Clin. Radiol. 2018, 73, 910.e915–910.e920. [Google Scholar] [CrossRef]
- Hutton, C. DISH … a state not a disease? Br. J. Rheumatol. 1989, 28, 277–278. [Google Scholar] [CrossRef][Green Version]
- Hirai, T.; Yoshii, T.; Ushio, S.; Mori, K.; Maki, S.; Katsumi, K.; Nagoshi, N.; Takeuchi, K.; Furuya, T.; Watanabe, K.; et al. Clinical characteristics in patients with ossification of the posterior longitudinal ligament: A prospective multi-institutional cross-sectional study. Sci. Rep. 2020, 10, 5532. [Google Scholar] [CrossRef]
- Mata, S.; Fortin, P.R.; Fitzcharles, M.A.; Starr, M.R.; Joseph, L.; Watts, C.S.; Gore, B.; Rosenberg, E.; Chhem, R.K.; Esdaile, J.M. A controlled study of diffuse idiopathic skeletal hyperostosis. Clinical features and functional status. Medicine 1997, 76, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Schlapbach, P.; Beyeler, C.; Gerber, N.J.; van der Linden, S.; Bürgi, U.; Fuchs, W.A.; Ehrengruber, H. Diffuse idiopathic skeletal hyperostosis (DISH) of the spine: A cause of back pain? A controlled study. Br. J. Rheumatol. 1989, 28, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Holton, K.F.; Denard, P.J.; Yoo, J.U.; Kado, D.M.; Barrett-Connor, E.; Marshall, L.M.; Osteoporotic Fractures in Men (MrOS) Study Group. Diffuse idiopathic skeletal hyperostosis and its relation to back pain among older men: The MrOS Study. Semin. Arthritis Rheum. 2011, 41, 131–138. [Google Scholar] [CrossRef] [PubMed]





| No DISH (n = 132) | DISH (n = 107) | p-Value | |
|---|---|---|---|
| Age (years) | 60.9 ± 11.6 | 67.6 ± 12.1 | <0.001 *** |
| Male (%) | 61.4 | 76.6 | 0.01 * |
| Body mass index | 26.1 ± 4.7 | 25.6 ± 4.2 | 0.38 |
| Diabetes mellitus (%) | 21.2 | 28.9 | 0.25 |
| Cervical JOA score | 12.5 (6–17) | 11.9 (6–17) | 0.22 |
| OP-index | 7.1 ± 0.5 | 10.5 ± 0.6 | <0.001 *** |
| Prevalence of symptoms (%) | |||
| Neck pain | 59.8 | 58.9 | 0.94 |
| Back pain | 25.8 | 30.8 | 0.52 |
| Low back pain | 54.5 | 52.3 | 0.81 |
| JOA-CMEQ score | |||
| Cervical spine function | 68.5 ± 28.2 | 62.5 ± 28.8 | 0.10 |
| Upper extremity function | 81.8 ± 20.6 | 78.0 ± 22.7 | 0.19 |
| Lower extremity function | 69.0 ± 29.5 | 62.3 ± 31.9 | 0.10 |
| Bladder function | 76.5 ± 19.8 | 72.0 ± 24.4 | 0.11 |
| Quality of life | 49.3 ± 20.0 | 50.7 ± 20.0 | 0.60 |
| JOA-BPEQ score | |||
| Lumbar spine function | 72.3 ± 28.2 | 62.5 ± 35.0 | 0.02 * |
| Social dysfunction | 57.7 ± 28.6 | 54.6 ± 30.4 | 0.47 |
| Mentality | 49.3 ± 19.5 | 49.0 ± 20.6 | 0.90 |
| Locomotive function | 67.1 ± 33.0 | 60.6 ± 37.7 | 0.19 |
| Body pain | 71.8 ± 32.7 | 69.6 ± 34.9 | 0.63 |
| VAS score | |||
| Neck pain | 39.1 ± 30.2 | 38.4 ± 32.5 | 0.83 |
| Upper extremity numbness | 47.5 ± 32.8 | 42.1 ± 33.8 | 0.20 |
| Chest constriction | 11.1 ± 22.2 | 9.2 ± 21.3 | 0.48 |
| Numbness below the chest | 35.3 ± 32.7 | 39.0 ± 35.6 | 0.41 |
| Low back pain | 25.8 ± 26.6 | 30.0 ± 31.6 | 0.29 |
| Lower extremity numbness | 29.5 ± 32.7 | 32.8 ± 35.1 | 0.47 |
| Lower extremity pain | 24.0 ± 30.5 | 22.0 ± 29.9 | 0.57 |
| Grade 1 (n = 23) | Grade 2 (n = 65) | Grade 3 (n = 19) | p-Value | |
|---|---|---|---|---|
| Age (years) | 65.4 ± 12.7 | 66.9 ± 12.3 | 72.8 ± 9.9 | <0.001 *** |
| Male (%) | 78.3 | 75.4 | 78.9 | 0.74 |
| Body mass index | 25.7 ± 5.0 | 25.9 ± 3.7 | 24.7 ± 4.9 | 0.55 |
| Diabetes mellitus (%) | 30.4 | 32.3 | 15.8 | 0.41 |
| Cervical JOA score | 12.4 (7.5–17) | 11.8 (−2, 17) | 11.9 (6–16) | 0.36 |
| OP-index | 8.7 ± 1.1 | 10.4 ± 0.8 | 12.6 ± 1.0 | <0.001 *** |
| Grade 1 (n = 23) | Grade 2 (n = 65) | Grade 3 (n = 19) | p-Value | |
|---|---|---|---|---|
| Prevalence of symptoms (%) | ||||
| Neck pain | 78.3 | 56.9 | 36.8 | <0.05 * |
| Back pain | 30.4 | 32.3 | 26.3 | 0.65 |
| Low back pain | 60.9 | 53.8 | 36.8 | 0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirai, T.; Nishimura, S.; Yoshii, T.; Nagoshi, N.; Hashimoto, J.; Mori, K.; Maki, S.; Katsumi, K.; Takeuchi, K.; Ushio, S.; et al. Associations between Clinical Findings and Severity of Diffuse Idiopathic Skeletal Hyperostosis in Patients with Ossification of the Posterior Longitudinal Ligament. J. Clin. Med. 2021, 10, 4137. https://doi.org/10.3390/jcm10184137
Hirai T, Nishimura S, Yoshii T, Nagoshi N, Hashimoto J, Mori K, Maki S, Katsumi K, Takeuchi K, Ushio S, et al. Associations between Clinical Findings and Severity of Diffuse Idiopathic Skeletal Hyperostosis in Patients with Ossification of the Posterior Longitudinal Ligament. Journal of Clinical Medicine. 2021; 10(18):4137. https://doi.org/10.3390/jcm10184137
Chicago/Turabian StyleHirai, Takashi, Soraya Nishimura, Toshitaka Yoshii, Narihito Nagoshi, Jun Hashimoto, Kanji Mori, Satoshi Maki, Keiichi Katsumi, Kazuhiro Takeuchi, Shuta Ushio, and et al. 2021. "Associations between Clinical Findings and Severity of Diffuse Idiopathic Skeletal Hyperostosis in Patients with Ossification of the Posterior Longitudinal Ligament" Journal of Clinical Medicine 10, no. 18: 4137. https://doi.org/10.3390/jcm10184137
APA StyleHirai, T., Nishimura, S., Yoshii, T., Nagoshi, N., Hashimoto, J., Mori, K., Maki, S., Katsumi, K., Takeuchi, K., Ushio, S., Furuya, T., Watanabe, K., Nishida, N., Watanabe, K., Kaito, T., Kato, S., Nagashima, K., Koda, M., Nakashima, H., ... Kawaguchi, Y. (2021). Associations between Clinical Findings and Severity of Diffuse Idiopathic Skeletal Hyperostosis in Patients with Ossification of the Posterior Longitudinal Ligament. Journal of Clinical Medicine, 10(18), 4137. https://doi.org/10.3390/jcm10184137