Endometriosis Is Associated with an Increased Risk of Coronary Artery Disease in Asian Women
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Data Source
2.3. Study Participants
2.4. Outcomes and Comorbidities
2.5. Statistical Analyses
3. Results
3.1. Subject Characteristics
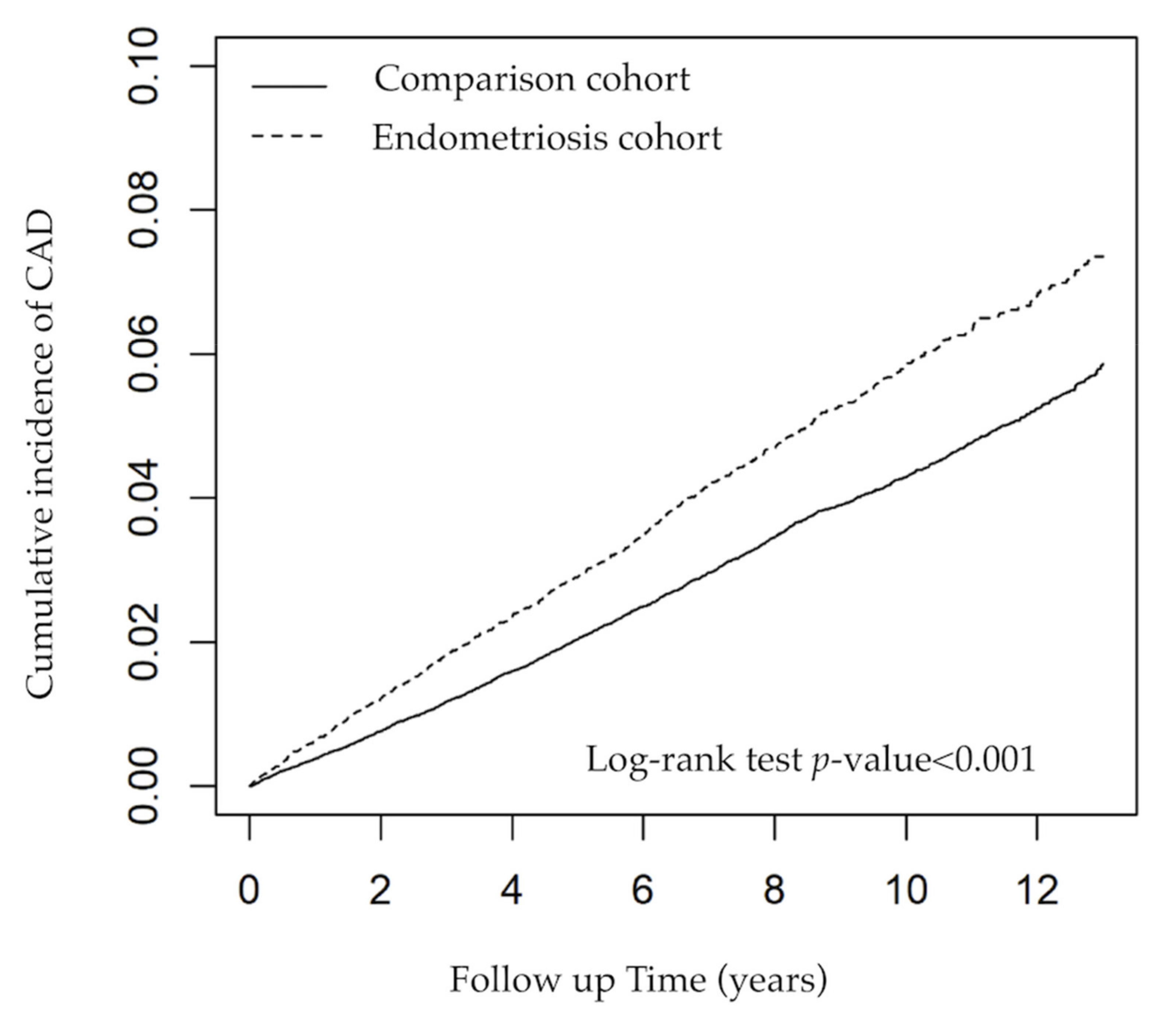
3.2. Risk of CAD
3.3. Subgroup Analysis According to Age
3.4. Subgroup Analysis According to Comorbidities and Medicine
3.5. Subgroup Analysis According to Comorbidities and Medicine in Endometriosis Patients
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taylor, H.S.; Kotlyar, A.M.; Flores, V.A. Endometriosis Is a Chronic Systemic Disease: Clinical Challenges and Novel Innovations. Lancet 2021, 397, 839–852. [Google Scholar] [CrossRef]
- Yamamoto, A.; Johnstone, E.B.; Bloom, M.S.; Huddleston, H.G.; Fujimoto, V.Y. A Higher Prevalence of Endometriosis among Asian Women Does Not Contribute to Poorer IVF Outcomes. J. Assist. Reprod. Genet. 2017, 34, 765–774. [Google Scholar] [CrossRef]
- Yen, C.-F.; Kim, M.-R.; Lee, C.-L. Epidemiologic Factors Associated with Endometriosis in East Asia. Gynecol Minim. Invasive Ther. 2019, 8, 4–11. [Google Scholar]
- Andrews, W.C.; Buttram, V.C., Jr.; Weed, J.C.; Hammond, C.B.; Thomas, H.H.; Behrman, S.J.; Carmichael, E.; Cohen, M.R.; Dmowski, P.; Eward, R.D.; et al. Revised American Fertility Society Classification of Endometriosis: 1985. Fertil. Steril. 1985, 43, 351–352. [Google Scholar]
- Santanam, N.; Song, M.; Rong, R.; Murphy, A.A.; Parthasarathy, S. Atherosclerosis, Oxidation and Endometriosis. Free Radic. Res. 2002, 36, 1315–1321. [Google Scholar] [CrossRef]
- Kvaskoff, M.; Mu, F.; Terry, K.L.; Harris, H.R.; Poole, E.M.; Farland, L.; Missmer, S.A. Endometriosis: A High-Risk Population for Major Chronic Diseases? Hum. Reprod. Update 2015, 21, 500–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mu, F.; Rich-Edwards, J.; Rimm, E.B.; Spiegelman, D.; Missmer, S.A. Endometriosis and Risk of Coronary Heart Disease. Circ. Cardiovasc. Qual. Outcomes 2016, 9, 257–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mu, F.; Rich-Edwards, J.; Rimm, E.B.; Spiegelman, D.; Forman, J.P.; Missmer, S.A. Association Between Endometriosis and Hypercholesterolemia or Hypertension. Hypertension 2017, 70, 59–65. [Google Scholar] [CrossRef]
- Tiniakou, E.; Costenbader, K.H.; Kriegel, M.A. Sex-Specific Environmental Influences on the Development of Autoimmune Diseases. Clin. Immunol. 2013, 149, 182–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.S.; Kim, T.H.; Chung, H.H.; Song, Y.S. Risk and Prognosis of Ovarian Cancer in Women with Endometriosis: A Meta-Analysis. Br. J. Cancer 2014, 110, 1878–1890. [Google Scholar] [CrossRef]
- Cirillo, M.; Coccia, M.E.; Petraglia, F.; Fatini, C. Role of Endometriosis in Defining Cardiovascular Risk: A Gender Medicine Approach for Women’s Health. Hum. Fertil. 2021, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Agic, A.; Xu, H.; Finas, D.; Banz, C.; Diedrich, K.; Hornung, D. Is Endometriosis Associated with Systemic Subclinical Inflammation? Gynecol. Obstet. Investig. 2006, 62, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Scutiero, G.; Iannone, P.; Bernardi, G.; Bonaccorsi, G.; Spadaro, S.; Volta, C.A.; Greco, P.; Nappi, L. Oxidative Stress and Endometriosis: A Systematic Review of the Literature. Oxid. Med. Cell. Longev. 2017, 2017, 7265238. [Google Scholar] [CrossRef]
- Melo, A.S.; Rosa-e-Silva, J.C.; Rosa-e-Silva, A.C.J.D.S.; Poli-Neto, O.B.; Ferriani, R.A.; Vieira, C.S. Unfavorable Lipid Profile in Women with Endometriosis. Fertil. Steril. 2010, 93, 2433–2436. [Google Scholar] [CrossRef]
- Pathak, L.A.; Shirodkar, S.; Ruparelia, R.; Rajebahadur, J. Coronary Artery Disease in Women. Indian Heart J. 2017, 69, 532–538. [Google Scholar] [CrossRef]
- Aggarwal, A.; Srivastava, S.; Velmurugan, M. Newer Perspectives of Coronary Artery Disease in Young. World J. Cardiol. 2016, 8, 728–734. [Google Scholar] [CrossRef]
- Wilmot, K.A.; O’Flaherty, M.; Capewell, S.; Ford, E.S.; Vaccarino, V. Coronary Heart Disease Mortality Declines in the United States From 1979 Through 2011: Evidence for Stagnation in Young Adults, Especially Women. Circulation 2015, 132, 997–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harvey, R.E.; Coffman, K.E.; Miller, V.M. Women-Specific Factors to Consider in Risk, Diagnosis and Treatment of Cardiovascular Disease. Womens. Health 2015, 11, 239–257. [Google Scholar] [CrossRef] [Green Version]
- Sachdev, M.; Sun, J.L.; Tsiatis, A.A.; Nelson, C.L.; Mark, D.B.; Jollis, J.G. The Prognostic Importance of Comorbidity for Mortality in Patients with Stable Coronary Artery Disease. J. Am. Coll. Cardiol. 2004, 43, 576–582. [Google Scholar] [CrossRef] [Green Version]
- Jankowski, J.; Floege, J.; Fliser, D.; Böhm, M.; Marx, N. Cardiovascular Disease in Chronic Kidney Disease: Pathophysiological Insights and Therapeutic Options. Circulation 2021, 143, 1157–1172. [Google Scholar] [CrossRef]
- Hajar, R. Risk Factors for Coronary Artery Disease: Historical Perspectives. Heart Views 2017, 18, 109–114. [Google Scholar] [CrossRef]
- Ambrose, J.A.; Singh, M. Pathophysiology of Coronary Artery Disease Leading to Acute Coronary Syndromes. F1000Prime Rep. 2015, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Rea, I.M.; Gibson, D.S.; McGilligan, V.; McNerlan, S.E.; Alexander, H.D.; Ross, O.A. Age and Age-Related Diseases: Role of Inflammation Triggers and Cytokines. Front. Immunol. 2018, 9, 586. [Google Scholar] [CrossRef]
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799. [Google Scholar] [CrossRef]
- Ramji, D.P.; Davies, T.S. Cytokines in Atherosclerosis: Key Players in All Stages of Disease and Promising Therapeutic Targets. Cytokine Growth Factor Rev. 2015, 26, 673–685. [Google Scholar] [CrossRef] [Green Version]
- Tousoulis, D.; Oikonomou, E.; Economou, E.K.; Crea, F.; Kaski, J.C. Inflammatory Cytokines in Atherosclerosis: Current Therapeutic Approaches. Eur. Heart J. 2016, 37, 1723–1732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fatkhullina, A.R.; Peshkova, I.O.; Koltsova, E.K. The Role of Cytokines in the Development of Atherosclerosis. Biochemistry 2016, 81, 1358–1370. [Google Scholar] [CrossRef]
- Hogg, C.; Horne, A.W.; Greaves, E. Endometriosis-Associated Macrophages: Origin, Phenotype, and Function. Front. Endocrinol. 2020, 11, 7. [Google Scholar] [CrossRef]
- Tariverdian, N.; Siedentopf, F.; Rücke, M.; Blois, S.M.; Klapp, B.F.; Kentenich, H.; Arck, P.C. Intraperitoneal Immune Cell Status in Infertile Women with and without Endometriosis. J. Reprod. Immunol. 2009, 80, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Weisheng, B.; Nezhat, C.H.; Huang, G.F.; Mao, Y.-Q.; Sidell, N.; Huang, R.-P. Discovering Endometriosis Biomarkers with Multiplex Cytokine Arrays. Clin. Proteom. 2019, 16, 28. [Google Scholar] [CrossRef]
- Santoro, L.; D’Onofrio, F.; Flore, R.; Gasbarrini, A.; Santoliquido, A. Endometriosis and Atherosclerosis: What We Already Know and What We Have yet to Discover. Am. J. Obstet. Gynecol. 2015, 213, 326–331. [Google Scholar] [CrossRef]
- Santoro, L.; D’Onofrio, F.; Campo, S.; Ferraro, P.M.; Flex, A.; Angelini, F.; Forni, F.; Nicolardi, E.; Campo, V.; Mascilini, F.; et al. Regression of Endothelial Dysfunction in Patients with Endometriosis after Surgical Treatment: A 2-Year Follow-up Study. Hum. Reprod. 2014, 29, 1205–1210. [Google Scholar] [CrossRef] [Green Version]
- Tani, A.; Yamamoto, S.; Maegawa, M.; Kunimi, K.; Matsui, S.; Keyama, K.; Kato, T.; Uemura, H.; Kuwahara, A.; Matsuzaki, T.; et al. Arterial Stiffness Is Increased in Young Women with Endometriosis. J. Obstet. Gynaecol. 2015, 35, 711–715. [Google Scholar] [CrossRef]
- Ittaman, S.V.; VanWormer, J.J.; Rezkalla, S.H. The Role of Aspirin in the Prevention of Cardiovascular Disease. Clin. Med. Res. 2014, 12, 147–154. [Google Scholar] [CrossRef] [Green Version]
- De Gaetano, G.; Collaborative Group of the Primary Prevention Project. Low-Dose Aspirin and Vitamin E in People at Cardiovascular Risk: A Randomised Trial in General Practice. Collaborative Group of the Primary Prevention Project. Lancet 2001, 357, 89–95. [Google Scholar] [PubMed]
- Hansson, L.; Zanchetti, A.; Carruthers, S.G.; Dahlöf, B.; Elmfeldt, D.; Julius, S.; Ménard, J.; Rahn, K.H.; Wedel, H.; Westerling, S.; et al. Effects of Intensive Blood-Pressure Lowering and Low-Dose Aspirin in Patients with Hypertension: Principal Results of the Hypertension Optimal Treatment (HOT) Randomised Trial. Lancet 1998, 351, 1755–1762. [Google Scholar] [CrossRef]
- The Medical Research Council’s General Practice Research Framework. Thrombosis Prevention Trial: Randomised Trial of Low-Intensity Oral Anticoagulation with Warfarin and Low-Dose Aspirin in the Primary Prevention of Ischaemic Heart Disease in Men at Increased Risk. Lancet 1998, 351, 233–241. [Google Scholar] [CrossRef]
- Steering Committee of the Physicians’ Health Study Research Group Final Report on the Aspirin Component of the Ongoing Physicians’ Health Study. N. Engl. J. Med. 1989, 321, 129–135. [CrossRef] [PubMed]
- Ridker, P.M.; Cook, N.R.; Lee, I.-M.; Gordon, D.; Gaziano, J.M.; Manson, J.E.; Hennekens, C.H.; Buring, J.E. A Randomized Trial of Low-Dose Aspirin in the Primary Prevention of Cardiovascular Disease in Women. N. Engl. J. Med. 2005, 352, 1293–1304. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, X.; Li, X.; Chen, H.; Hu, Y.; Zhang, X.; Tang, X.; Miao, Y.; Tian, G.; Shang, H. Statins for the Primary Prevention of Coronary Heart Disease. Biomed. Res. Int. 2019, 2019, 4870350. [Google Scholar] [CrossRef]
- Vercellini, P.; Eskenazi, B.; Consonni, D.; Somigliana, E.; Parazzini, F.; Abbiati, A.; Fedele, L. Oral Contraceptives and Risk of Endometriosis: A Systematic Review and Meta-Analysis. Hum. Reprod. Update 2011, 17, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Arnold, S.V.; Bhatt, D.L.; Barsness, G.W.; Beatty, A.L.; Deedwania, P.C.; Inzucchi, S.E.; Kosiborod, M.; Leiter, L.A.; Lipska, K.J.; Newman, J.D.; et al. Clinical Management of Stable Coronary Artery Disease in Patients With Type 2 Diabetes Mellitus: A Scientific Statement From the American Heart Association. Circulation 2020, 141, e779–e806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, H.-J.; Lan, K.-C.; Yang, Y.-H.; Chiang, J.Y.; Kung, F.-T.; Huang, F.-J.; Lin, Y.-J.; Su, Y.-T.; Sung, P.-H. Risk of Major Adverse Cardiovascular and Cerebrovascular Events in Taiwanese Women with Endometriosis. J. Formos. Med. Assoc. 2021, 120, 327–336. [Google Scholar] [CrossRef]
- Waller, K.G.; Shaw, R.W. Risk Factors for Endometriosis. Med. Princ. Pract. 1998, 7, 127–133. [Google Scholar] [CrossRef]
- Parazzini, F.; Vercellini, P.; Pelucchi, C. Endometriosis: Epidemiology, and Etiological Factors. In Endometriosis; Wiley-Blackwell: Oxford, UK, 2012; pp. 19–26. ISBN 9781444398519. [Google Scholar]
- Treloar, S.A.; Bell, T.A.; Nagle, C.M.; Purdie, D.M.; Green, A.C. Early Menstrual Characteristics Associated with Subsequent Diagnosis of Endometriosis. Am. J. Obstet. Gynecol. 2010, 202, 534.e1-6. [Google Scholar] [CrossRef] [PubMed]
| Endometriosis | Comparison Group | ||
|---|---|---|---|
| (n = 19,454) | (n = 77,816) | p-Value | |
| Age, Years, n (%) | >0.999 | ||
| <40 | 11,049 (56.80) | 44,196 (56.80) | |
| 40–49 | 7268 (37.36) | 29,072 (37.36) | |
| 50–64 | 1137 (5.84) | 4548 (5.84) | |
| Mean (SD) | 37.37 (8.95) | 37.34 (9.13) | 0.658 |
| Comorbidity, n (%) | |||
| Obesity | 232 (1.19) | 641 (0.82) | <0.001 |
| CKD | 68 (0.35) | 263 (0.34) | 0.804 |
| Hypertension | 1472 (7.57) | 4894 (6.29) | <0.001 |
| Hyperlipidemia | 1269 (6.52) | 3862 (4.96) | <0.001 |
| DM | 965 (4.96) | 3003 (3.86) | <0.001 |
| Hysterectomy | 4709 (24.21) | 1769 (2.27) | <0.001 |
| Oophorectomy | 4861 (24.99) | 1802 (2.32) | <0.001 |
| Medication, n (%) | |||
| Hormone (estradiol, premarin) | 11,331 (58.25) | 28,362 (36.45) | <0.001 |
| Statin | 1886 (9.69) | 5728 (7.36) | <0.001 |
| Aspirin | 9074 (46.64) | 29,210 (37.54) | <0.001 |
| Antihypertensive | 11,639 (59.83) | 35,399 (45.49) | <0.001 |
| Antihyperglycemic | 1441 (7.41) | 4565 (5.87) | <0.001 |
| Insulin | 399 (2.05) | 1401 (1.80) | 0.020 |
| Follow-up duration, years | |||
| Mean (SD) | 7.36 (3.82) | 7.02 (3.86) | <0.001 |
| Endometriosis | N | CAD Event | Person-Years | IR | HR (95% CI) | |
|---|---|---|---|---|---|---|
| Crude | Adjusted † | |||||
| No | 77,816 | 2392 | 546,412 | 4.38 | 1.00 (reference) | 1.00 (reference) |
| Yes | 19,454 | 853 | 143,169 | 5.96 | 1.36 (1.26, 1.47) *** | 1.34 (1.22, 1.47) *** |
| Endometriosis Cohort | Comparison Cohort | Crude | Adjusted † | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CAD Event | PY | IR | CAD Event | PY | IR | HR (95% CI) | p-Value | HR (95% CI) | p-Value | p-Value for Interaction | |
| Age, years | <0.001 | ||||||||||
| <40 | 230 | 84,798 | 2.71 | 529 | 314,233 | 1.68 | 1.60 (1.37, 1.86) | <0.001 | 1.42 (1.19, 1.70 | <0.001 | |
| 40–49 | 505 | 51,333 | 9.84 | 1425 | 204,036 | 6.98 | 1.41 (1.27, 1.56) | <0.001 | 1.33 (1.18, 1.49) | <0.001 | |
| 50–64 | 118 | 7038 | 16.77 | 438 | 28,143 | 15.56 | 1.08 (0.88, 1.32) | 0.475 | 1.02 (0.81, 1.29) | 0.870 | |
| Comorbidity | |||||||||||
| Obesity | 0.438 | ||||||||||
| No | 831 | 141,770 | 5.86 | 2358 | 542,807 | 4.34 | 1.35 (1.24, 1.46) | <0.001 | 1.17 (1.07, 1.28) | <0.001 | |
| Yes | 22 | 1399 | 15.73 | 34 | 3605 | 9.43 | 1.66 (0.97, 2.84) | 0.064 | 1.42 (0.76, 2.65) | 0.270 | |
| CKD | 0.096 | ||||||||||
| No | 844 | 142,741 | 5.91 | 2351 | 544,958 | 4.31 | 1.37 (1.26, 1.48) | <0.001 | 1.18 (1.08, 1.30) | <0.001 | |
| Yes | 9 | 428 | 21.03 | 41 | 1454 | 28.21 | 0.76 (0.37, 1.56) | 0.454 | 0.80 (0.35, 1.87) | 0.611 | |
| Hypertension | 0.047 | ||||||||||
| No | 632 | 133,732 | 4.73 | 1753 | 514,840 | 3.40 | 1.38 (1.26, 1.51) | <0.001 | 1.16 (1.04, 1.29) | 0.007 | |
| Yes | 221 | 9437 | 23.42 | 639 | 31,572 | 20.24 | 1.16 (0.99, 1.35) | 0.062 | 1.24 (1.04, 1.48) | 0.017 | |
| Hyperlipidemia | <0.001 | ||||||||||
| No | 735 | 135,336 | 5.43 | 2032 | 523,695 | 3.88 | 1.40 (1.28, 1.52) | <0.001 | 1.22 (1.11, 1.35) | <0.001 | |
| Yes | 118 | 7833 | 15.06 | 360 | 22,716 | 15.85 | 0.96 (0.78, 1.18) | 0.690 | 0.96 (0.76, 1.22) | 0.747 | |
| DM | 0.030 | ||||||||||
| No | 734 | 136,463 | 5.38 | 2065 | 526,736 | 3.92 | 1.37 (1.26, 1.49) | <0.001 | 1.18 (1.07, 1.30) | 0.001 | |
| Yes | 119 | 6706 | 17.74 | 327 | 19,676 | 16.62 | 1.07 (0.87, 1.32) | 0.523 | 1.16 (0.91, 1.48) | 0.222 | |
| Hysterectomy | 0.267 | ||||||||||
| No | 540 | 105,475 | 5.12 | 2281 | 532,435 | 4.28 | 1.19 (1.09, 1.31) | <0.001 | 1.23 (1.11, 1.36) | <0.001 | |
| Yes | 313 | 37,694 | 8.30 | 111 | 13,977 | 7.94 | 1.04 (0.84, 1.30) | 0.690 | 1.09 (0.88, 1.35) | 0.443 | |
| Oophorectomy | 0.004 | ||||||||||
| No | 662 | 104,720 | 6.32 | 2323 | 533,103 | 4.36 | 1.45 (1.33, 1.58) | <0.001 | 1.22 (1.11, 1.35) | <0.001 | |
| Yes | 191 | 38,449 | 4.97 | 69 | 13,309 | 5.18 | 0.95 (0.72, 1.25) | 0.724 | 1.05 (0.79, 1.39) | 0.735 | |
| Medication | |||||||||||
| Hormone (estradiol + premarin) | 0.089 | ||||||||||
| No | 342 | 56,806 | 6.02 | 1424 | 340,439 | 4.18 | 1.44 (1.28, 1.62) | <0.001 | 1.29 (1.12, 1.47) | <0.001 | |
| Yes | 511 | 86,363 | 5.92 | 968 | 205,972 | 4.70 | 1.25 (1.12, 1.39) | <0.001 | 1.11 (0.98, 1.25) | 0.103 | |
| Statin | 0.001 | ||||||||||
| No | 704 | 127,306 | 5.53 | 1948 | 498,615 | 3.91 | 1.42 (1.30, 1.54) | <0.001 | 1.20 (1.09, 1.33) | <0.001 | |
| Yes | 149 | 15,863 | 9.39 | 444 | 47,797 | 9.29 | 1.01 (0.84, 1.22) | 0.917 | 1.08 (0.87, 1.35) | 0.494 | |
| Aspirin | 0.004 | ||||||||||
| No | 379 | 75,191 | 5.04 | 1145 | 336,187 | 3.41 | 1.48 (1.32, 1.66) | <0.001 | 1.27 (1.11, 1.46) | <0.001 | |
| Yes | 474 | 67,978 | 6.97 | 1247 | 210,225 | 5.93 | 1.17 (1.06, 1.30) | 0.003 | 1.11 (0.98, 1.25) | 0.105 | |
| Antihypertensive | 0.008 | ||||||||||
| No | 157 | 54,941 | 2.86 | 548 | 282,990 | 1.94 | 1.48 (1.24, 1.76) | <0.001 | 1.46 (1.20, 1.79) | <0.001 | |
| Yes | 696 | 88,228 | 7.89 | 1844 | 263,422 | 7.00 | 1.13 (1.03, 1.23) | 0.008 | 1.12 (1.01, 1.24) | 0.029 | |
| Antihyperglycemic | <0.001 | ||||||||||
| No | 755 | 131,763 | 5.73 | 2043 | 510,470 | 4.00 | 1.43 (1.32, 1.55) | <0.001 | 1.22 (1.11, 1.34) | <0.001 | |
| Yes | 98 | 11,406 | 8.59 | 349 | 35,942 | 9.71 | 0.88 (0.71, 1.11) | 0.285 | 0.94 (0.72, 1.22) | 0.652 | |
| Insulin | 0.152 | ||||||||||
| No | 813 | 140,141 | 5.80 | 2262 | 536,065 | 4.22 | 1.37 (1.27, 1.49) | <0.001 | 1.18 (1.08, 1.30) | <0.001 | |
| Yes | 40 | 3028 | 13.21 | 130 | 10,347 | 12.56 | 1.06 (0.74, 1.51) | 0.764 | 1.16 (0.76, 1.79) | 0.491 | |
| Endometriosis Cohort | Crude | Adjusted † | |||||
|---|---|---|---|---|---|---|---|
| CAD Events | PY | IR | HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Age, years | |||||||
| <40 | 230 | 84,798 | 2.71 | 1.00 (reference) | - | 1.00 (reference) | - |
| 40–49 | 505 | 51,333 | 9.84 | 3.63 (3.10, 4.24) | <0.001 | 2.96 (2.50, 3.50) | <0.001 |
| 50–64 | 118 | 7038 | 16.77 | 6.19 (4.96, 7.74) | <0.001 | 3.88 (3.04, 4.95) | <0.001 |
| Comorbidity | |||||||
| Obesity | |||||||
| No | 831 | 141,770 | 5.86 | 1.00 (reference) | - | 1.00 (reference) | - |
| Yes | 22 | 1399 | 15.73 | 2.67 (1.75, 4.08) | <0.001 | 1.85 (1.20, 2.85) | 0.005 |
| CKD | |||||||
| No | 844 | 142,741 | 5.91 | 1.00 (reference) | - | 1.00 (reference) | - |
| Yes | 9 | 428 | 21.03 | 3.54 (1.84, 6.83) | <0.001 | 1.50 (0.77, 2.91) | 0.234 |
| Hypertension | |||||||
| No | 632 | 133,732 | 4.73 | 1.00 (reference) | - | 1.00 (reference) | - |
| Yes | 221 | 9437 | 23.42 | 4.95 (4.24, 5.77) | <0.001 | 2.57 (2.15, 3.07) | <0.001 |
| Hyperlipidemia | |||||||
| No | 735 | 135,336 | 5.43 | 1.00 (reference) | - | 1.00 (reference) | - |
| Yes | 118 | 7833 | 15.06 | 2.77 (2.28, 3.36) | <0.001 | 1.26 (1.01, 1.58) | 0.038 |
| DM | |||||||
| No | 734 | 136,463 | 5.38 | 1.00 (reference) | - | 1.00 (reference) | - |
| Yes | 119 | 6706 | 17.74 | 3.29 (2.71, 4.00) | <0.001 | 1.95 (1.55, 2.44) | <0.001 |
| Hysterectomy | |||||||
| No | 540 | 105,475 | 5.12 | 1.00 (reference) | - | 1.00 (reference) | - |
| Yes | 313 | 37,694 | 8.3 | 1.63 (1.42, 1.87) | <0.001 | 0.97 (0.84, 1.13) | 0.699 |
| Oophorectomy | |||||||
| No | 662 | 104,720 | 6.32 | 1.00 (reference) | - | 1.00 (reference) | - |
| Yes | 191 | 38,449 | 4.97 | 0.79 (0.67, 0.92) | 0.004 | 0.91 (0.77, 1.07) | 0.24 |
| Medication | |||||||
| Hormone (estradiol, premarin) | |||||||
| No | 342 | 56,806 | 6.02 | 1.00 (reference) | - | 1.00 (reference) | - |
| Yes | 511 | 86,363 | 5.92 | 0.98 (0.86, 1.13) | 0.824 | 0.86 (0.75, 0.99) | 0.032 |
| Statin | |||||||
| No | 704 | 127,306 | 5.53 | 1.00 (reference) | - | 1.00 (reference) | - |
| Yes | 149 | 15,863 | 9.39 | 1.70 (1.43, 2.03) | <0.001 | 0.70 (0.57, 0.87) | <0.001 |
| Aspirin | |||||||
| No | 379 | 75,191 | 5.04 | 1.00 (reference) | - | 1.00 (reference) | - |
| Yes | 474 | 67,978 | 6.97 | 1.38 (1.21, 1.58) | <0.001 | 1.19 (1.03, 1.37) | 0.016 |
| Antihypertensive | |||||||
| No | 157 | 54,941 | 2.86 | 1.00 (reference) | - | 1.00 (reference) | - |
| Yes | 696 | 88,228 | 7.89 | 2.77 (2.33, 3.29) | <0.001 | 1.88 (1.56, 2.26) | <0.001 |
| Antihyperglycemic | |||||||
| No | 755 | 131,763 | 5.73 | 1.00 (reference) | - | 1.00 (reference) | - |
| Yes | 98 | 11,406 | 8.59 | 1.50 (1.22, 1.85) | <0.001 | 0.68 (0.52, 0.89) | 0.005 |
| Insulin | |||||||
| No | 813 | 140,141 | 5.8 | 1.00 (reference) | - | 1.00 (reference) | - |
| Yes | 40 | 3028 | 13.21 | 2.27 (1.66, 3.12) | <0.001 | 1.13 (0.76, 1.66) | 0.546 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.-C.; Yang, Y.-C.; Wang, J.-H.; Lin, S.-Z.; Ding, D.-C. Endometriosis Is Associated with an Increased Risk of Coronary Artery Disease in Asian Women. J. Clin. Med. 2021, 10, 4173. https://doi.org/10.3390/jcm10184173
Li P-C, Yang Y-C, Wang J-H, Lin S-Z, Ding D-C. Endometriosis Is Associated with an Increased Risk of Coronary Artery Disease in Asian Women. Journal of Clinical Medicine. 2021; 10(18):4173. https://doi.org/10.3390/jcm10184173
Chicago/Turabian StyleLi, Pei-Chen, Yu-Cih Yang, Jen-Hung Wang, Shinn-Zong Lin, and Dah-Ching Ding. 2021. "Endometriosis Is Associated with an Increased Risk of Coronary Artery Disease in Asian Women" Journal of Clinical Medicine 10, no. 18: 4173. https://doi.org/10.3390/jcm10184173
APA StyleLi, P.-C., Yang, Y.-C., Wang, J.-H., Lin, S.-Z., & Ding, D.-C. (2021). Endometriosis Is Associated with an Increased Risk of Coronary Artery Disease in Asian Women. Journal of Clinical Medicine, 10(18), 4173. https://doi.org/10.3390/jcm10184173







