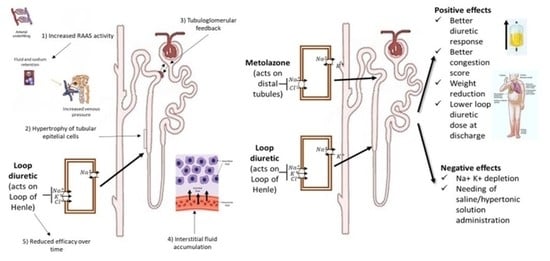
Effects of Metolazone Administration on Congestion, Diuretic Response and Renal Function in Patients with Advanced Heart Failure
Abstract
:1. Introduction
2. Methods
2.1. Patient Screening and Evaluation
2.2. Laboratory Assessment
2.3. Follow-Up
2.4. End Points
2.5. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gheorghiade, M.; Filippatos, G.; De Luca, L.; Burnett, J. Congestion in acute heart failure syndromes: An essential target of evaluation and treatment. Am. J. Med. 2006, 119, S3–S10. [Google Scholar] [CrossRef]
- Cleland, J.C.; Pellicori, P.; Januzzi, J.L.; Zannand, F.; Clark, A.; Richards, M.; McMurray, J.J.; Mueller, C. The conceptual basis for a universal definition of heart failure: Congestion due to cardiac dysfunction. Eur. Heart J. 2021, 42, 2331–2343. [Google Scholar] [CrossRef]
- Palazzuoli, A.; Ruocco, G.; Ronco, C.; McCullough, P.A. Loop diuretics in acute heart failure: Beyond the decongestive relief for the kidney. Crit. Care. 2015, 19, 296. [Google Scholar] [CrossRef] [Green Version]
- Chiong, J.R.; Cheung, R.J. Loop Diuretic Therapy in Heart Failure: The Need for Solid Evidence on a Fluid Issue. Clin. Cardiol. 2010, 33, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Felker, G.M.; Ellison, D.H.; Mullens, W.; Cox, Z.L.; Testani, J.M. Diuretic Therapy for Patients with Heart Failure: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 1178–1195. [Google Scholar] [CrossRef] [PubMed]
- Mullens, W.; Damman, K.; Harjola, V.P.; Mebazaa, A.; Brunner-La Rocca, H.P.; Martens, P.; Testani, J.M.; Tang, W.H.W.; Orso, F.; Rossignol, P.; et al. The use of diuretics in heart failure with congestion—A position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2019, 21, 137–155. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Qadir, H.M.; Tu, J.V.; Yun, L.; Austin, P.C.; Newton, G.E.; Lee, D.S. Diuretic dose and long-term outcomes in elderly patients with heart failure after hospitalization. Am. Heart J. 2010, 160, 264–271. [Google Scholar] [CrossRef]
- Shah, R.V.; McNulty, S.; O’Connor, C.M.; Felker, G.M.; Braunwald, E.; Givertz, M.M. Effect of admission oral diuretic dose on response to continuous versus bolus intravenous diuretics in acute heart failure: An analysis from diuretic optimization strategies in acute heart failure. Am. Heart J. 2012, 164, 862–868. [Google Scholar] [CrossRef] [Green Version]
- Felker, G.M.; O’Connor, C.M.; Braunwald, E. Loop diuretics in acute decompensated heart failure: Necessary? Evil? A necessary evil? Circ. Heart Fail. 2009, 2, 56–62. [Google Scholar] [CrossRef] [Green Version]
- Testani, J.M.; Brisco, M.A.; Turner, J.M.; Spatz, E.S.; Bellumkonda, L.; Parikh, C.R.; Tang, W.H. Loop diuretic efficiency: A metric of diuretic responsiveness with prognostic importance in acute decompensated heart failure. Circ. Heart Fail. 2014, 7, 261–270. [Google Scholar] [CrossRef] [Green Version]
- Dauw, J.; Martens, P.; Tersalvi, G.; Schouteden, J.; Deferm, S.; Gruwez, H.; De Moor, B.; Nijst, P.; Dupont, M.; Mullens, W. Diuretic response and effects of diuretic omission in ambulatory heart failure patients on chronic low-dose loop diuretic therapy. Eur. J. Heart Fail. 2021, 23, 1110–1119. [Google Scholar] [CrossRef]
- Ter Maaten, J.M.; Dunning, A.M.; Valente, M.A.; Damman, K.; Ezekowitz, J.A.; Califf, R.M.; Starling, R.C.; van der Meer, P.; O’Connor, C.M.; Schulte, P.J.; et al. Diuretic response in acute heart failure-an analysis from ASCEND-HF. Am. Heart J. 2015, 170, 313–321. [Google Scholar] [CrossRef]
- Valente, M.A.; Voors, A.A.; Damman, K.; Van Veldhuisen, D.J.; Massie, B.M.; O’Connor, C.M.; Metra, M.; Ponikowski, P.; Teerlink, J.R.; Cotter, G.; et al. Diuretic response in acute heart failure: Clinical characteristics and prognostic significance. Eur. Heart J. 2014, 35, 1284–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jentzer, J.C.; DeWald, T.A.; Hernandez, A.F. Combination of loop diuretics with thiazide-type diuretics in heart failure. J. Am. Coll. Cardiol. 2010, 56, 1527–1534. [Google Scholar] [CrossRef] [Green Version]
- Crespo-Leiro, M.G.; Metra, M.; Lund, L.H.; Milicic, D.; Costanzo, M.R.; Filippatos, G.; Gustafsson, F.; Tsui, S.; Barge-Caballero, E.; De Jonge, N.; et al. Advanced heart failure: A position statement of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2018, 20, 1505–1535. [Google Scholar] [CrossRef] [PubMed]
- Palazzuoli, A.; Ruocco, G.; Vescovo, G.; Valle, R.; Di Somma, S.; Nuti, R. Rationale and study design of intravenous loop diuretic administration in acute heart failure: DIUR-AHF. ESC Heart Fail. 2017, 4, 479–486. [Google Scholar] [CrossRef] [Green Version]
- Gheorghiade, M.; Follath, F.; Ponikowski, P.; Barsuk, J.H.; Blair, J.E.; Cleland, J.G.; Dickstein, K.; Drazner, M.H.; Fonarow, G.C.; Jaarsma, T.; et al. Assessing and grading congestion in acute heart failure: A scientific statement from the acute heart failure committee of the heart failure association of the European Society of Cardiology and endorsed by the European Society of Intensive Care Medicine. Eur. J. Heart Fail. 2010, 12, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Damman, K.; Tang, W.H.; Testani, J.M.; McMurray, J.J. Terminology and definition of changes renal function in heart failure. Eur. Heart J. 2014, 35, 3413–3416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ter Maaten, J.M.; Valente, M.A.; Damman, K.; Hillege, H.L.; Navis, G.; Voors, A.A. Diuretic response in acute heart failure-pathophysiology, evaluation, and therapy. Nat. Rev. Cardiol. 2015, 12, 184–192. [Google Scholar] [CrossRef]
- Francis, G.S.; Siegel, R.M.; Goldsmith, S.R.; Olivari, M.T.; Levine, T.B.; Cohn, J.N. Acute vasoconstriction response to intravenous furosemide in patients with chronic congestive heart failure. Activation of neurohormonal axis. Ann. Intern. Med. 1985, 103, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hasselblad, V.; Stough, W.G.; Shah, M.R.; Lokhnygina, Y.; O’Connor, C.M.; Califf, R.M.; Adams, K.F., Jr. Relation between dose of loop diuretics and outcomes in heart failure population: Results of the ESCAPE trial. Eur. J. Heart Fail. 2007, 9, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Bikdeli, B.; Strait, K.M.; Dharmarajan, K.; Li, S.X.; Mody, P.; Partovian, C.; Coca, S.G.; Kim, N.; Horwitz, L.I.; Testani, J.M.; et al. Intravenous fluids in acute decompensated heart failure. JACC Heart Fail. 2015, 3, 127–133. [Google Scholar] [CrossRef]
- Griffin, M.; Soufer, A.; Goljo, E.; Colna, M.; Rao, V.S.; Jeon, S.; Raghavendra, P.; D’Ambrosi, J.; Riello, R.; Coca, S.G.; et al. Real World Use of Hypertonic Saline in Refractory Acute Decompensated Heart Failure: A US. Center’s Experience. JACC Heart Fail. 2020, 8, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.; Mosleh, W.; Myers, R.B. Hypertonic saline with furosemide for the treatment of acute congestive heart failure: A systematic review and meta-analysis. Int. J. Cardiol. 2014, 173, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, M.R. Hypertonic Saline: The Genesis of the Exodus of Fluid in Heart Failure? JACC Heart Fail. 2020, 8, 209–211. [Google Scholar] [CrossRef]
- Yilmaz, M.B.; Gayat, E.; Salem, R.; Lassus, J.; Nikolaou, M.; Laribi, S.; Parissis, J.; Follath, F.; Peacock, W.F.; Mebazaa, A. Impact of diuretic dosing on mortality in acute heart failure using a propensity matched study. Eur. J. Heart Fail. 2011, 13, 1244–1252. [Google Scholar] [CrossRef]
- Palazzuoli, A.; Testani, J.M.; Ruocco, G.; Pellegrini, M.; Ronco, C.; Nuti, R. Different diuretic dose and response in acute decompensated heart failure: Clinical characteristics and prognostic significance. Int. J. Cardiol. 2016, 224, 213–219. [Google Scholar] [CrossRef]
- Pellicori, P.; Cleland, J.G.; Zhang, J.; Kallvikbacka-Bennett, A.; Urbinati, A.; Shah, P.; Kazmi, S.; Clark, A.L. Cardiac Dysfunction, Congestion and Loop Diuretics: Their Relationship to Prognosis in Heart Failure. Cardiovasc. Drugs Ther. 2016, 30, 599–609. [Google Scholar] [CrossRef] [Green Version]
- Hanberg, J.S.; Tang, W.W.; Wilson, F.P.; Coca, S.G.; Ahmad, T.; Brisco, M.A.; Testani, J.M. An exploratory analysis of the competing effects of aggressive decongestion and high-dose loop diuretic therapy in the DOSE trial. Int. J. Cardiol. 2017, 241, 277–282. [Google Scholar] [CrossRef]
- Cox, Z.L.; Testani, J.M. Loop diuretic resistance complicating acute heart failure. Heart Fail. Rev. 2020, 25, 133–145. [Google Scholar] [CrossRef]
- Boorsma, E.M.; Ter Maaten, J.M.; Damman, K.; Dinh, W.; Gustafsson, F.; Goldsmith, S.; Burkhoff, D.; Zannad, F.; Udelson, J.E.; Voors, A.A. Congestion in heart failure: A contemporary look at physiology, diagnosis and treatment. Nat. Rev. Cardiol. 2020, 17, 641–655. [Google Scholar] [CrossRef]
- Metra, M.; O’Connor, C.M.; Davison, B.A.; Cleland, J.G.; Ponikowski, P.; Teerlink, J.R.; Voors, A.A.; Givertz, M.M.; Mansoor, G.A.; Bloomfield, D.M.; et al. Early dyspnoea relief in acute heart failure: Prevalence, association with mortality, and effect of rolofylline in the P.RO.TE.CT Study. Eur. Heart J. 2011, 32, 1519–1534. [Google Scholar] [CrossRef] [Green Version]
- Rubio-Gracia, J.; Demissei, B.G.; Ter Maaten, J.M.; Cleland, J.G.; O’Connor, C.M.; Metra, M.; Ponikowski, P.; Teerlink, J.R.; Cotter, G.; Davison, B.A.; et al. Prevalence, predictors and clinical outcome of residual congestion in acute decompensated heart failure. Int. J. Cardiol. 2018, 258, 185–191. [Google Scholar] [CrossRef] [Green Version]
- Lala, A.; McNulty, S.E.; Mentz, R.J.; Dunlay, S.M.; Vader, J.M.; AbouEzzeddine, O.F.; DeVore, A.D.; Khazanie, P.; Redfield, M.M.; Goldsmith, S.R.; et al. Relief and Recurrence of Congestion During and After Hospitalization for Acute Heart Failure: Insights from Diuretic Optimization Strategy Evaluation in Acute Decompensated Heart Failure (DOSE-AHF) and Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARESS-HF). Circ. Heart Fail. 2015, 8, 741–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mentz, R.J.; Kjeldsen, K.; Rossi, G.P.; Voors, A.A.; Cleland, J.G.; Anker, S.D.; Gheorghiade, M.; Fiuzat, M.; Rossignol, P.; Zannad, F.; et al. Decongestion in acute heart failure. Eur. J. Heart Fail. 2014, 16, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Pang, P.; Cleland, J.G.; Teerlink, J.R.; Collins, S.P.; Lindsell, C.J.; Sopko, G.; Peacock, W.F.; Fonarow, G.C.; Aldeen, A.Z.; Kirk, J.D.; et al. measurement in clinical trial of acute heart failure syndromes: The need for an uniform approach. Eur. Heart J. 2008, 29, 816–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harjola, V.P.; Mullens, W.; Banaszewski, M.; Bauersachs, J.; Brunner-La Rocca, H.P.; Chioncel, O.; Collins, S.P.; Doehner, W.; Filippatos, G.S.; Flammer, A.J.; et al. Organ dysfunction, injury and failure in acute heart failure: From pathophysiology to diagnosis and management. A review on behalf of the Acute Heart Failure Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (E.SC). Eur. J. Heart Fail. 2017, 19, 821–836. [Google Scholar] [CrossRef] [PubMed]
- Brisco-Bacik, M.A.; Ter Maaten, J.M.; Houser, S.R.; Vedage, N.A.; Rao, V.; Ahmad, T.; Wilson, F.P.; Testani, J.M. Outcomes Associated with a Strategy of Adjuvant Metolazone or High-Dose Loop Diuretics in Acute Decompensated Heart Failure: A Propensity Analysis. J. Am. Heart Assoc. 2018, 7, e009149. [Google Scholar] [CrossRef] [Green Version]
- Shulenberger, C.E.; Jiang, A.; Devabhakthuni, S.; Ivaturi, V.; Liu, T.; Reed, B.N. Efficacy and Safety of Intravenous Chlorothiazide versus Oral Metolazone in Patients with Acute Decompensated Heart Failure and Loop Diuretic Resistance. Pharmacotherapy 2016, 36, 852–860. [Google Scholar] [CrossRef]
- Cox, Z.L.; Hung, R.; Lenihan, D.J.; Testani, J.M. Diuretic Strategies for Loop Diuretic Resistance in Acute Heart Failure: The 3T Trial. JACC Heart Fail. 2020, 8, 157–168. [Google Scholar] [CrossRef]



| n = 132 Patients | GROUP F (67 pz) | GROUP M (65 pz) |
|---|---|---|
| AGE | 69 ± 16 (median 70 IQR 60–82) | 71 ± 21 (median 70 IQR 58–84) |
| GENDER | 32 F–38 M | 17 F–46 M |
| BMI (kg/m2) | 27 ± 7 (median 27 IQR 24–32) | 28 ± 6 (median 28 IQR 24–32) |
| HYPERTENSION | 55 (77%) | 46 (75%) |
| DYSLIPIDEMIA | 35 (49%) | 28 (45%) |
| DIABETES | 22 (31%) | 16 (26%) |
| CMD | 36 (51%) | 32 (51%) |
| PREVIOUS CAD | 28 (39%) | 25 (40%) |
| VALVULAR DISEASE | 7 (10%) | 3 (9%) |
| ATRIAL FIBRILLATION | 23 (32%) | 21 (33%) |
| MEAN EF (%) | 34 ± 6 | 32 ± 7 |
| PRO-BNP (pg/mL) | 10,316 ± 8815 (median 10,500 IQR 550–14,900) | 12,177 ± 6283 (median 11,200 IQR 5600–14,300) |
| CONGESTION SCORE | 3.16 ± 0.71 (median 3 IQR 2.5–3.5) | 3.08 ± 0.81 (median 3 IQR 2–3.2) |
| CREATININE ADMISSION (mg/dL) | 1.76 ± 1.05 (median 1.8 IQR 1.3–2.1) | 1.67 ± 1.2 (median 1.6 IQR 1.3–2.1) |
| eGFR (mL/min) | 39.3 ± 18 (median 38 IQR 25–45) | 40.5 ± 20 (median 40 IQR 22–55) |
| CKD | 35 (57%) | 32 (51%) |
| Blood UREA (mg/dL) | 80 ± 31 (median 78 IQR 50–75) | 66 ± 17 (median 68 IQR 48–70) |
| K (mEq/L) | 4.3 ± 0.5 (median 4.2 IQR 4–4.5) | 4.1 ± 0.8 (median 4.1 IQR 3.6–4.4) |
| Na (mEq/L) | 136 ± 5 (median 137 IQR 133–141) | 137 ± 5 (median 138 IQR 136–140) |
| BLOOD PRESSURE (mmHg) | SYS 145 ± 20 DIA 81 ± 15 | SYS 138 ± 18 DIA 75 ± 15 |
| NYHA class | CLASS III = 17 pz (28%) CLASS IV = 51 pz (72%) | CLASS III = 15 pz (27%) CLASS IV = 50 pz (73%) |
| Loop diuretic dose at admission (mg/day) | 230 ± 150 (median 225 IQR 120–300) | 250 ± 120 (median 230 IQR 125–350) |
| ACE-Inhibitors | 39 (58%) | 36 (55%) |
| Angiotensin receptor Blocker (ARB) | 20 (30%) | 20 (31%) |
| Beta-blockers | 41 (61%) | 43 (66%) |
| Digoxin | 20 (30%) | 17 (26%) |
| Mineralocorticoid antagonists (MRA) | 28 (42%) | 26 (40%) |
| Angiotensin receptor–neprilysin inhibitors (ARNIs) | 8 (12%) | 9 (14%) |
| GROUP F | GROUP M | ||
|---|---|---|---|
| DIURETIC RESPONSE (mL/40 mg furosemide) | 541 ± 314 [median 540 IQR 940–240] | 940 ± 149 [median 944 IQR 550–1080] | p < 0.001 ANOVA p < 0.03 ANCOVA |
| CONGESTION SCOREAT DISCHARGE | 2.4 ± 1 [median 2 IQR 1.0–3.2] | 1 ± 1 [median 0.8 IQR 0.5–1.2] | p < 0.001 ANOVA p = 0.03 ANCOVA |
| NT-PRO-BNP (pg/mL) difference from admission to discharge | −3954 ± 5560 [median 3900 IQR 2120–7500] | −4819 ± 8718 [median 4780 IQR 2880–12,700] | 0.1 |
| NT-PRO-BNP (Δ/%) | −25.1 ± 25 | −26.6 ± 27.3 | 0.1 |
| GROUP F | GROUP M | ||
|---|---|---|---|
| Blood Urea (mg/dL) | 82 ± 40 [median 76 IQR 58–95] | 66 ± 18 [median 67 IQR 52–75] | p = 0.005 |
| Mean eGFR (mL/min/m2) | 36.8 ± 22 [median 38 IQR 25–52] | 38.5 ± 24 [median 39 IQR 26–50] | 0.8 |
| CREATININE (mg/dL) | 1.69 ± 0.62 [median 1.5 IQR 1.3–2.2] | 1.72 ± 0.78 [median 1.5 IQR 1.2–2.1] | 0.5 |
| K+DISCHARGE (mEq/L) | 3.87 ± 0.55 [median 3.9 IQR 3.7–4.4] | 4.05 ± 0.67 [median 4.1 IQR 3.6–4.2] | 0.4 |
| NA+DISCHARGE (mEq/L) | 138.6 ± 4.45 [median 138 IQR 135–141] | 137.8 ± 4.3 [median 138 IQR 135–141] | 0.6 |
| HYPERTONIC SOLUTION | 8 (12%) | 22 (33%) | p = 0.03 |
| MEAN DIURESIS (mL) | 2050 ± 1120 [median 2080 IQR 1650–2800] | 2820 ± 900 [median 2450 IQR 1900–3000] | p < 0.05 |
| Δ WEIGHT (kg) | −3 ± 1.5 | −6 ± 2.3 | p < 0.01 |
| LOOP DIURETIC DOSEAT DISCHARGE(mg) | 223.9 ± 121.7 [median 175 IQR 125–275] | 175 ± 104.8 [median 150 IQR 100–250] | p < 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palazzuoli, A.; Ruocco, G.; Severino, P.; Gennari, L.; Pirrotta, F.; Stefanini, A.; Tramonte, F.; Feola, M.; Mancone, M.; Fedele, F. Effects of Metolazone Administration on Congestion, Diuretic Response and Renal Function in Patients with Advanced Heart Failure. J. Clin. Med. 2021, 10, 4207. https://doi.org/10.3390/jcm10184207
Palazzuoli A, Ruocco G, Severino P, Gennari L, Pirrotta F, Stefanini A, Tramonte F, Feola M, Mancone M, Fedele F. Effects of Metolazone Administration on Congestion, Diuretic Response and Renal Function in Patients with Advanced Heart Failure. Journal of Clinical Medicine. 2021; 10(18):4207. https://doi.org/10.3390/jcm10184207
Chicago/Turabian StylePalazzuoli, Alberto, Gaetano Ruocco, Paolo Severino, Luigi Gennari, Filippo Pirrotta, Andrea Stefanini, Francesco Tramonte, Mauro Feola, Massimo Mancone, and Francesco Fedele. 2021. "Effects of Metolazone Administration on Congestion, Diuretic Response and Renal Function in Patients with Advanced Heart Failure" Journal of Clinical Medicine 10, no. 18: 4207. https://doi.org/10.3390/jcm10184207
APA StylePalazzuoli, A., Ruocco, G., Severino, P., Gennari, L., Pirrotta, F., Stefanini, A., Tramonte, F., Feola, M., Mancone, M., & Fedele, F. (2021). Effects of Metolazone Administration on Congestion, Diuretic Response and Renal Function in Patients with Advanced Heart Failure. Journal of Clinical Medicine, 10(18), 4207. https://doi.org/10.3390/jcm10184207