1. Introduction
Febrile neutropenia (FN) is a major threat to patients that are treated with chemotherapy, as it can result in subsequent hospital admissions, life-threatening infections, treatment delays, and chemotherapy dose reduction. To prevent chemotherapy-related FN, the primary prophylactic use of granulocyte colony-stimulating factor (G-CSF) has been employed. A large-scale meta-analysis of 61 randomized controlled trials of chemotherapy with or without initial G-CSF support revealed that all-cause mortality was lower among patients who received chemotherapy with primary G-CSF support compared to that of without primary G-CSF [
1]. The primary prophylactic administration of G-CSF with pegylated granulocyte colony-stimulating factor (PEG-G-CSF) has been approved for the prevention of FN in clinical practice. However, PEG-G-CSF is not used routinely because it is expensive. According to the guidelines developed by the American Society of Clinical Oncology (ASCO) [
2], European Organisation for Research and Treatment of Cancer (EORTC) [
3], National Comprehensive Cancer Network (NCCN) [
4], and Japanese Society of Medical Oncology (JSMO) [
5], the prophylactic administration of PEG-G-CSF is recommended during regimens in which the risk of FN is ≥20%.
Amrubicin is a completely synthetic anthracycline derivative, which is characterized by a nine-amino group and a simple sugar moiety. The chemical structure and acute toxicity of amrubicin are similar to those of doxorubicin [
6,
7]; however, it causes almost no cardiotoxicity [
8,
9]. Single-agent amrubicin chemotherapy is used to treat small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC), and it is especially important as the standard second-line regimen for SCLC [
10,
11]. As the incidence rate of FN in key clinical trials of single-agent amrubicin chemotherapy was lower than 20%, i.e., 14% and 10%, respectively [
10,
11], the prophylactic administration of G-CSF is not recommended during single-agent amrubicin therapy. However, single-agent amrubicin can cause severe hematological toxicities, such as grade 4 neutropenia and FN, and is associated with a poor prognosis in the clinical setting. In addition, the incidence rates of FN during single-agent amrubicin chemotherapy varied from 0% to 33% in previous studies [
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24], and the necessity of the prophylactic use of G-CSF in patients that are treated with single-agent amrubicin in real-world settings remains unclear.
Based on these results, we conducted a retrospective multi-institutional study, involving patients with thoracic malignancies that were treated with single-agent amrubicin chemotherapy. The primary objective of the study was to determine the real-world incidence rate of FN in this population.
2. Patients and Methods
2.1. Study Design
This retrospective study was performed at four institutions (Nagasaki University Hospital, Sasebo City General Hospital, National Hospital Organization Nagasaki, and Ureshino Medical Center). The study protocol was reviewed and approved by the ethics committee of each institution. Whenever possible, the fact that the study was being conducted and the purpose of the study were disclosed to the subjects, and they were provided with an opportunity to refuse to participate. This was an independent collaborative (unsponsored) group study.
2.2. Patients and Treatment
The cases of consecutive patients with thoracic malignancies who were treated with single-agent amrubicin between January 2010 and March 2020 were retrospectively analyzed. Medical information regarding the following factors were collected: age; sex; diagnosis; clinical stage; the patients’ history of chemotherapy; the treatment line; the dose of amrubicin (mg/m2); pretreatment renal function; the duration of amrubicin therapy; bone marrow toxicities, including FN, leukopenia, neutropenia, thrombocytopenia, and anemia; urinalysis; the results of biochemical tests of renal and hepatic function and electrolyte levels; the use statuses of G-CSF and antibacterial drugs; progression-free survival (PFS); and overall survival (OS). Filgrastim, lenograstim, and nartograstim were used as therapeutic G-CSF drugs. FN was defined as being present in cases in which the patient experienced a single febrile episode involving fever ≥38.0 °C and had an absolute neutrophil count (ANC) of ≤500 cells/mm3 (or ≤1000 cells/mm3 with an expected decrease ≤ 500 cells/mm3). Neutrophil counts were checked each time to determine if a patient had a fever, and clinically expected FNs with only episodes of fever were excluded. Amrubicin was dissolved in 20 mL of normal saline and administered intravenously as a 5 min infusion at a dose of 25–45 mg/mm2 on days 1 to 3 every 3–4 weeks. Hematological toxicities were evaluated according to the Common Terminology Criteria for Adverse Events, version 4.03. The data were once collected by the researchers belonging to each hospital, then collected by the central data center, and quality checked, and inquiries about missing items and suspicious sections were addressed. In some cases, the patient was transferred to another hospital during treatment, but we contacted the transferee and collected data. All members were selected as experts who can handle the data properly.
2.3. Statistical Analysis
The primary endpoint was the incidence rate of FN. The secondary endpoints included the duration of hospitalization, whether chemotherapy dose reduction or a treatment delay was required due to hematological adverse events, PFS, and OS. All statistical analyses were performed using IBM SPSS Statistics Advanced, version 27, Japan. Two-sided p-values of <0.05 were considered statistically significant. The Kaplan–Meier method was used for the survival analyses of PFS and OS. Welch t and log-rank tests were used for the duration of hospitalization period and survival, respectively. Univariate and multivariate Cox proportional hazards analyses were used for potential risk factors. Progression-free survival was measured from the day chemotherapy commenced until the day the attending physician determined the progression of disease. Overall survival was measured from the day chemotherapy commenced until death by any cause.
4. Discussion
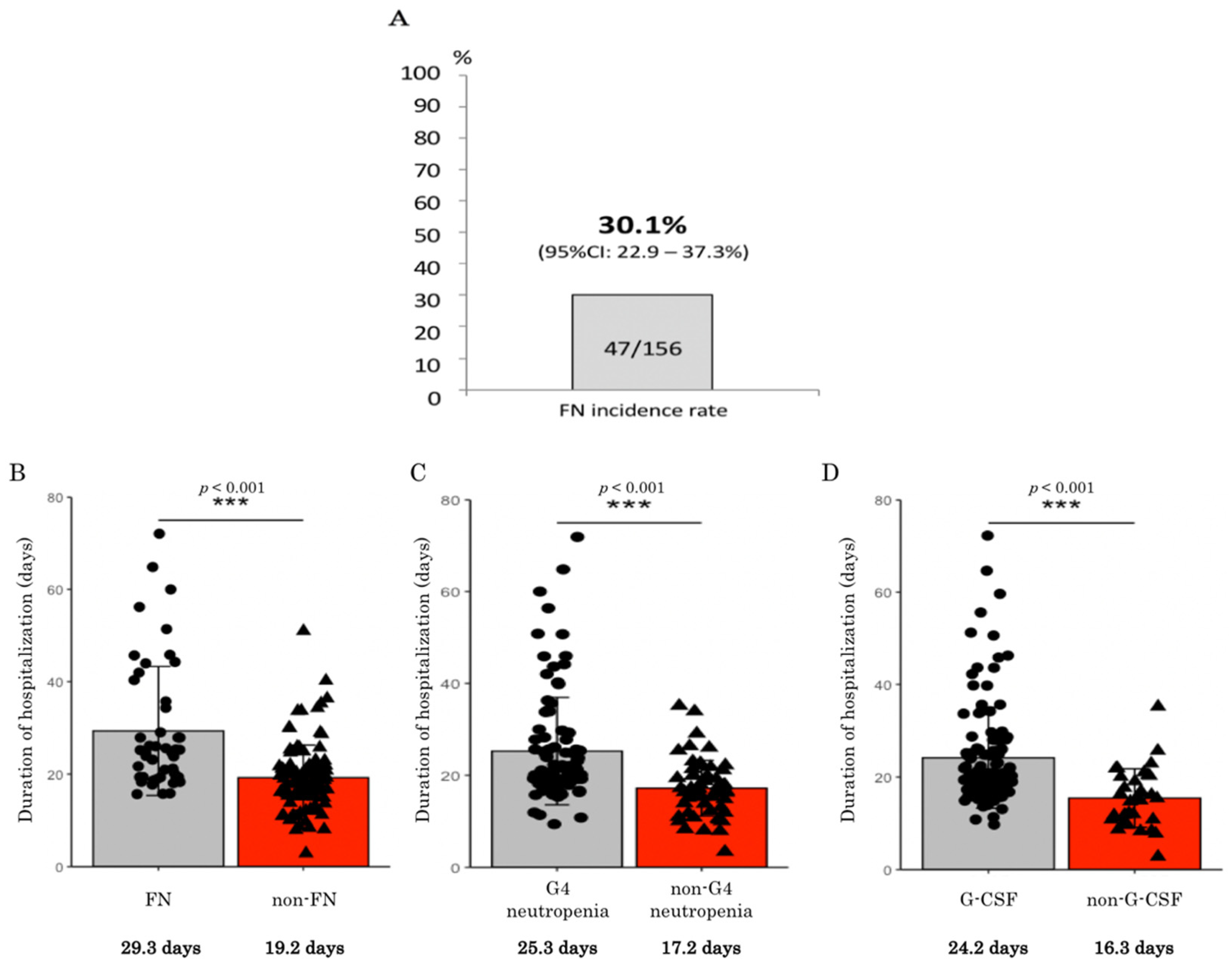
In the present study, the real-world incidence rate of FN among patients that were treated with single-agent amrubicin chemotherapy was found to be 30%, which is higher than the 20% cut-off level for the prophylactic use of PEG-G-CSF recommended in the relevant guidelines. Therefore, it is recommended that prophylactic PEG-G-CSF should be administered during single-agent amrubicin chemotherapy for patients with thoracic malignancies. In addition, the patients who developed FN exhibited significantly shorter PFS in SCLC.
Regarding FN, the incidence rates of FN in 17 prospective clinical trials of single-agent amrubicin therapy, involving 1151 patients with lung cancer, are shown in
Table 7 [
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25]. The total FN incidence rate was 12% (n = 1151, range: 0–33%). The studies conducted in Japan and other countries reported FN incidence rates of 14% (n = 625) and 10% (n = 526), respectively. The FN incidence rates in Japan tended to be higher than those seen in other countries, but the incidence rate of FN was below 20% in both types of studies. The incidence rates of FN among patients with SCLC and NSCLC were 11% (n = 780) and 14% (n = 338), respectively. The FN incidence rates in SCLC tended to be lower than those seen in NSCLC because the former included studies in other countries and first-line treatment. Most guidelines, including the ASCO, EORTC, NCCN, and JSMO guidelines, suggest that the prophylactic administration of G-CSF for regimens that carry a high risk of FN (≥20%) can improve OS, but this is not the case for regimens that carry an intermediate risk of FN (10–20%) [
2,
3,
4,
5]. Therefore, the prophylactic administration of G-CSF is not currently recommended during single-agent amrubicin chemotherapy. However, we demonstrated that the real-world FN rate was higher than 20%, the patients with FN developed shorter OS, and an episode of FN tends to be a risk factor for shorter OS in the present study. Moreover, the potential risk factors for FN were found to be PS ≥ 2 and neutrophils < 2000/µL via the multivariate Cox proportional hazards analysis. Age ≥ 75 was found to be a potential risk factor for FN via the univariate analysis. The most probable reason for the high frequency of FN in the current study than that previously reported in
Table 7 would be whether these were clinical trials or a real-world study. Patients entering clinical trials may have a PS of 0–1 and age restrictions, and are considered to be in good condition. In the actual clinical setting, elderly patients with a PS of 2 and complications present more frequently. We know that there are institutions that provide lung cancer chemotherapy under the policy of not using therapeutic G-CSF. However, we use therapeutic G-CSF when grade 4 neutropenia or FN occurs or is expected to occur, such as on weekends in the current study, so the cause of the high frequency of FN is not due to not using therapeutic G-CSF.
Dosing of amrubicin shows variability in our study; while the full dose of 45 mg/m2 was used for 19% of patients, the most commonly used dose was 35 mg/m2 because each attending physician adjusts the dose according to the patient’s condition. This is lower than previous reports of 40 mg/m2. Real-world setting studies have shown that doses will be reduced due to an increased proportion of elderly patients (median age 68 years in current study), poor PS (include PS 2 of 25% in current study), and complications compared to previously reported clinical trials. This is considered to be one of the reasons that the FN incidence rate was higher than that of studies in the same country.
Regarding the administration of prophylactic G-CSF in the present real-world study, none of the 156 patients were given prophylactic PEG-G-CSF in the first amrubicin cycle. Even when all cycles are considered, only one patient received prophylactic PEG-G-CSF during single-agent amrubicin treatment (due to severe neutropenia in the previous cycle). In Japan, the hospitalization costs paid to each institution are fixed according to the type of anticancer drug regimen that the patient receives; thus, their income is not affected by whether PEG-G-CSF, which costs JPY 108,635, is administered. This economic factor and the recommendations outlined in the treatment guidelines have contributed to the fact that PEG-G-CSF is not widely administered during single-agent amrubicin chemotherapy in clinical practice. Nevertheless, as revealed in the present study, the prognosis of the patients who developed FN was significantly shortened to a median of 1.9 months for PFS and a median of 7.2 months for OS (compared with 3.5 and 10 months for the patients without FN, respectively), and a randomized phase III study showed that prophylactic G-CSF was effective in reducing the risk of FN and infections in SCLC patients, despite the addition of prophylactic antibiotics [
26]; we should consider the administration of prophylactic PEG-G-CSF. Chemotherapy for thoracic malignancies is increasingly being administered in an outpatient setting, and the administration of primary prophylactic G-CSF will enable safer outpatient chemotherapy [
27]. On the other hand, it is also important to consider changes in regimen, drug dose, and drug schedule, instead of primary prophylactic administration of G-CSF if the purpose of chemotherapy is symptom relief.
Regarding the myelosuppression encountered in the present study, 86% of the patients that were treated with single-agent amrubicin developed grade 3 or worse neutropenia, and 62% experienced grade 4 neutropenia. Among the patients treated with single-agent amrubicin in previous studies, 39–94% developed grade 3 or worse neutropenia, and 18–79% experienced grade 4 neutropenia [
10,
11,
12,
13,
14,
15,
16,
17,
18,
19], and efforts are being made to determine appropriate amrubicin doses based on its bone marrow toxicity [
28,
29]. In studies in which amrubicin was used to treat patients with diseases other than lung cancer, grade 3 or worse neutropenia was reported to occur in 4% of the patients that were treated for thymic tumors and 60% of those that were treated for malignant pleural mesothelioma [
30,
31]. It might be difficult to complete amrubicin treatment without any problems, as clinicians will want to maintain the standard dose intensity to achieve a strong response. In the current study, 30% of patients developed FN, resulting in longer periods of hospitalization and therapeutic G-CSF and antibiotics being required for longer periods. This suggests that treatment without prophylactic PEG-G-CSF might end up being more expensive, despite the cost of PEG-G-CSF itself being avoided. Furthermore, the development of FN due to amrubicin therapy can adversely affect patients’ performance statuses and quality of life. Thirteen percent of the patients in the present study were unable to receive continuous chemotherapy due to FN. This demonstrated that FN can have an impact on survival in some cases. Consequently, we should consider the prophylactic administration of PEG-G-CSF during single-agent amrubicin chemotherapy for thoracic malignancies.
Regarding the risk factors in the present study, PS ≥ 2 was a risk factor for shorter OS. It is interesting to note that single-agent amrubicin treatment in elderly patients was not a risk factor in the present study, whether they were 65 years or older or 75 years or older. Although chemotherapy in elderly patients is known to have different results than in younger patients [
32,
33,
34], it is presumed that chemotherapy can safely be administered to even elderly patients with good PS and an appropriate dose setting. An amrubicin dose of 45 mg/m
2 was a factor for longer OS, and decrease in dose before treatment was a risk factor. This may be attributed to not only the higher efficacity of the high dose of amrubicin, but also to the attending physician’s belief that the patients had sufficient organ function, were young enough, and had a sufficiently small number of complications to be able to withstand full-dose amrubicin treatment. Patients whose dose of amrubicin is reduced may have same cause, such as poor general condition, which the attending physician considers necessary to reduce the dose. Thus, amrubicin dose is considered to be a confounding factor.
This study had several limitations. First, it was a small-scale investigation conducted at four institutions, and therefore, it was not possible to draw definitive conclusions. However, it can provide some hypotheses for future research. An external validation study involving a larger number of patients might be needed to confirm our findings. Second, this study was only conducted in one country (Japan). The FN rates in Japan tend to be higher than those observed elsewhere, as shown in
Table 7, and it is hoped that studies will be conducted in real-world settings in other regions as well. Third, we did not evaluate the cost-effectiveness of using prophylactic PEG-G-CSF. The primary prophylactic use of PEG-G-CSF is expensive compared to therapeutic G-CSF treatment, and this issue should be considered in future.
In conclusion, the real-world incidence rate of FN among patients with thoracic malignancies that were treated with single-agent amrubicin chemotherapy was 30%. It is suggested that prophylactic G-CSF should be administered during the practical use of single-agent amrubicin for patients who have already received chemotherapy.